Abstract
Background
Acute kidney injury (AKI) occurs frequently in ST-elevation myocardial infarction with cardiogenic shock (CS-STEMI) and is a strong independent prognostic marker for short and intermediate-term outcomes. Owing to the delayed presentation and limited facilities for primary percutaneous coronary intervention in low- and middle-income countries, the incidence, predictors, and outcome of AKI are likely to be different compared to the developed countries. We performed a post hoc analysis of patients presenting with CS-STEMI over 7 years (2016–2022) at a tertiary referral center in North India. The primary outcome assessed was AKI and the secondary outcome was in-hospital mortality.
Results
Of the 426 patients, 194 (45.5%) patients developed AKI, as defined by the Kidney Disease Improving Global Outcomes criteria. Left ventricular (LV) pump failure with pulmonary edema [Odds ratio (OR) 1.67; 95% confidence interval (CI) 1.04–2.67], LV ejection fraction (OR 1.35 per 10% decrease in ejection fraction; CI 1.04–1.73), complete heart block (OR 2.06; CI 1.2–3.53), right ventricular infarction (OR 2.76; CI 1.39–5.49), mechanical complications (OR 3.89; CI 1.85–8.21), ventricular tachycardia (OR 2.80; CI 1.57–4.99), and non-revascularization (OR 2.2; CI 1.33–3.67) were independent predictors of AKI in multivariate logistic regression analysis. Additionally, AKI was a strong predictor of in-hospital mortality (univariate OR 30.61, CI 17.37–53.95).
Conclusions
There is a higher incidence of AKI in CS-STEMI in resource-limited settings and is associated with adverse short-term outcomes. Additional studies are needed to address the optimal strategies for the prevention and management of AKI in such settings.
Keywords: Cardiogenic shock, ST-elevation myocardial infarction, AKI, Mortality
Background
Cardiogenic shock (CS) is the leading cause of mortality and adverse outcomes in ST-elevation myocardial infarction (STEMI). Although the outcomes of cardiogenic shock complicating STEMI (CS-STEMI) have improved considerably over the past two decades, they remain sub-optimal [1–4], especially in low- and middle-income countries such as India, which have limited access to primary percutaneous coronary intervention (PCI) and mechanical circulatory support (MCS) devices [5]. Renal function is an important variable and end-point in the context of CS [6]. In contrast with chronic heart failure, the interplay between acute myocardial dysfunction, hypotension, and renal function gets magnified in CS-STEMI. The renal hypoperfusion secondary to low cardiac output, and raised central and renal venous pressures causes acute deterioration of renal function and acute kidney injury (AKI) [7–9]. Other contributory factors may include contrast use and systemic atheroembolism during percutaneous coronary intervention (PCI), cardiopulmonary bypass (CPB) during coronary artery bypass grafting (CABG), and positive pressure ventilation (PPV) [10–12].
Studies have demonstrated that AKI is one of the strongest predictors of both short- and long-term outcomes and the long-term need for chronic dialysis in CS-STEMI [5, 7, 13–18] and acts not only as a surrogate marker of the severity of CS but also as a direct mediator of adverse outcomes. Early (< 24 h) development of AKI after the onset of CS is associated with an especially high likelihood of mortality [19]. Additionally, AKI is also associated with a longer duration of hospital stay and hospitalization costs [15].
Compared to the developed world, patients with STEMI in India present later, and are less likely to receive primary PCI or emergency CABG. Hence, they have a higher likelihood of developing AKI. [5, 20–22] We have previously demonstrated the role of AKI in the prognosis of CS-STEMI in a smaller subgroup of late-presenting patients with CS-STEMI [5, 23]. We aimed to study the predictive role of multiple variables for the incidence of AKI and its outcomes in Indian patients with CS-STEMI.
Methods
Study design
We performed a post hoc analysis of a single-center registry of patients with CS-STEMI presenting to a tertiary referral hospital in North India. The study conforms to the ethical guidelines of the Declaration of Helsinki and was reviewed and cleared by the institutional ethics committee of the Postgraduate Institute of Medical Education and Research, Chandigarh, India. Informed written consent was obtained from all patients or appropriate legally authorized representatives.
Patient selection and evaluation
The study enrolled consecutive patients with CS-STEMI over 7 years (January 2016 to December 2022). STEMI was defined by the European Society of Cardiology/ the American College of Cardiology / the American Heart Association/ World Heart Federation (ESC/ACCF/AHA/WHF) third universal definition [24]. We considered CS as systolic blood pressure (SBP) < 90 mm Hg for at least 30 min or the need for supportive measures to maintain SBP > 90 mm Hg despite adequate filling pressures, and signs of end-organ hypoperfusion (oliguria/cold extremities/altered sensorium) [25]. Patients with non-ST-elevation MI (NSTEMI), prior resuscitated cardiac arrest, and known end-stage renal disease (ESRD) were excluded (Fig. 1). Left ventricular (LV) ejection fraction was calculated by the Simpson's method using a 2-D transthoracic echocardiogram (Vivid Q, GE Healthcare, New York, USA). The severity of mitral regurgitation (MR) was graded by the established criteria [26].
Fig. 1.
STROBE diagram of the patients in the study. ACS = acute coronary syndrome; CS = cardiogenic shock; NSTEMI = Non-ST-elevation myocardial infarction; CS-STEMI = ST-elevation myocardial infarction complicated by cardiogenic shock
Standard tests including hemoglobin, complete blood counts, renal function tests (serum urea and creatinine), liver function tests, high-sensitivity troponin-I, creatine kinase-MB (CK-MB), and coagulation parameters were performed upon hospital admission. Serum urea and creatinine were repeated during the hospital stay every 24–48 h for the initial 3–4 days and then as dictated by the clinical circumstances. The monitoring of urine output was done hourly by the trained nursing staff. The estimated glomerular filtrate rate (eGFR) was calculated using the chronic kidney disease epidemiology collaboration (CKD-EPI) equation [27]. AKI was defined as per the Kidney Disease: Improving Global Outcomes (KDIGO) definition as an increase in serum creatinine by 0.3 mg/dl within 48 h or 1.5 times baseline in last 7 days or urine output £ 0.5 ml/kg/h for 6 h [28].
Management strategy
The management, including the use of inotropes and circulatory assist devices, and timing of revascularization was decided by the treating physician, based on the delay in presentation, hemodynamic variables, end-organ failure including AKI, neurological status, mechanical complications, and patient willingness [5]. The decision regarding the initiation of renal replacement therapy was taken in consultation with a nephrologist based on acid–base balance, fluid and electrolyte status, urine output, and renal function tests. All patients were treated according to guideline-directed medical treatment and PCI or fibrinolysis for patients not consenting to PCI. The default revascularization strategy was culprit vessel primary PCI. Non-ionic, iso-osmolar (Iodixanol), or low-osmolar (Iohexol) contrast agents were used for performing angiograms and PCI.
Baseline characteristics and in-hospital courses were recorded by trained physicians (cardiology fellows). The angiographic profile was analyzed by trained interventional cardiologists. The primary outcome was AKI; while, the secondary outcome included in-hospital mortality.
Statistical analyses
Data was entered into a spreadsheet (Microsoft Excel 2016, Microsoft Corporation, USA), and statistical analysis was done using the Statistical Package for Social Sciences (SPSS Inc. version 23.0, IBM Corporation, Chicago, USA). All continuous variables were described as mean (SD) or median [interquartile range (IQ)] and categorical variables as percentages and counts. The difference between two groups for a continuous variable was assessed using the independent t test (parametric) or Mann–Whitney test (non parametric) and between two categorical variables with chi-square or Fisher's exact test. A binary logistic regression analysis was performed to determine the independent predictors for AKI and included variables with p ≤ 0.10 on univariate analysis. All other p-values are two-tailed and set at a statistical significance of 0.05.
Results
A total of 426 patients were included, of whom 194 (45.5%) developed AKI (Fig. 1). The cohort was divided into two groups: Group 1 [those who did not develop AKI (n = 232)] and Group 2 [those who developed AKI (n = 194)].
Baseline clinical characteristics
The patients in group 2 who developed AKI were older, more likely to be female, diabetic, hypertensive, late presenters, had lower LV ejection fraction, a higher chance of LV pump failure with pulmonary edema, and right ventricular MI compared to group 1. The group 2 had a higher incidence of MI-associated left bundle branch block (LBBB) (5.2% vs. 0.9%; p = 0.008), complete heart block (CHB) [28.9% vs. 19.8%; p = 0.03], ventricular tachycardia (VT) [26.3% vs. 11.2%; p = < 0.001], and mechanical complications [severe MR (10.2% vs. 3%; p = 0.002) and ventricular septal rupture (5.2% vs. 1.7%; p = 0.048)]. The differences in the location of MI (57.8% vs. 60.3; p = 0.59) and the proportion of patients who received fibrinolysis (50.4% vs. 45.9%; p = 0.34) between the two groups were statistically insignificant (Table 1).
Table 1.
Comparison between patients with and without AKI
| Characteristic | Group 1 (No AKI) N = 232 |
Group 2 (AKI) N = 194 |
P-value |
|---|---|---|---|
| Age, years, mean (SD) | 58.33 (12.18) | 60.63 (11.38) | 0.046 |
| Sex, n (%) | |||
| Male | 181 (78) | 125 (65.4) | 0.002 |
| Female | 51 (22) | 69 (35.6) | |
| Risk factors, n (%) | |||
| Diabetes Mellitus | 77 (33.2) | 88 (45.4) | 0.01 |
| Hypertension | 89 (38.4) | 98 (50.5) | 0.01 |
| Smoking | 113 (48.7) | 73 (37.6) | 0.02 |
| Family History of CAD | 19 (8.2) | 15 (7.7) | 0.86 |
| Prior CVA | 5 (2.2) | 7 (3.6) | 0.36 |
| Prior MI | 20 (8.6) | 17 (8.8) | 0.95 |
| Prior PCI/CABG | 5 (2.2) | 7 (3.6) | 0.36 |
| Time to presentation, hours, median (IQ) | 12 (6–24) | 15 (6–36) | 0.07 |
| Anterior MI, n (%) | 134 (57.8) | 117 (60.3) | 0.59 |
| LV pump failure with pulmonary edema, n (%) | 109 (47) | 129 (66.5) | < 0.001 |
| LV ejection fraction, %, mean ± SD | 32.65 ± 10.2 | 29.8 ± 10.03 | 0.004 |
| Fibrinolysis, n (%) | 117 (50.4) | 89 (45.9) | 0.34 |
| Conduction abnormalities, n (%) | |||
| RBBB | 25 (10.9) | 31 (16) | 0.11 |
| LBBB | 2 (0.9) | 10 (5.2) | 0.008 |
| CHB | 46 (19.8) | 56 (28.9) | 0.03 |
| Right ventricular MI, n (%) | 22 (9.5) | 33 (17) | 0.02 |
| Ventricular tachycardia, n (%) | 26 (11.2) | 51 (26.3) | < 0.001 |
| Mechanical complications, n (%) | 13 (5.6) | 35 (18) | < 0.001 |
| Severe MR | 7 (3) | 20 (10.2) | 0.002 |
| Ventricular septal rupture | 4 (1.7) | 10 (5.2) | 0.048 |
| Cardiac rupture | 2 (0.9) | 5 (2.6) | 0.16 |
| Coronary angiogram, n (%) | 182 (78.4) | 81 (41.8) | < 0.001 |
| Creatinine, mg/dl, mean ± SD | 1.1 ± 0.61 | 2.0 ± 1.29 | < 0.001 |
| eGFR, mL/min/1.73m2, median (IQ) | 76 (52.3–99.3) | 39.5 (25.9–58.1) | < 0.001 |
AKI Acute kidney injury, CABG Coronary artery bypass grafting, CAD Coronary artery disease, CHB Complete heart block, CVA Cerebrovascular accident, eGFR estimated glomerular filtrate rate, IQ Interquartile range, LBBB Left bundle branch block, LV Left ventricular, MI Myocardial infarction, MR Mitral regurgitation, PCI Percutaneous coronary intervention, RBBB Right bundle branch block
Angiographic profile
Patients in group 2 were less likely to undergo an angiogram (41.8% vs. 78.4%; p = 0.001) and PCI (24.7% vs. 48.7%; p = 0.001) and more likely to have an intra-aortic balloon pump (IABP) implanted. The IABP was the only assist device used because of the non-availability of other support devices. The culprit vessel and pattern of coronary involvement were not significantly different between the two groups (Table 2).
Table 2.
Comparison of angiographic characteristics between patients with and without AKI
| Characteristic | No AKI N = 232 |
AKI N = 194 |
P-value |
|---|---|---|---|
| Coronary angiogram, n (%) | 182 (78.4) | 81 (41.8) | < 0.001 |
| Culprit lesion, n (%)* | |||
| LAD | 100 (54.9) | 42 (51.9) | 0.80 |
| LCX | 12 (6.6) | 7 (8.6) | |
| RCA | 70 (38.5) | 32 (39.5) | |
| Coronary artery involved, n (%)* | |||
| LMCA | 6 (3.3) | 7 (8.6) | 0.11 |
| LAD | 136 (74.7) | 60 (74.1) | 0.91 |
| LCX | 64 (35.2) | 37 (45.7) | 0.10 |
| RCA | 115 (63.2) | 52(64.2) | 0.87 |
| Type of vessel involvement, n (%)* | |||
| Single-vessel disease | 88 (48.4) | 32 (39.5) | 0.18 |
| Double-vessel disease | 55 (30.2) | 29 (35.8) | 0.37 |
| Triple-vessel disease | 39 (21.5) | 20 (24.7) | 0.60 |
| LMCA/Multi-vessel disease | 95 (52.2) | 52 (64.2) | 0.07 |
| Occlusion of culprit vessel, n (%)* | 66 (36.5) | 36 (44.4) | 0.22 |
| CTO of non-culprit vessel, n (%)* | 15 (8.2) | 13 (16) | 0.058 |
| PCI, n (%) | 113 (48.7) | 48 (24.7) | < 0.001 |
| GP IIb/IIIa inhibitor use, n (%) | 72 (31) | 48 (24.7) | 0.15 |
| IABP use, n (%) | 14 (6) | 29 (14.9) | 0.002 |
AKI Acute kidney injury, CTO Chronic total occlusion, IABP Intra-aortic balloon pump, LAD Left anterior descending artery, LCX Left circumflex artery, LMCA Left main coronary artery, PCI Percutaneous coronary intervention, RCA Right coronary artery
*Values are based on 263 patients (182 without and 81 with AKI) who underwent an angiogram. More than 70% stenosis of the LAD, RCA, LCX, and more than 50% stenosis of the LMCA was considered significant
Multivariate analysis
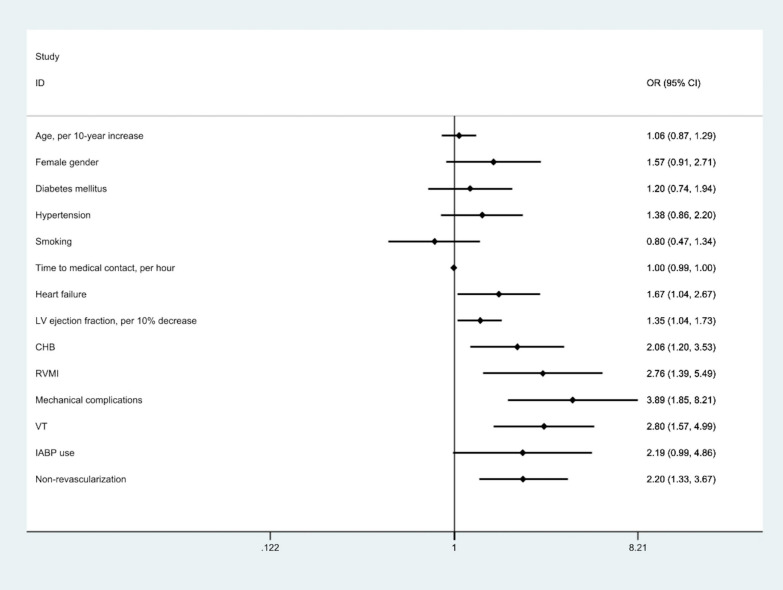
A binary logistic regression analysis was performed to determine the effects of age, gender, diabetes, hypertension, smoking, time to presentation, LV ejection fraction, LV failure, LBBB, CHB, RVMI, mechanical complications, VT, IABP use, left main/multivessel disease and non-revascularization on AKI. Of these, left ventricular (LV) pump failure with pulmonary edema [Odds ratio (OR) 1.67; 95% confidence interval (CI) 1.04–2.67], LV ejection fraction (OR 1.35 per 10% decrease in ejection fraction; CI 1.04–1.73), CHB (OR 2.06; CI 1.2–3.53), RVMI (OR 2.76; CI 1.39–5.49), mechanical complications (OR 3.89; CI 1.85–8.21), VT (OR 2.80; CI 1.57–4.99), and non-revascularization (OR 2.2; CI 1.33–3.67) were independent predictors of AKI in multivariate regression analysis (Table 3 and Fig. 2). The overall in-hospital mortality was 38% and was significantly higher in patients with AKI compared to those without (73.2% vs. 8.2%; p < 0.001; univariate OR of AKI for in-hospital mortality 30.61, CI 17.37–53.95).
Table 3.
Logistic regression analysis for independent predictors of AKI
| Variable | Odds Ratios | C.I | P-value |
|---|---|---|---|
| Mechanical complications | 3.89 | 1.85–8.21 | < 0.001 |
| Ventricular tachycardia | 2.80 | 1.57–4.99 | < 0.001 |
| Right ventricular MI | 2.76 | 1.39–5.49 | 0.004 |
| Non-revascularization | 2.2 | 1.33–3.67 | 0.002 |
| Complete heart block | 2.06 | 1.2–3.53 | 0.009 |
| Left ventricular (LV) pump failure with pulmonary edema | 1.67 | 1.04–2.67 | 0.03 |
| LV ejection fraction, per 10% decrease | 1.35 | 1.04–1.73 | 0.027 |
| Age, per 10-year increase | 1.06 | 0.87–1.29 | 0.55 |
| Female gender | 1.57 | 0.91–2.71 | 0.10 |
| Diabetes mellitus | 1.20 | 0.74–1.94 | 0.45 |
| Hypertension | 1.38 | 0.86–2.20 | 0.179 |
| Smoking | 0.80 | 0.47–1.34 | 0.40 |
| Time to medical contact, per hour | 0.997 | 0.99–1.003 | 0.32 |
| LBBB | 4.74 | 0.79–28.49 | 0.09 |
| IABP use | 2.19 | 0.99–4.86 | 0.052 |
| LMCA/multi-vessel disease | 1.56 | 0.8–3.01 | 0.184 |
Italic value indicates p < 0.05
AKI Acute kidney injury, CI Confidence interval, IABP Intra-aortic balloon pump, LBBB Left bundle branch block, LMCA Left main coronary artery, LV Left ventricular, MI Myocardial infarction
The linearity of the continuous variables with respect to the logit of the dependent variable was analyzed using the Box–Tidwell procedure. A Bonferroni correction was applied using all 20 elements in the model resulting in statistical significance being accepted when p < .0025. Based on this assessment, all continuous independent variables were found to be linearly related to the logit of the dependent variable. The logistic regression model was statistically significant [χ2(4) = 28.36, p < .0001]. The model explained 52% (Nagelkerke R2) of the variance in in-hospital mortality and correctly classified 87.5% of cases
Fig. 2.
Predictors of acute kidney injury in STEMI with cardiogenic shock. CHB = complete heart block; CI = confidence interval; IABP = intra-aortic balloon pump; LV = left ventricular; OR = odds ratio; RVMI = right ventricular myocardial infarction; VT = ventricular tachycardia
Discussion
In a 7-year study of CS-STEMI at a tertiary referral hospital, we found that 45% of an unselected cohort of patients either presented with/developed AKI during the hospital stay and had significantly higher in-hospital mortality compared to those without AKI. Left ventricular failure, LV ejection fraction, CHB, RVMI, mechanical complications, VT, and non-revascularization were associated with a higher risk of AKI.
“The incidence rate of AKI in our study was significantly higher than that reported from studies performed in the developed countries [15, 17, 22]. This may be attributed to a significant delay in presentation and lower rates of revascularization. Also, the predominance of fibrinolysis as a revascularization strategy may have resulted in a larger number of patients with unsuccessful revascularization persistent occlusion of the culprit vessel, and higher chances of AKI. Previous studies have demonstrated that single- or multi-organ failure, especially renal failure is associated with higher in-hospital mortality and resource utilization in CS-STEMI [5, 7, 15, 18, 19, 29]. Also, AKI is associated with significant post-discharge resource utilization and 30-day readmission [15, 17, 30]. Our findings of significantly higher in-hospital mortality in patients with AKI are in line with the established literature (Table 4). The lower rates of coronary angiogram and PCI in patients with AKI reflect the reluctance to perform an angiogram due to the higher anticipated risk [15].
Table 4.
Overview of studies assessing predictors and outcomes of AKI in CS-STEMI
| Study | No. of patients | AKI incidence (%) | Predictors of AKI | Mortality (AKI vs. no AKI) |
|---|---|---|---|---|
| Hochman et al. [22] | 302 | 18 | – | – |
| Koreny et al. [19] | 118 | 33 | Age > 75, diabetes, mechanical complications, mechanical ventilation |
87% versus 53% AKI only independent predictor of mortality |
| Marenzi et al. [18] | 97 | 55 | Age > 75, LV ejection fraction < 40%, mechanical ventilation |
50% versus 2.2% AKI strongest predictor of mortality |
| Lauridosn et al. [17] | 5079 | 13 | – |
62 versus 36% 5-year risk of dialysis 11% versus 1% |
| Arbel et al. [34] | 48 | 60 | Positive fluid balance | Positive fluid balance associated with higher mortality (68% vs. 10%) and low likelihood of recovery of renal function |
| Margolis et al. [35] | 84 | 60 | Positive fluid balance | Positive fluid balance associated with low likelihood of recovery of renal function |
| Tarvasmaki et al. [9] | 125 | 31 | Age, CKD, lower arterial pH, higher CVP | AKI associated with high mortality |
| Van den Akker et al. [8] | 39 | 61.5 | CVP | 38% versus 7% |
| Vallabhajosyula et al. [15] | 440257 | 35 | Age, black race, non-private insurance, higher comorbidity, organ failure, cardiac and non-cardiac organ support | 46% versus 34% |
| Adegbala et al. [7] | 8900 | 6 | – | 76% versus 51% |
| Current study | 426 | 45 | LV failure with pulmonary edema, LV ejection fraction, CHB, RVMI, mechanical complications, VT, non-revascularization | 73% vs 8% |
AKI Acute kidney injury, CHB Complete heart block, CKD Chronic kidney disease, CS-STEMI Cardiogenic shock complicating ST-elevation myocardial infarction, CVP Central venous pressure, LV Left ventricular, MI Myocardial infraction, RVMI Right ventricular MI, VT Ventricular tachycardia
Table 4 shows a comparison of the current with the results of our study with the results of a number of previous relevant studies. Although higher age, male sex, and comorbidities are associated with AKI [9, 15, 19, 31], our study may not have been adequately powered to reveal these differences. The slightly higher rates of AKI in females may have been due to lower rates of revascularization compared to males. Heart failure, LV ejection fraction, and ventricular arrhythmias have previously been shown to be independent correlates of AKI [18, 19, 32]. Complete heart block, mechanical complications, and RVMI cause CS due to low cardiac output and predispose to the development of AKI. High right atrial pressure in RVMI has been shown to lead to worsening renal functions in CS-STEMI [33]. Although central venous pressure and mechanical ventilation are other strong predictors of AKI, we did not assess these in our study. Since baseline serum creatinine and eGFR were not available for many patients, we did not assess the prognostic role of chronic kidney disease (CKD) in the study.
We assessed the renal function and eGFR using serum creatinine measurement only. Although several novel biomarkers such as neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), and cystatin C help in the assessment of renal function, serum creatinine is the most widely utilized marker and is the strongest predictor of 30-day and 12-month mortality and need of renal replacement therapy at 30 days [6, 13, 16]. Hence, the newer biomarkers do not provide any incremental prognostic value over the measurement of serum creatinine.
There are several limitations to the study. This was a post hoc analysis of single-center data with a possible referral bias since it caters to a large population and most of the patients are referred from smaller centers. The low rates of revascularization due to late presentation, lack of adequate health insurance, and non-availability of assistive devices other than IABP may have affected the findings of the study. Also, we did not distinguish between AKI due to CS or due to the use of contrast agents during cardiac catheterization. Also, since we did not record the contrast volume, its impact on renal function remains unknown. A detailed assessment of hemodynamics may have yielded additional prognostic variables. Additionally, we did not assess other biomarkers such as NGAL, KIM-1, and cystatin C due to logistic issues. The impact of Extracorporeal membrane oxygenation (ECMO) and mechanical circulatory support on the incidence and outcomes of AKI was not assessed, as done by several authors [9, 36–38]. Also, the impact of renal replacement therapy on intermediate-term outcomes remains unknown in the current study since we only assessed the in-hospital outcomes. Since follow-up details were not recorded, intermediate- and long-term mortality and the need for renal replacement therapy are unknown.
Conclusions
We found a high incidence of AKI in patients presenting at a tertiary center with CS-STEMI in a low-middle-income country. Acute kidney injury was a strong predictor of in-hospital mortality. Heart failure, LV ejection fraction, CHB, RVMI, mechanical complications, VT, and non-revascularization were independent predictors of the development of AKI. As AKI is likely to be a common problem in CS-STEMI in resource-limited settings, establishing the ideal prevention and management strategies with appropriate studies remains pertinent.
Acknowledgements
Not applicable.
Abbreviations
- AKI
Acute kidney injury
- CS-STEMI
ST-elevation myocardial infarction with cardiogenic shock
- LV
Left ventricular
- KDIGO
Kidney Disease Improving Global Outcomes
- OR
Odds ratio
- PCI
Primary percutaneous coronary intervention
- MCS
Mechanical circulatory support
- CPB
Cardiopulmonary bypass
- PPV
Positive pressure ventilation
- CABG
Coronary artery bypass grafting
- MR
Mitral regurgitation
- ESC/ACCF/AHA/WHF
European Society of Cardiology/ the American College of Cardiology / the American Heart Association/ World Heart Federation
- SBP
Systolic blood pressure
- ESRD
End-stage renal disease
- CK-MB
Creatine kinase–myocardial band
- eGFR
Estimated glomerular filtrate rate
- CKD-EPI
Chronic kidney disease epidemiology collaboration
- LBBB
Left bundle branch block
- CHB
Complete heart block
- VT
Ventricular tachycardia
- IABP
Intra-aortic balloon pump
- RVMI
Right ventricular myocardial infarction
- CKD
Chronic kidney disease
- NGAL
Neutrophil gelatinase-associated lipocalin
- KIM-1
Kidney injury molecule-1
- ECMO
Extracorporeal membrane oxygenation
Author contributions
KK contributed to conceptualization, writing, analysis and interpretation of the manuscript. YP and DK contributed to data acquisition, content development, review and editing manuscript; while, MD contributed to data collection, analysis and follow-up.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study confirms to the ethical guidelines of the Declaration of Helsinki and was reviewed and cleared by the institutional ethics committee of the Postgraduate Institute of Medical Education and Research, Chandigarh, India (INT/IEC/2017/330). Written inform consent was obtained from all patients or appropriate legally authorized representatives.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S et al (2014) Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc 3(1):e000590 10.1161/JAHA.113.000590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS, NRMI Investigators (2005) Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 294(4):448–54 10.1001/jama.294.4.448 [DOI] [PubMed] [Google Scholar]
- 3.Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, LeJemtel TH (1999) Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med 341:625–634 10.1056/NEJM199908263410901 [DOI] [PubMed] [Google Scholar]
- 4.Thiele H, Zeymer U, Neumann F-J, Ferenc M, Olbrich H-G, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Böhm M, Ebelt H, Schneider S, Schuler G, Werdan K (2012) Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 367:1287–1296 10.1056/NEJMoa1208410 [DOI] [PubMed] [Google Scholar]
- 5.Sharma YP, Krishnappa D, Kanabar K, Kasinadhuni G, Sharma R, Kishore K et al (2019) Clinical characteristics and outcome in patients with a delayed presentation after ST-elevation myocardial infarction and complicated by cardiogenic shock. Indian Heart J 71(5):387–393 10.1016/j.ihj.2019.11.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheikh O, Nguyen T, Bansal S, Prasad A (2020) Acute kidney injury in cardiogenic shock: a comprehensive review. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 10.1002/ccd.29141 10.1002/ccd.29141 [DOI] [PubMed] [Google Scholar]
- 7.Adegbala O, Inampudi C, Adejumo A, Otuonye G, Akintoye E, Elsayed R et al (2019) Characteristics and outcomes of patients with cardiogenic shock utilizing hemodialysis for acute kidney injury. Am J Cardiol 123(11):1816–21 10.1016/j.amjcard.2019.02.038 [DOI] [PubMed] [Google Scholar]
- 8.van den Akker JPC, Bakker J, Groeneveld ABJ, den Uil CA (2019) Risk indicators for acute kidney injury in cardiogenic shock. J Crit Care 50:11–16 10.1016/j.jcrc.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 9.Tarvasmäki T, Haapio M, Mebazaa A, Sionis A, Silva-Cardoso J, Tolppanen H et al (2018) Acute kidney injury in cardiogenic shock: definitions, incidence, haemodynamic alterations, and mortality. Eur J Heart Fail 20(3):572–581 10.1002/ejhf.958 [DOI] [PubMed] [Google Scholar]
- 10.Seeliger E, Sendeski M, Rihal CS, Persson PB (2012) Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J 33(16):2007–2015 10.1093/eurheartj/ehr494 [DOI] [PubMed] [Google Scholar]
- 11.Pannu N, Mehta RL (2004) Effect of mechanical ventilation on the kidney. Best Pract Res Clin Anaesthesiol 18(1):189–203 10.1016/j.bpa.2003.08.002 [DOI] [PubMed] [Google Scholar]
- 12.Krawczeski CD (2019) Cardiopulmonary bypass and AKI: AKI is bad, so let’s get beyond the diagnosis. Front Pediatr 26(7):492 10.3389/fped.2019.00492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acharya D (2018) Predictors of outcomes in myocardial infarction and cardiogenic shock. Cardiol Rev 26(5):255 10.1097/CRD.0000000000000190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein LW, Shaw RE, Krone RJ, Brindis RG, Anderson HV, Block PC et al (2005) Mortality after emergent percutaneous coronary intervention in cardiogenic shock secondary to acute myocardial infarction and usefulness of a mortality prediction model. Am J Cardiol 96(1):35–41 10.1016/j.amjcard.2005.02.040 [DOI] [PubMed] [Google Scholar]
- 15.Vallabhajosyula S, Dunlay SM, Barsness GW, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR et al (2019) Temporal trends, predictors, and outcomes of acute kidney injury and hemodialysis use in acute myocardial infarction-related cardiogenic shock. PLoS One 14(9):e0222894 10.1371/journal.pone.0222894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuernau G, Poenisch C, Eitel I, Denks D, de Waha S, Pöss J et al (2015) Prognostic impact of established and novel renal function biomarkers in myocardial infarction with cardiogenic shock: a biomarker substudy of the IABP-SHOCK II-trial. Int J Cardiol 15(191):159–166 10.1016/j.ijcard.2015.04.242 [DOI] [PubMed] [Google Scholar]
- 17.Lauridsen MD, Gammelager H, Schmidt M, Rasmussen TB, Shaw RE, Bøtker HE et al (2015) Acute kidney injury treated with renal replacement therapy and 5-year mortality after myocardial infarction-related cardiogenic shock: a nationwide population-based cohort study. Crit Care Lond Engl 30(19):452 10.1186/s13054-015-1170-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marenzi G, Cosentino N, Marinetti A, Leone AM, Milazzo V, Rubino M et al (2017) Renal replacement therapy in patients with acute myocardial infarction: Rate of use, clinical predictors and relationship with in-hospital mortality. Int J Cardiol 1(230):255–261 10.1016/j.ijcard.2016.12.130 [DOI] [PubMed] [Google Scholar]
- 19.Koreny M, Karth GD, Geppert A, Neunteufl T, Priglinger U, Heinz G et al (2002) Prognosis of patients who develop acute renal failure during the first 24 hours of cardiogenic shock after myocardial infarction. Am J Med 112(2):115–119 10.1016/S0002-9343(01)01070-1 [DOI] [PubMed] [Google Scholar]
- 20.Guha S, Sethi R, Ray S, Bahl VK, Shanmugasundaram S, Kerkar P et al (2017) Cardiological Society of India: position statement for the management of ST elevation myocardial infarction in India. Indian Heart J 69(Suppl 1):S63-97 10.1016/j.ihj.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xavier D, Pais P, Devereaux PJ, Xie C, Prabhakaran D, Reddy KS et al (2008) Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet Lond Engl 371(9622):1435–1442 10.1016/S0140-6736(08)60623-6 [DOI] [PubMed] [Google Scholar]
- 22.Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD et al (1999) Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med 341(9):625–34 10.1056/NEJM199908263410901 [DOI] [PubMed] [Google Scholar]
- 23.Sharma YP, Kanabar K, Santosh K, Kasinadhuni G, Krishnappa D (2020) Role of N-terminal pro-B-type natriuretic peptide in the prediction of outcomes in ST-elevation myocardial infarction complicated by cardiogenic shock. Indian Heart J 72(4):302–305 10.1016/j.ihj.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD et al (2012) Third universal definition of myocardial infarction. Circulation 126(16):2020–2035 10.1161/CIR.0b013e31826e1058 [DOI] [PubMed] [Google Scholar]
- 25.Alpert JS, Becker RC (1993) Cardiogenic shock: elements of etiology, diagnosis, and therapy. Clin Cardiol 16(3):182–190 10.1002/clc.4960160305 [DOI] [PubMed] [Google Scholar]
- 26.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA et al (2014) 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 129(23):e521-643 [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–12 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Disease K (2012) Improving Global Outcomes (KDIGO) acute kidney injury work group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138 [Google Scholar]
- 29.Hayıroğlu Mİ, Bozbeyoglu E, Yıldırımtürk Ö, Tekkeşin Aİ, Pehlivanoğlu S (2020) Effect of acute kidney injury on long-term mortality in patients with ST-segment elevation myocardial infarction complicated by cardiogenic shock who underwent primary percutaneous coronary intervention in a high-volume tertiary centre. Turk Kardiyol Dernegi Arsivi Turk Kardiyol Derneginin Yayin Organidir 48(1):1–9 [DOI] [PubMed] [Google Scholar]
- 30.Atti V, Patel NJ, Kumar V, Tripathi B, Basir MB, Voeltz M et al (2019) Frequency of 30-day readmission and its causes after percutaneous coronary intervention in acute myocardial infarction complicated by cardiogenic shock. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv 94(2):E67-77 [DOI] [PubMed] [Google Scholar]
- 31.Vallabhajosyula S, Ya’Qoub L, Dunlay SM, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR et al (2019) Sex disparities in acute kidney injury complicating acute myocardial infarction with cardiogenic shock. ESC Heart Fail 6(4):874–7 10.1002/ehf2.12482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matějka J, Varvařovský I, Rozsíval V, Herman A, Bláha K, Večeřa J et al (2016) Heart failure is the strongest predictor of acute kidney injury in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction. Kardiol Pol 74(1):18–24 10.5603/KP.a2015.0115 [DOI] [PubMed] [Google Scholar]
- 33.Ivey-Miranda JB, Posada-Martínez EL, Almeida-Gutiérrez E, Borrayo-Sánchez G, Flores-Umanzor E (2018) Right atrial pressure predicts worsening renal function in patients with acute right ventricular myocardial infarction. Int J Cardiol 01(264):25–27 10.1016/j.ijcard.2017.12.087 [DOI] [PubMed] [Google Scholar]
- 34.Arbel Y, Mass R, Ziv-Baran T, Khoury S, Margolis G, Sadeh B et al (2017) Prognostic implications of fluid balance in ST elevation myocardial infarction complicated by cardiogenic shock. Eur Heart J Acute Cardiovasc Care 6(5):462–467 10.1177/2048872616652312 [DOI] [PubMed] [Google Scholar]
- 35.Margolis G, Kofman N, Gal-Oz A, Arbel Y, Khoury S, Keren G et al (2017) Relation of positive fluid balance to the severity of renal impairment and recovery among ST elevation myocardial infarction complicated by cardiogenic shock. J Crit Care 40:184–188 10.1016/j.jcrc.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 36.Levy B, Clere-Jehl R, Legras A, Morichau-Beauchant T, Leone M, Frederique G et al (2018) Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 72(2):173–82 10.1016/j.jacc.2018.04.051 [DOI] [PubMed] [Google Scholar]
- 37.Mandawat A, Rao SV (2017) Percutaneous mechanical circulatory support devices in cardiogenic shock. Circ Cardiovasc Interv. 10.1161/CIRCINTERVENTIONS.116.004337 10.1161/CIRCINTERVENTIONS.116.004337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muller G, Flecher E, Lebreton G, Luyt C-E, Trouillet J-L, Bréchot N et al (2016) The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med 42(3):370–378 10.1007/s00134-016-4223-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.