Abstract
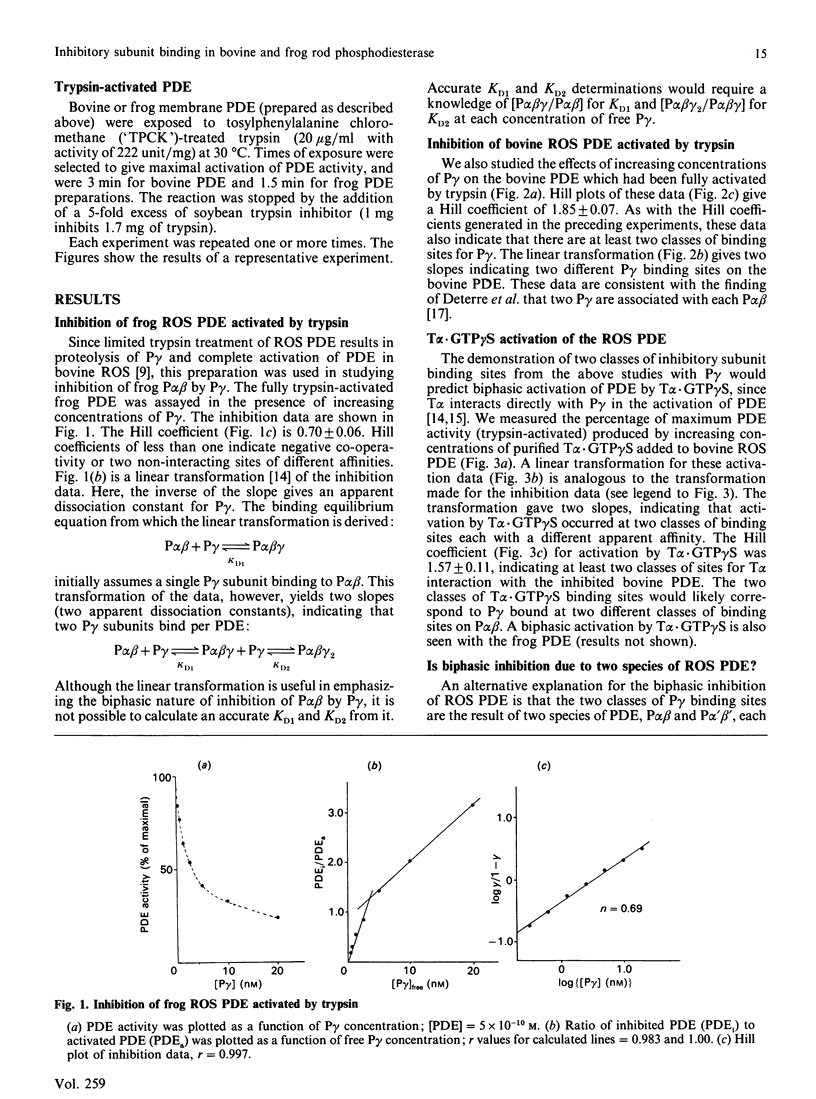
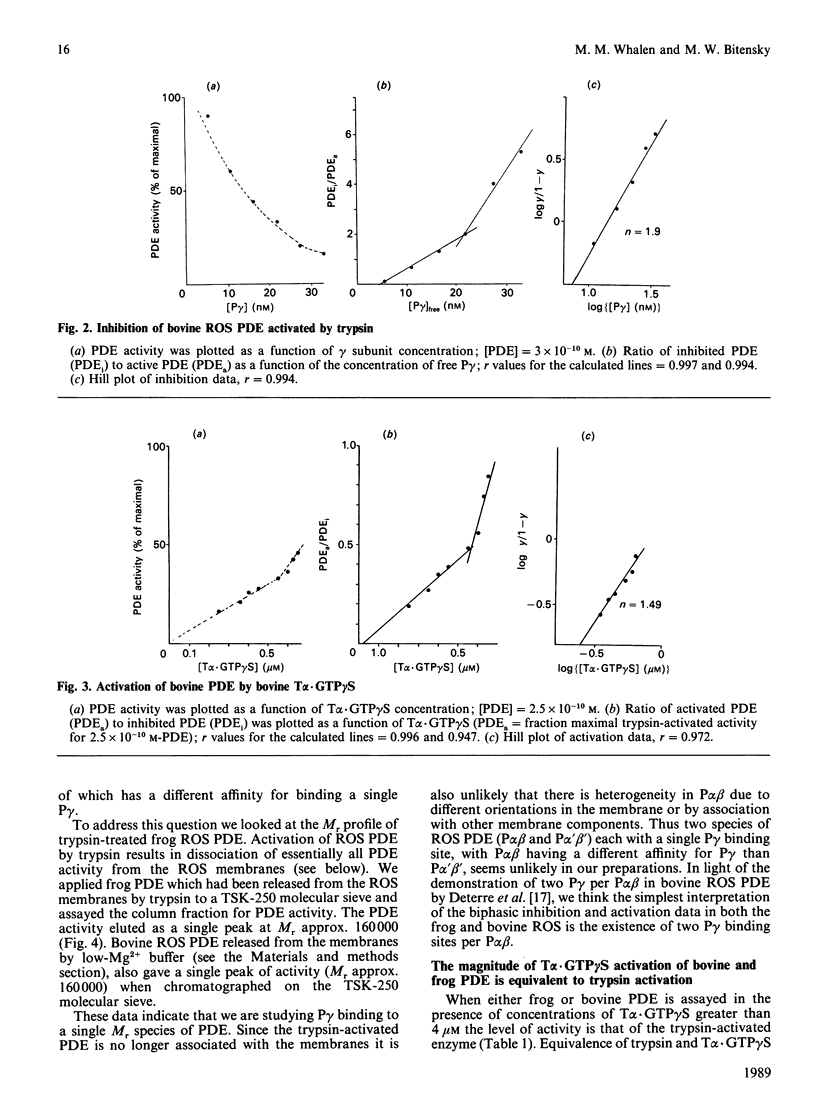
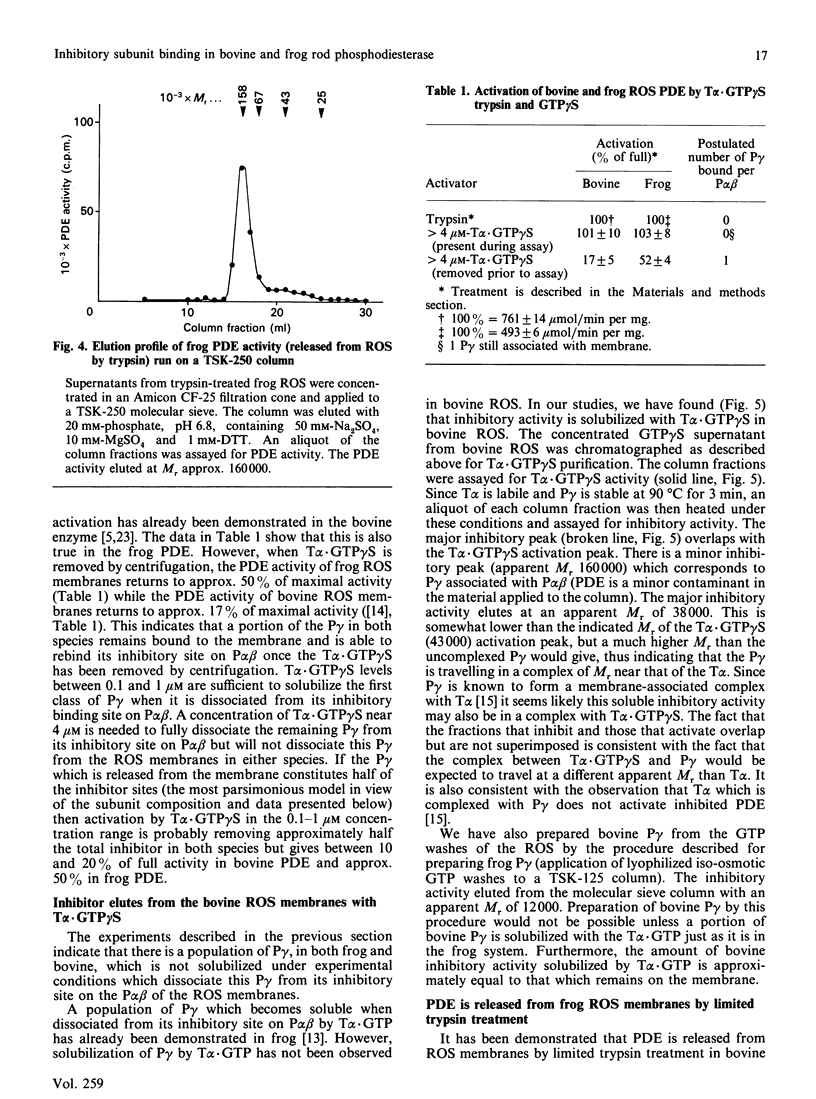
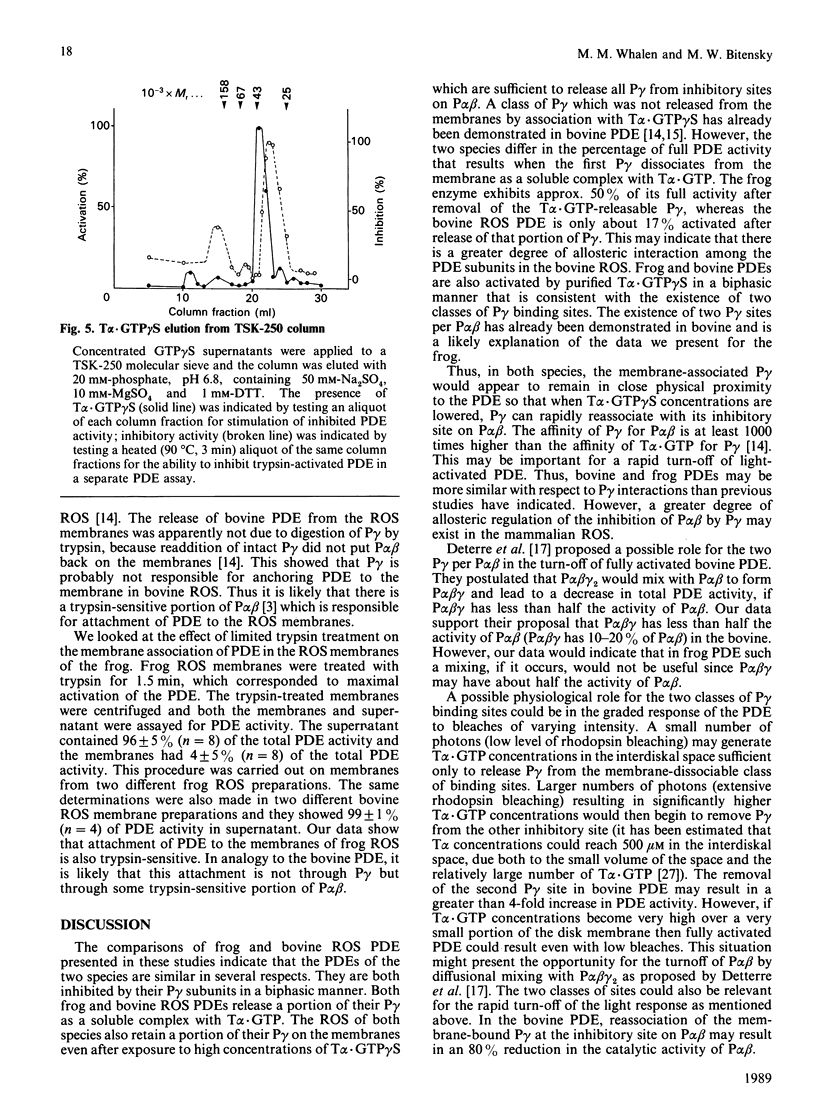
The rod outer segments of the bovine and frog retina possess a cyclic GMP phosphodiesterase (PDE) that is composed of two larger subunits, alpha and beta (P alpha beta), which contain the catalytic activity and a smaller gamma (P gamma) subunit which inhibits the catalytic activity. We studied the binding of P gamma to P alpha beta in both the bovine and frog rod outer segment membranes. Analysis of these data indicates that there are two classes of P gamma binding sites per P alpha beta in both species. The activation of PDE by the guanosine 5'-[gamma-thio]triphosphate form of the alpha subunit of transducin, T alpha.GTP gamma S, was also studied. These data indicate that the two classes of P gamma binding sites contribute to the formation of two classes of binding sites for T alpha.GTP gamma S. We demonstrate solubilization of a portion of the P gamma by T alpha.GTP gamma S in both species. There is also present, in both species, a second class of P gamma which is not solubilized even when it is dissociated from its inhibitory site on P alpha beta by T alpha.GTP gamma S. The amount of full PDE activity which results from release of the solubilizable P gamma is about 50% in the frog PDE but only approx. 17% in the bovine PDE. We also show that activation of frog rod outer segment PDE by trypsin treatment releases the PDE from the membranes. This type of release by trypsin has already been demonstrated in bovine rod outer segments [Wensel & Stryer (1986) Proteins: Struct. Funct. Genet. 1, 90-99].
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Devlin M. J., Applebury M. L. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979 Nov 25;254(22):11669–11677. [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chabre M. Trigger and amplification mechanisms in visual phototransduction. Annu Rev Biophys Biophys Chem. 1985;14:331–360. doi: 10.1146/annurev.bb.14.060185.001555. [DOI] [PubMed] [Google Scholar]
- Deterre P., Bigay J., Forquet F., Robert M., Chabre M. cGMP phosphodiesterase of retinal rods is regulated by two inhibitory subunits. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2424–2428. doi: 10.1073/pnas.85.8.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deterre P., Bigay J., Robert M., Pfister C., Kühn H., Chabre M. Activation of retinal rod cyclic GMP-phosphodiesterase by transducin: characterization of the complex formed by phosphodiesterase inhibitor and transducin alpha-subunit. Proteins. 1986 Oct;1(2):188–193. doi: 10.1002/prot.340010210. [DOI] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Fung B. K., Hurley J. B., Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A. 1981 Jan;78(1):152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godchaux W., 3rd, Zimmerman W. F. Membrane-dependent guanine nucleotide binding and GTPase activities of soluble protein from bovine rod cell outer segments. J Biol Chem. 1979 Aug 25;254(16):7874–7884. [PubMed] [Google Scholar]
- Halliday K. R., Stein P. J., Chernoff N., Wheeler G. L., Bitensky M. W. Limited trypsin proteolysis of photoreceptor GTP-binding protein. Light- and GTP-induced conformational changes. J Biol Chem. 1984 Jan 10;259(1):516–525. [PubMed] [Google Scholar]
- Hamm H. E., Bownds M. D. Protein complement of rod outer segments of frog retina. Biochemistry. 1986 Aug 12;25(16):4512–4523. doi: 10.1021/bi00364a010. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Stryer L. Purification and characterization of the gamma regulatory subunit of the cyclic GMP phosphodiesterase from retinal rod outer segments. J Biol Chem. 1982 Sep 25;257(18):11094–11099. [PubMed] [Google Scholar]
- Kohnken R. E., Eadie D. M., Revzin A., McConnell D. G. The light-activated GTP-dependent cyclic GMP phosphodiesterase complex of bovine retinal rod outer segments. Dark resolution of the catalytic and regulatory proteins. J Biol Chem. 1981 Dec 10;256(23):12502–12509. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miki N., Baraban J. M., Keirns J. J., Boyce J. J., Bitensky M. W. Purification and properties of the light-activated cyclic nucleotide phosphodiesterase of rod outer segments. J Biol Chem. 1975 Aug 25;250(16):6320–6327. [PubMed] [Google Scholar]
- Northup J. K., Smigel M. D., Gilman A. G. The guanine nucleotide activating site of the regulatory component of adenylate cyclase. Identification by ligand binding. J Biol Chem. 1982 Oct 10;257(19):11416–11423. [PubMed] [Google Scholar]
- Papermaster D. S. Preparation of retinal rod outer segments. Methods Enzymol. 1982;81:48–52. doi: 10.1016/s0076-6879(82)81010-0. [DOI] [PubMed] [Google Scholar]
- Shinozawa T., Uchida S., Martin E., Cafiso D., Hubbell W., Bitensky M. Additional component required for activity and reconstitution of light-activated vertebrate photoreceptor GTPase. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1408–1411. doi: 10.1073/pnas.77.3.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaramayya A., Harkness J., Parkes J. H., Gonzalez-Oliva C., Liebman P. A. Kinetic studies suggest that light-activated cyclic GMP phosphodiesterase is a complex with G-protein subunits. Biochemistry. 1986 Feb 11;25(3):651–656. doi: 10.1021/bi00351a021. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Wensel T. G., Stryer L. Reciprocal control of retinal rod cyclic GMP phosphodiesterase by its gamma subunit and transducin. Proteins. 1986 Sep;1(1):90–99. doi: 10.1002/prot.340010114. [DOI] [PubMed] [Google Scholar]
- Wheeler G. L., Bitensky M. W. A light-activated GTPase in vertebrate photoreceptors: regulation of light-activated cyclic GMP phosphodiesterase. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4238–4242. doi: 10.1073/pnas.74.10.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki A., Bartucca F., Ting A., Bitensky M. W. Reciprocal effects of an inhibitory factor on catalytic activity and noncatalytic cGMP binding sites of rod phosphodiesterase. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3702–3706. doi: 10.1073/pnas.79.12.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki A., Stein P. J., Chernoff N., Bitensky M. W. Activation mechanism of rod outer segment cyclic GMP phosphodiesterase. Release of inhibitor by the GTP/GTP-binding protein. J Biol Chem. 1983 Jul 10;258(13):8188–8194. [PubMed] [Google Scholar]
- Yamazaki A., Tatsumi M., Bitensky M. W. Purification of rod outer segment GTP-binding protein subunits and cGMP phosphodiesterase by single-step column chromatography. Methods Enzymol. 1988;159:702–710. doi: 10.1016/0076-6879(88)59065-1. [DOI] [PubMed] [Google Scholar]
- Yamazaki A., Tatsumi M., Torney D. C., Bitensky M. W. The GTP-binding protein of rod outer segments. I. Role of each subunit in the GTP hydrolytic cycle. J Biol Chem. 1987 Jul 5;262(19):9316–9323. [PubMed] [Google Scholar]


