Abstract
Patient: Female, 45-year-old
Final Diagnosis: Pheochromocytoma
Symptoms: Abdominal pain • nausea • vomiting
Clinical Procedure: —
Specialty: Laboratory Diagnostics • Endocrinology and Metabolic • Gastroenterology and Hepatology
Objective:
Rare disease
Background:
Pheochromocytomas, rare tumors arising from the adrenal medulla, can present with highly variable symptoms; therefore, pheochromocytomas frequently remain undiagnosed, leaving the potential for physiological complications. Acutely, these complications include pheochromocytoma crisis, in which high levels of catecholamines are released and cause a life-threatening hypertensive emergency. Over time, undiagnosed pheochromocytomas can lead to cardiovascular damage and end-organ disease related to chronic exposure to elevated blood pressure.
Case Report:
We share a case of pheochromocytoma in a 45-year-old woman who presented with gastrointestinal symptoms of intractable nausea, vomiting, and abdominal pain. Imaging revealed an adrenal mass that had radiographic features that were most consistent with myelolipoma. Before exposing the patient to anesthesia and endoscopy for further diagnostic workup of her gastrointestinal symptoms, which can trigger a catecholamine surge in individuals with a pheochromocytoma, further biochemical testing was performed. Testing of plasma and urine confirmed pheochromocytoma, and surgical resection was performed for definitive treatment. Ultimately, the patient had resolution of her symptoms following the removal of the tumor.
Conclusions:
The resolution of symptoms following surgical resection suggests that symptoms may have been related to the mass effect of the tumor or as an atypical manifestation of increased catecholamine levels. Additionally, by screening for pheochromocytoma, the patient was able to avoid potential complications that can result from common gastroenterological diagnostic procedures. This case report highlights the potential benefit for screening for pheochromocytoma when faced with symptoms that may be non-specific or related to mass effect upon surrounding organs.
Key words: Adrenal Incidentaloma, Gastroenterology, Nausea, Pheochromocytoma, Vomiting
Introduction
Pheochromocytomas are rare tumors that originate from the adrenal medulla, occurring either sporadically or in the context of a genetic syndrome. The annual incidence ranges from 2 to 9.1 per 1 million adults, though this is suspected to only account for roughly 50% of people affected by the tumor [1,2]. Males and females are equally affected and are most often diagnosed in the 3rd to 5th decades of life [2]. Signs and symptoms of a pheochromocytoma are classically related to increased catecholamine release, resulting in physiological responses that include high blood pressure, palpitations, headaches, and diaphoresis, but can also be related to local mass effect or metastasis [3,4]. If undetected, these tumors carry the potential for acutely lethal complications and the long-term consequences of chronic hypertension [5]. Additionally, pheochromocytomas can pose life-threatening challenges to hemodynamics when routine diagnostic procedures involving anesthesia are performed [6]. The relative rarity, non-specificity of symptoms, and clinical significance of a pheochromocytoma make it a diagnostic challenge. We present a case that is unique for both its constellation of symptoms related to gastrointestinal effects and atypical imaging findings. This case report aims to demonstrate the relevance of considering this differential diagnosis by gastroenterologists, despite pheochromocytoma not typically being within their scope of practice.
Case Report
A 45-year-old woman with a past history of congenital ventricular septal defect with repair, recurrent pancreatitis with cholecystectomy, and well-controlled hypertension presented to the emergency room with a one-week history of epigastric abdominal pain and persistent nausea and vomiting. Over the last several years, she had experienced multiple similar bouts of symptoms, typically lasting 1–5 days each time, accompanied by low-grade fevers and night sweats. Upon admission, she was afebrile with a heart rate of 125 and blood pressure of 108/77.
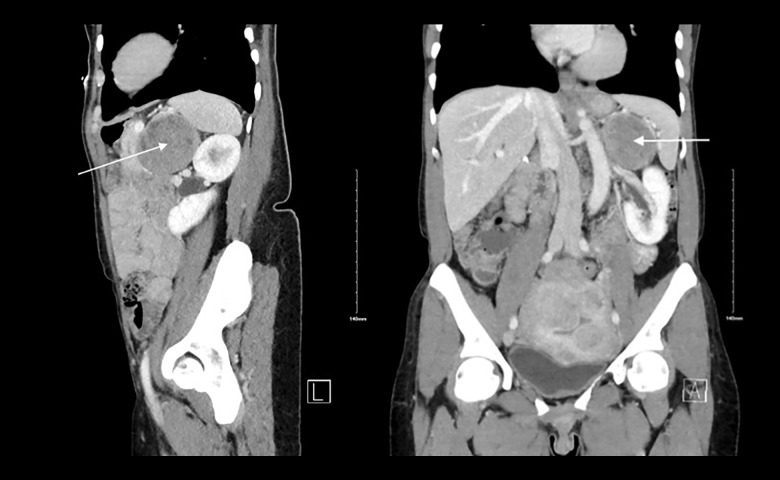
On review of outside records, a left upper-quadrant mass had been incidentally found on computed tomography (CT) at another facility 6 years before, measuring 3.5×3.2×3.0. The patient was subsequently lost to follow-up. Upon presenting to our facility, a repeat CT imaging showed the mass had now increased in size to 6.3×6.1×6.0. It had a heterogenous appearance, with low Hounsfield units (HU <10), which was consistent with a presumed diagnosis of adrenal myelolipoma (Figures 1, 2).
Figure 1.
Sagittal and coronal view of CT of the abdomen of a patient with left adrenal mass. White arrow indicates a 6.2×6.11×6.6 cm peripherally-enhancing mass abutting the pancreatic tail and gastric body, as well as the spleen and left kidney.
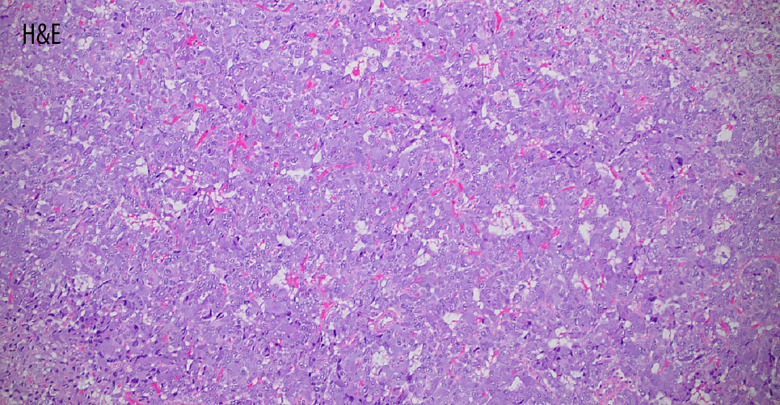
Figure 2.
The histopathological examination after resection at 100× magnification showing cellular monotony and diffuse growth, consistent with pheochromocytoma. The Pheochromocytoma of the Adrenal Gland Scaled Score (PASS) was ≥4, concerning for malignancy.
It was suspected that the patient’s persistent nausea, vomiting, and abdominal pain could be related to the mass effect on the stomach. As part of the diagnostic workup, endoscopy was planned. Although the radiologic appearance of her adrenal mass was most consistent with a myelolipoma, it was recommended to first pursue precautionary biochemical diagnostics. This was specifically recommended because endoscopy would expose the patient to anesthesia, which could trigger a catecholamine surge if the mass was a pheochromocytoma. The biochemical testing included plasma studies, as well as 24-hour urine studies, which were ultimately diagnostic of pheochromocytoma (Table 1).
Table 1.
Results of biochemical testing.
| Result | Reference range | |
|---|---|---|
| Plasma metanephrines | 0.87 nmol/L | 0.00–0.49 nmol/L |
| Plasma normetanephrines | 2.22 nmol/L | 0.00–0.89 nmol/L |
| 24-hour urine metanephrines | 483 µg/d | 36–229 µg/d |
| 24-hour urine normetanephrines | 495 µg/d | 114–865 µg/d |
The patient was discharged with plans for a scheduled left adrenalectomy for definitive treatment. As an outpatient, she was started on doxazosin for alpha-blockade. Her hypertension was also managed with carvedilol, amlodipine, and losartan. The surgery itself was uncomplicated, but on postoperative day 1, she developed hypertension with a blood pressure of 172/108. Her hypertension was suspected to be related to postoperative pain, with a possible component of residual catecholamine leak from the surgery. This was controlled with the use of labetalol. By postoperative day 3, her blood pressure returned to normal and the doxazosin was discontinued. Notably, the patient’s initial presenting symptoms of nausea, vomiting, and epigastric pain improved postoperatively. At future follow-up appointments, she continued to report the resolution of these symptoms. Additionally, catecholamine levels returned to normal range on repeat testing.
Discussion
Though the incidence of pheochromocytoma is low, it remains a critical differential to consider due to its associated physiological complications. Long-standing hypertension can lead to end-organ damage involving the eyes, heart, kidneys, and nervous system [2]. The most severe consequence is that of a pheochromocytoma crisis, which is caused by sudden excessive catecholamine release and can result in a hypertensive emergency, with the most commonly affected organs being the heart (99.0% of cases), lungs (44.%), and kidneys (21.5%) [7]. Among newly diagnosed pheochromocytomas, 7–18% present as a crisis, and mortality for these cases is estimated at 10–13.8% [7].
Pheochromocytoma’s highly variable presentation makes diagnosis challenge. The most common signs and symptoms associated with pheochromocytoma include high blood pressure (with sensitivity of 80.7%), headaches (sensitivity of 60.4%), palpitations (sensitivity of 59.3%), and diaphoresis (sensitivity of 52.4%) [8]. Although these symptoms may increase consideration of pheochromocytoma, they remain non-specific and overlap with many other common conditions. Additionally, pheochromocytomas display variance in the relative concentrations of norepinephrine, epinephrine, and dopamine secreted. This inconsistency of catecholamine concentrations in reported cases consequently results in a variety of phenotypic presentations and associated effects on blood pressure [8]. In this clinical case, our patient presented with symptoms of nausea, vomiting, and epigastric abdominal pain, which completely resolved following surgical resection of the adrenal medulla. This response supports the initial hypothesis that her gastrointestinal symptoms were related to either a mass effect upon the stomach, or as an atypical manifestation of increased catecholamine levels.
Currently, there is limited literature on the prevalence of pheochromocytoma symptoms being related to mass effect itself, though it has been documented in adrenal myelolipomas. In this study, only 5% of tumors were diagnosed following the presentation of mass effect symptoms, which included early satiety, abdominal pain, and nausea. This was most commonly seen with tumor size ≥6 cm [9]. It remains likely that this infrequency of mass effect leading to diagnosis would be similar among those with pheochromocytoma. There is also limited literature on the effects of catecholamines manifesting as gastrointestinal symptoms.
Notably, pheochromocytoma crises have been shown to be triggered by anesthesia, diagnostic and therapeutic gastrointestinal procedures, use of metoclopramide, and abdominal manipulation, all of which remain particularly relevant with both the treatment and diagnostic workups for abdominal pain, nausea, and vomiting [4,7]. This further emphasizes the importance of considering pheochromocytoma as a possible cause of these symptoms to avoid potentially catastrophic consequences related to crises.
Aside from the atypical presenting symptoms, our case was also remarkable for the radiologic findings of ≤10 Hounsfield units, which is highly unusual for imaging of pheochromocytoma (Figure 1, 2) [8,11]. However, in rare instances, adrenal pheochromocytomas containing microscopic fat may result in low attenuation on CT imaging [8]. Up to 7% of all adrenal incidentalomas are pheochromocytomas, prompting the North American Neuroendocrine Tumor Society guidelines to recommend further biochemical screening for all cases due to the potential harm caused by missing a diagnosis [1]. Pheochromocytomas with attenuation ≤10 HU have been rarely described in the literature, and other studies demonstrate that it is reasonable to abstain from further biochemical testing with these findings [8,10]. Our case contributes to the current evidence highlighting the potential benefit of a lower threshold for screening patients who are found to have adrenal incidentalomas and non-specific symptoms. Of note, despite the atypical radiologic findings in our pheochromocytoma case, the pathology remained consistent due to the inherently wide variability in histological appearance, ranging from small to large polygonal cells with basophilic to eosinophilic granular cytoplasm (Figure 2) [2].
Conclusions
Overall, the importance of recognizing pheochromocytoma as a differential diagnosis cannot be overstated when considering its significant morbidity and mortality implications. Moreover, the ease and relative simplicity of conducting diagnostic studies, including the use of plasma or 24-hour urinary fractionated metanephrines, makes the evaluation process readily accessible. Beyond the diagnostic process, adrenalectomy is the mainstay of treatment and has demonstrated good outcomes [5]. Given these considerations, advocating for a lower threshold for evaluating patients for pheochromocytoma, even with atypical presentations, is imperative for ensuring timely diagnosis and intervention. Additionally, while pheochromocytomas are not commonly encountered by the gastroenterologist, it is critical to consider this as a differential diagnosis, particularly when encountering atypical or unexplainable symptoms that may be related to catecholamine surge or mass effect.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Chen H, Sippel RS, O’Dorisio MS, et al. The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: Pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas. 2010;39:775–83. doi: 10.1097/MPA.0b013e3181ebb4f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrugia FA, Charalampopoulos A. Pheochromocytoma. Endocr Regul. 2019;53:191–212. doi: 10.2478/enr-2019-0020. [DOI] [PubMed] [Google Scholar]
- 3.Zelinka T, Eisenhofer G, Pacak K. Pheochromocytoma as a catecholamine producing tumor: Implications for clinical practice. Stress. 2007;10:195–203. doi: 10.1080/10253890701395896. [DOI] [PubMed] [Google Scholar]
- 4.Leonard JB, Munir KM, Kim HK. Metoclopramide induced pheochromocytoma crisis. Am J Emerg Med. 2018;36:1124 e1121–e.22. doi: 10.1016/j.ajem.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Farrugia FA, Martikos G, Tzanetis P, et al. Pheochromocytoma, diagnosis and treatment: Review of the literature. Endocr Regul. 2017;51:168–81. doi: 10.1515/enr-2017-0018. [DOI] [PubMed] [Google Scholar]
- 6.Myklejord DJ. Undiagnosed pheochromocytoma: The anesthesiologist nightmare. Clin Med Res. 2004;2:59–62. doi: 10.3121/cmr.2.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ando Y, Ono Y, Sano A, et al. Clinical characteristics and outcomes of pheochromocytoma crisis: A literature review of 200 cases. J Endocrinol Invest. 2022;45:2313–28. doi: 10.1007/s40618-022-01868-6. [DOI] [PubMed] [Google Scholar]
- 8.Buitenwerf E, Korteweg T, Visser A, et al. Unenhanced CT imaging is highly sensitive to exclude pheochromocytoma: a multicenter study. Eur J Endocrinol. 2018;178:431–37. doi: 10.1530/EJE-18-0006. [DOI] [PubMed] [Google Scholar]
- 9.Hamidi O, Raman R, Lazik N, et al. Clinical course of adrenal myelolipoma: A long-term Longitudinal follow-up study. Clin Endocrinol (Oxf) 2020;93(1):11–18. doi: 10.1111/cen.14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canu L, Van Hemert JAW, Kerstens MN, et al. CT Characteristics of pheochromocytoma: Relevance for the evaluation of adrenal incidentaloma. J Clin Endocrinol Metab. 2019;104:312–18. doi: 10.1210/jc.2018-01532. [DOI] [PubMed] [Google Scholar]
- 11.Blake MA, Krishnamoorthy SK, Boland GW, et al. Low-density pheochromocytoma on CT: A mimicker of adrenal adenoma. Am J Roentgenol. 2003;181:1663–68. doi: 10.2214/ajr.181.6.1811663. [DOI] [PubMed] [Google Scholar]