Abstract
Multiple investigations have demonstrated the crucial involvement of T-cell exhaustion (TEX) in anti-tumor immune response and their strong correlation with prognosis. This study aimed at creating a strong signature using TEX for gastric cancer through bioinformatics analysis and experimental validation. We utilized data from The Cancer Genome Atlas (TCGA) databases to retrieve RNA-seq data from patients with stomach adenocarcinoma (STAD). Genes related to TEX were discovered using gene set variance analysis (GSVA) and weighted gene correlation network analysis (WGCNA). Subsequently, prognostic signature based on TEX was developed using LASSO-Cox analysis. Relationship between key genes and immune cells were examined. Finally, biological function of a key TEX-related gene PTPRT in gastric cancer was verified by in vivo experiment. A total of 29 TEX-related biomarkers were screened by WGCNA and random forest. Among them, five core signatures (PTPRT, CAV2, PPIH, PRDM2, and FGF1), further identified by LASSO-Cox, were considered as strong predictors of prognosis for gastric cancer and associated with immune infiltration. PTPRT gene had the largest number of SNPs, with the most mutation types. In vivo experiments revealed that PTPRT overexpression significantly inhibited tumor malignant progression and accelerated apoptosis through stimulating the secretion of killer cytokines such as TNF-α and IFN-γ. In addition, flow cytometry revealed that PTPRT overexpression alleviated TEX by increasing the abundance of CD8+ T cells, with inhibition of cell surface PD-1 and Tim-3. The predictive prognostic value of TEX gene expression levels was evaluated in patients with gastric cancer, providing a new perspective for precision immuno-oncology studies.
Keywords: Gastric cancer, T-cell exhaustion, Prognosis, Immune microenvironment, PTPRT
Subject terms: Cancer microenvironment, Tumour biomarkers, Tumour immunology, Cancer, Gastric cancer
Introduction
Ranked as the third highest cause of cancer-related deaths globally, the occurrence of gastric cancer is influenced by factors such as ethnicity, dietary habits, and genetic predisposition1. In recent years, targeted therapy, surgical resection, and systemic chemotherapy have emerged as primary treatment modalities for gastric cancer2. In the management of advanced or metastatic gastric cancer, the primary treatment option often involves palliative chemotherapy combined with targeted therapy3. However, when determining the appropriate chemotherapeutic agents, healthcare providers typically take into account the patient's overall health status and any existing comorbidities4. Hence, treatment outcomes vary considerably and are unpredictable due to the absence of effective prediction tools for assessing patients' treatment responses and prognosis.
T cell exhaustion (TEX) is a state of effective diminished T cell response during chronic infections5. The diminished responsiveness is attributed to pathogens persisting in chronic inflammation and perpetuating T cell stimulation. This sustained stimulation leads to the upregulation of numerous immune checkpoints on T cell surfaces, diminishing their proliferation and pathogen-capturing capacity6. TEX triggers immune dysfunction in cancer patients, and recent research indicates that revitalizing the cytolytic function of depleted CD8+ T cells can be achieved by blocking co-inhibitory receptors like programmed death receptor 1 (PD-1)7.
Nowadays, here has been growing recognition of the pivotal role played by tumor immune microenvironment (TIME) in cancer advancement8. Tumor-infiltrating T cells play a crucial role in TIME and are essential for recognizing and eliminating tumor cells. However, the expression of inhibitory receptors (IRS) at high levels and quantities often results in the depletion of infiltrating T cells, facilitating immune evasion by cancer cells9. These depleted T cells exhibit a distinct epigenetic profile, potentially leading to adverse reactions to immunotherapy10. As illustrated by a comprehensive pan-cancer analysis, a lower proportion of CD8+ TEX and a higher abundance of tissue-resident memory CD8+ T cells in gastric cancer tissues suggest a more favorable composition of anti-tumor immune cells within the TIME. This insight holds promise for the development of targeted immunotherapies tailored to gastric cancer patients11. Ongoing research in gastrointestinal cancers explores CD8+ TEX involvement in immune checkpoint blockade, CAR T cell therapy, and synergistic approaches with chemotherapy, but many underlying mechanisms remain elusive12.
Herein, we constructed a TEX-based prognostic model employing bioinformatics methodologies and animal models were established for validation. Potential underlying mechanisms of TEX-related gene involved in immunomodulation in gastric cancer were preliminarily investigated, laying the groundwork for investigating the mechanism of TEX in gastric cancer.
Materials and methods
Unsupervised cluster analysis
Download stomach adenocarcinoma (STAD)-related data (n = 450) from TCGA database (https://portal.gdc.cancer.gov/). According to TCGA dataset, ConsensusClusterPlus13 in R Version 4.3.1 package was used for consistent cluster analysis. The range of clusters (k) varied from 2 to 10, and optimal cluster size was explored was identified as k = 4 according to the cumulative distribution function (CDF). Kaplan–Meier (KM) survival analysis was constructed for the four subtypes utilizing survival data. The GSVA pathway scores of the four subtypes were calculated based on the TEX pathway, and the R Version 4.3.1 ComplexHeatmapPackage was used for visualization14.
Weighted gene co-expression network analysis (WGCNA)
By grouping information from four subtypes in the TCGA dataset, scale-free weighted network analysis was carried out by WGCNA in R4.3.115, and modular clustering was performed for gene expression patterns according to hierarchical clustering algorithm. Identify key modules by the Pearson correlation coefficient between modules and sample groups. The network was built using a soft threshold of 3 conform to a scale-free distribution. Then, the scale-free network was converted into co-expression networks by WGCNA algorithm. Finally, modules were recognized using hierarchical clustering and dynamic tree cutting functions.
Classifier and diagnostic model development and validation
To choose an appropriate classifier, both random forest and lasso regression algorithms were utilized to evaluate key TEXs based on their performance metrics. RandomForest R Version 4.3.116 was used to construct diagnostic models for the four subtypes. Half of the samples were randomly allocated to the training set, while the remaining half comprised the testing set. Subsequently, the receiver operating characteristic (ROC) curve was created using the R package pROC to evaluate the model's predictive performance. Optimal outcomes were attained through LASSO regression analysis of the training group data using the R package "glmnet", followed by multivariate Cox regression analysis to ascertain the association between TEXs-related gene expression levels and correlation coefficients. Patients in the training set were categorized into high- and low-risk groups based on the median risk score. Kaplan–Meier survival analysis was performed to evaluate survival disparities. Subsequently, the model's predictive capability was validated in the testing group.
Immune infiltration analysis
R package Version 4.3.2 ssgsea was employed to conduct immune infiltration analysis for diverse subtypes17. Besides, a t-test was employed to assess discrepancies in immune cell infiltration among subtypes.
Correlation analysis between prognostic genes and immune cells
Pearson correlation coefficient was utilized to evaluate the relationship between prognostic genes and immune cells18.
Somatic mutation analysis
We utilized GSCA database (http://bioinfo.life.hust.edu.cn/GSCA/#/mutation) to evaluate somatic variant information of prognostic genes saved in mutation annotation format (MAF).
Validation in single-cell transcriptome
Single-cell sequencing data were obtained from the GSE183904 dataset19 that was downloaded from GEO database, including 29 gastric cancer tissues and 11 paracancerous normal samples. Seurat was employed to conduct quality control of single-cell sequencing data, with the following screening criteria: nCount_RNA < 30,000, 300 < nFeature_RNA < 6000, and percent.MT < 0.25. After the data were normalized with "NormalizeData", the "Find Variable Features" program was utilized to identify top 2000 highly variable genes. Dimensionality reduction analysis was performed via Principal Component Analysis (PCA) to identify the 30 most significant principal components for further analysis. Next, Uniform Manifold Approximation and Projection (UMAP) was applied to screen cell subpopulations. Cell clusters were finally annotated using Seurat package. The expression distribution of five prognostic genes in UMAP of single cell data was observed, followed by comparison of the differences in gene expression between tumor and normal samples using T-test.
Cell culture
Mouse Forestomach Carcinoma cells (MFCs, No. CL-0156) were obtained from the Wuhan Pricella Biotechnology Co., Ltd. (Wuhan, China). The cells were cultured in RPMI-1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA), 1% penicillin, and 1% streptomycin. Incubation was carried out in the 37 °C and 5% CO2 environment.
Cell transfection and grouping
Two lentiviral expression vector oe-PTPRT were constructed according to the NCBI website and transfected into MFC cells for 48 h. Cells were divided into four groups: (1) Control; (2) oe-NC; (3) oe-PTPRT#1; (4) oe-PTPRT#2. Plasmids without any target sequence were used as negative control (NC). The oe-NC, oe-PTPRT#1, and oe-PTPRT#2 plasmids were transfected into MFC cells via Lipofectamine™ 2000 Transfection Reagent (Invitrogen) for 48 h by instructions.
Quantitative real-time PCR (RT-qPCR)
To detect PTPRT expression in cells or tissues, RT-qPCR was conducted. RNA extraction from samples was conducted via TRIzol reagent (Invitrogen, Carlsbad, CA). Subsequently, 1 μg of RNA was transcribed into cDNA using SYBR Green PCR Master Mix (Invitrogen, Carlsbad, CA), with GAPDH as the internal reference. The relative expression level of each gene was determined using the 2−ΔΔCt method. qRT-PCR reactions were conducted in triplicate with the following steps: denaturation at 95 °C for 3 min, followed by 40 cycles of 95 °C for 12 s and 62 °C for 40 s. The primer sequences utilized were as follows: PTPRT, 5′-GAACCCTCCTCTCTCCCCAT-3′ (forward primer) and 5′-ACGGCTGCCATCTGTGAATC-3′ (reverse) and GAPDH, 5′-GGCAAATTCAACGGCACAGT-3′ (forward primer) and 5′-TGAAGTCGCAGGAGACAA CC-3′ (reverse).
Animals modeling and grouping
The animal experiments were performed in accordance with ARRIVE guidelines and approved by the Experimental Animal Ethics Committee of Medical School of Huanghe S&T university (Approval number 2023-006). We confirm that all methods were performed in accordance with the relevant guidelines and regulations. Healthy female C57BL/6 mice, aged 6 weeks and weighing 22 ± 2 g, were obtained from the Animal Medical Center of Yangzhou University. Twelve mice were randomly allocated into two groups: oe-NC and oe-PTPRT#1. To establish cancer models, a total of 100 µL oe-NC transferred or oe-PTPRT#1 transferred MFC cells (4 × 106 cells) were administered via subcutaneous injection into the right flank of the mice. Tumor sizes was expected to be 50–100 mm3 seven days after inoculation, and monitored every 7 days thereafter. The mice were sacrificed 28 days later and tumor tissues were collected, weighed, and their volume was calculated using the specified formula:
| 1 |
TUNEL staining
TUNEL method was used to detect apoptosis of tumor cells by the In Situ Cell Death Detection Kit, Fluorescein (Beyotime Biotechnology) according to manufacturer's recommended instructions. Briefly, after treatment, tumor tissues underwent fixation using a 4% polyformaldehyde solution, followed by deparaffinization and hydration. Then, brain tissues were made into paraffin blocks that were cut into 4–7 μm sections. Subsequently, cells underwent treatment with the TUNEL reaction mixture and then stained with DAPI, with intermittent washing steps. The TUNEL-positive cells were observed using fluorescence microscope.
Immunohistochemistry staining
CD8 and PD-1 expression in tumor tissues was detected through immunohistochemistry Staining. Tumor tissues fixed with 4% PFA and subjected to antigen retrieval were further treated with 100% methanol for 10 min, followed by a 1-h incubation with 1% bovine serum albumin (BSA). Subsequently, the tumor sections underwent overnight incubation with primary antibodies (CD8: 85336T, 1: 200; PD-1: 86163T, 1: 200, Cell Signaling Technology) at 4 °C, followed by a 1-h incubation at 37 °C with fluorescent-conjugated secondary antibodies. After staining with DAPI solution, laser scanning confocal microscopy was utilized to examine all sections.
Elisa assay
Whole blood of mice was collected in a blood collection tube without anticoagulants. The samples were centrifuged at 4℃ and 2000 rpm for 10 min to obtain the serum supernatant. TNF-α and IFN-γ levels in the serum were measured using commercial ELISA kits (Beyotime, Shanghai, China).
Tumor digestion and cell isolation
Tumor samples were rapidly transferred to RPMI-1640 medium (containing 10% fetal bovine serum) under sterile conditions. The samples were cut into small pieces (1–2 mm3) with sterile scalpel and digested with 0.3 μg/mL at 37 °C for 30 min. Next, the samples were filtered through a 70 μm mesh filter, followed by centrifugation at 300g for 5 min to obtain a single-cell suspension.
Flow cytometry
To evaluate the proportion of CD8+ T cells in the tumors, we performed flow cytometry analysis as previously described20. The single-cell suspension was incubated with the following fluorescent-coupled antibodies at 4 °C for 30 min in the dark: CD4-PE (catalog no. 100407) and CD8-FITC (catalog no. 100803). In addition, we detected the expression levels of PD-1 and Tim-3 on the surface of T cells by flow cytometry. Briefly, cell suspension was incubated with specific antibodies, including CD8-FITC (catalog no. 100803), CD279 (PD-1)-PE (catalog no. 135205), and CD366 (Tim-3)-PE (catalog no. 134003), at 4 °C for 30 min in the dark, and the proportion of CD8+ T cells expression PD-1 or Tim-3 was measured. All antibodies used in this study were purchased from Biolegend (San Diego, USA). Stained cells were assayed using a CytoFLEX (Beckman Coulter, Brea, USA), followed by data analysis with CytExpert software.
Data analysis
Data were presented as mean ± standard deviation (SD). Group differences were assessed using one-way analysis of variance (ANOVA). Statistical analysis was conducted using GraphPad 8.0 software, with significance set at a P-value < 0.05.
Results
Unsupervised cluster analysis and identification of candidate TEX-related genes
Unsupervised clustering analysis classified 450 STAD samples into four different subtypes (Fig. 1A). Then, we explored GSVA enrichment score of the four subtypes based on TEX-related pathway and found that subtype1 and subtybe2 had the highest TEX scores (Fig. 1B). The KM plot showed that subtype1 had the highest survival rate among clusters (Fig. 1C, P < 0.05). While constructing co-expression network, a soft threshold power β of 3 (Fig. 1D) and three modules (magenta, turquoise, and greenyellow) were obtained which were significantly related to four subtypes (Fig. 1E, F). After evaluating correlation coefficient and P-value, we identified a total of 286 candidate genes associated with TEX.
Fig. 1.
Identified T cell depletion (TEX)-related clusters. (A) Delta area curves for consensus clustering indicating the relative change in area under the cumulative distribution function (CDF) curve for each category number k compared to k-1. The horizontal axis represents the category number k and the vertical axis represents the relative change in area under CDF curve. (B) Heatmap of GSVA analysis of four clusters showed TEX-related pathways. (C) Kaplan–Meier (KM) curve of patients in four subtypes. **P < 0.01. (D) Scale independence and average connectivity. (E) Cluster dendrogram. (F) Heatmap of the correlation between four subtypes and modules.
Construction of TEX-related gene predictive model
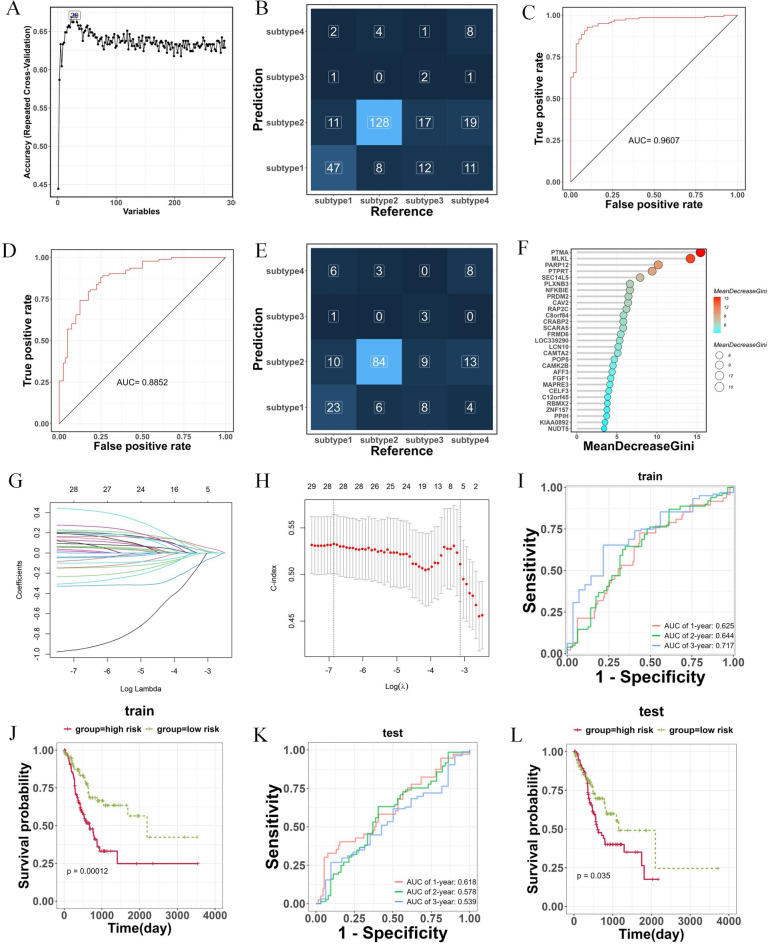
Based on 286 genes screened by WGCNA, a RF classification model was constructed. When the variable was equal to 29 (Fig. 2A), the model had the highest accuracy. In the training set, the model was able to accurately distinguish patients from different subtypes, especially subtype 1 and subtype 2 (Fig. 2B). The ROC curve showed an AUC of 0.9607, confirming that the model displayed satisfactory diagnostic performance (Fig. 2C). Same results were observed in the testing set. ROC analysis indicated that the AUC value of this model was 0.8852, with effective differentiation between subtype 1 and subtype 2 (Fig. 2D, E). The importance of the 29 candidate TEX-related genes was ranked according to the MeanDecreaseGini value (Fig. 2F).
Fig. 2.
Construction of TEX-related gene predictive model by random forest and lasso regression algorithm. (A) The change curve of prediction accuracy of model as variables increases. (B) The confusion matrix for predictive model in training set. (C) The area under the ROC curve for model in training set. (D) The area under the ROC curve for model in testing set. (E) The confusion matrix for predictive model in testing set. (F) 29 candidate TEX-associated genes identified by random forest. (G) tenfold cross-validation of Lasso regression profiles of TEXs to avoid over-fitting. (H) The change curve of the mean-squared error of the model as the λ value changes. (I,J) Kaplan–Meier curve compares the overall gastric cancer patients between LR and HR groups in the training and testing cohorts. (K,L) Time-dependent ROC curves analysis in the training and testing cohorts.
Additionally, classifiers underwent additional screening using the lasso regression algorithm (Fig. 2G), and the change curve of the mean-squared error was depicted in Fig. 2H. Following that, we constructed a predictive prognostic model consisting of five TEX genes via lasso regression and multivariate analysis: PTPRT, CAV2, PPIH, PRDM2, and FGF1. Kaplan–Meier curves in both the training and testing cohorts illustrated that patients classified into the low-risk group exhibited significantly better survival outcomes compared to those in the high-risk group (Fig. 2J, L, P < 0.05). The area under the curve (AUC) for patient survival at 1 year, 2 years, and 3 years in the training set were 0.625, 0.644, and 0.717, respectively. Similarly, in the testing set, the AUC for patient survival at 1 year, 2 years, and 3 years were 0.618, 0.578, and 0.539, respectively (Fig. 2I, K). This indicated that the model had certain predictive value.
Immune cell infiltration analysis and correlations between TEX-related genes and immune cells
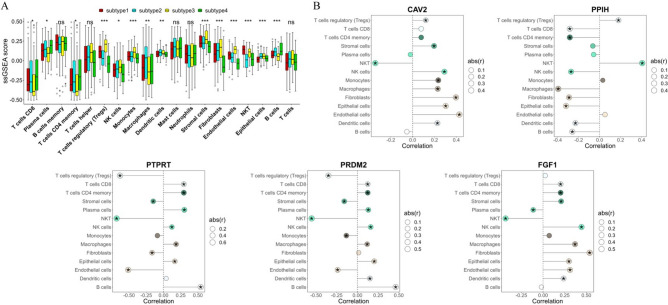
The variances among 19 types of immune cells in various subtypes were analyzed by ssgsea and t-test, and 14 kinds of differential immune cells were screened (Fig. 3A, P < 0.05), including CD8 T cells, plasma cells, memory CD4 T cells, Tregs, NK cells, Monocytes, Macrophages, Dendritic cells, stromal cells, Fibroblasts, NKT, Epithelial cells, and B cells. Pearson correlation analysis unveiled a notable correlation between prognostic genes and distinct immune cells, as illustrated in Fig. 3B (P < 0.05). Notably, CD8 T cells demonstrated a positive correlation with PTPRT (Fig. 3B, P < 0.05).
Fig. 3.
Immune cell infiltration analysis and correlations between TEX-related genes and immune cells. (A) Boxplot of immune infiltration cells between subtypes. (B) Dot plot of correlation between prognostic genes and immune infiltration. *P < 0.05, **P < 0.01, and ***P < 0.001.
MAF analysis
Genetic mutations are recognized as a precursor to tumorigenesis. Using TCGA database data, we visualized and correlated somatic mutations with the TEX signature. Notably, the PPIH gene exhibited no single nucleotide polymorphisms (SNPs), while PTPRT displayed the highest mutation frequencies, as illustrated in Fig. 4.
Fig. 4.
Mutation annotation format (MAF) analysis. (A) Heat map of SNV scale. (B) Heat map of mutation type. (C) Bar chart of mutation type.
Validation in single-cell RNA sequencing
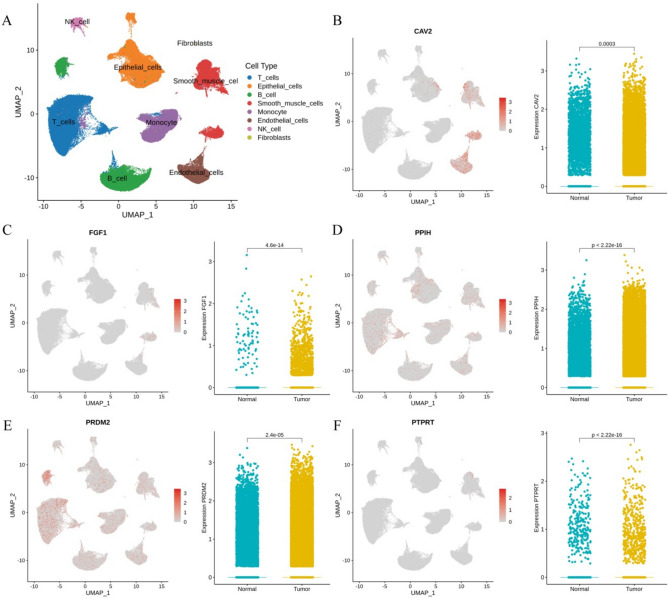
We assessed the immune microenvironment using single-cell sequencing data GSE183904. After quality control and meticulous screening, 154,319 single cells were grouped into 12 cell clusters. These clusters were annotated using SingleR and resulted in eight cell types, including T cells, Epithelial cells, B cell, Monocyte, Smooth muscle cells, Endothelial cells, NK cell, and Fibroblasts. We utilized UMAP plots to demonstrate the overall distribution of cell populations in the samples (Fig. 5A). In addition, we evaluated the cellular distribution and expression of five prognostic genes in gastric cancer and normal samples (Fig. 5B–F). The characteristic maps showed that PRDM2, PPIH, and PTPRT were mainly expressed in T cells. The expression levels of five genes were significantly different between tumor and normal samples. Expect for PTPRT and FGF1, the rest of the genes had elevated expression levels in tumor samples.
Fig. 5.
Validation of five hub genes at the single-cell level. (A) UMAP plot of the annotated eight cell types in the single-cell RNA sequencing dataset GSE183904. UMAP visualization and violin plot of CAV2 (B), FGF1 (C), PPIH (D), PRDM2 (E), and PTPRT (F).
In vivo antitumor efficacy of TEX-related gene PTPRT
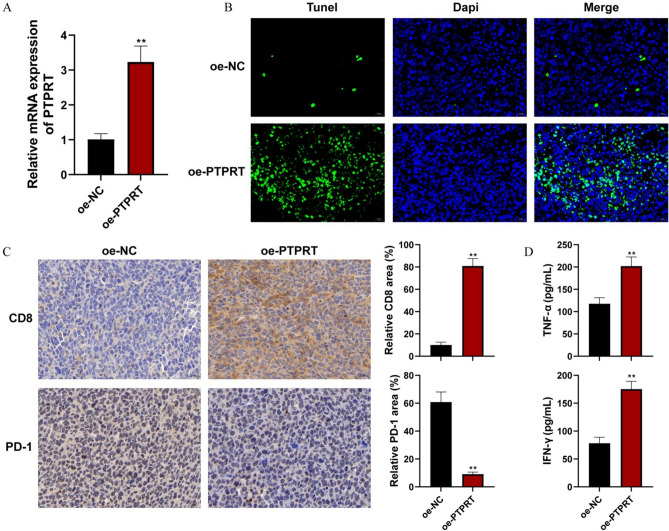
Two PTPRT overexpression plasmids were constructed, and the overexpression efficiency was verified in MFC cells. RT-qPCR confirmed that both oe-PTPRT#1 and oe-PTPRT#2 clearly up-regulated the expression of PTPRT, especially #1 (P < 0.01, Fig. 6A), thus oe-PTPRT#1 was selected for in vivo experiment. To evaluate the antitumor efficacy, the mice received the subcutaneous injection of oe-PTPRT-transferred cells. Compared with the oe-NC group, the oe-PTPRT group exhibited an obvious inhibition effect on the tumor growth (Fig. 6B–D).
Fig. 6.
In vivo experiments reveal antitumor efficacy of TEX-related gene PTPRT. (A) The overexpression efficiency of two PTPRT overexpression plasmids was verified in HGC-27 cells by qPCR. **P < 0.01 vs control; #P < 0.01 vs oe-NC; &&P < 0.01 vs oe-PTPRT#. (B) Photographs of all the tumors in different groups (a: oe-NC, b: oe-PTPRT) were harvested. (C) Tumor weight variations of tumor-bearing mice in different groups. (D) Tumor volume variations of tumor-bearing mice in different groups. Data are shown as mean ± SD, n = 6, **P < 0.01 vs oe-NC.
Over-expression of PTPRT promoted the cell apoptosis and regulating the CD8/PD-1 expression in vivo
In comparison to the oe-NC group, PTPRT expression was indeed increased in oe-PTPRT group (Fig. 7A, P < 0.01). Additionally, the apoptosis rate of cells in the oe-PTPRT group was higher than control group (Fig. 7B). Moreover, results of immunohistochemistry staining indicated CD8 expression of the tumor tissue in oe-PTPRT group was obviously evaluated when compared to the oe-NC group, while PD-1 expression was suppressed (Fig. 7C). In comparison to the oe-NC group, TNF-α and IFN-γ levels in the oe-PTPRT group exhibited a significant increase (Fig. 7D, P < 0.01). The above results showed that PTPRT promoted cell apoptosis and regulated the levels of immune therapy related markers: CD8 and PD-1.
Fig. 7.
Over-expression of PTPRT promoted the cell apoptosis and upregulating the CD8+ T cells expression. (A) The PTPRT expression in the tumor tissue was detected by qRT-qPCR. (B) The apoptosis rate of tumor tissue in the oe-NC and oe-PTPRT groups was detected by TUNEL method. Scale bar, 20 µm. (C) Representative images and quantification of CD8 and PD-1 in the tumor tissue was detected by immunohistochemistry staining. Scale bar, 20 µm. (D) The level of TNF-α and IFN-γ in serum was detected by Elisa. **P < 0.01.
Over-expression of PTPRT alleviates CD8 + TEX in vivo
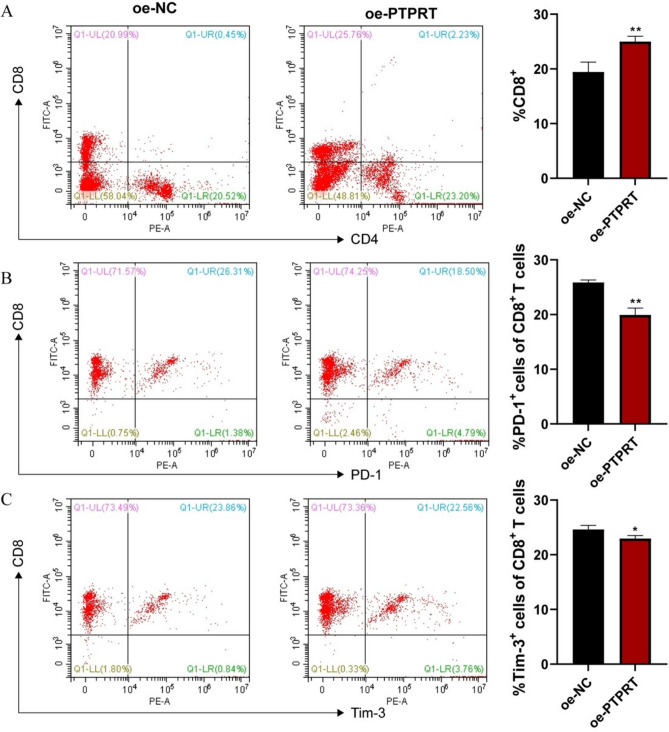
The above results preliminarily confirmed that PTPRT expression was related to the regulation of CD8+ T cell function. A previous study has reported that attenuation of CD8+ T cell proliferation and effector function is accompanied by the expression of multiple inhibitory receptors, known as CD8+ TEX, which often leads to loss of tumor control21. Hence, we further revealed the role of PTPRT in modulating CD8+ T cell depletion using flow cytometry. The results showed that overexpression of PTPRT dramatically increased the number of CD8+ T cells in tumor-bearing mice (Fig. 8A). Meanwhile, the expression of common markers of CD8+ TEX, including PD-1 and Tim-3, was clearly reduced (Fig. 8B, C). These data represent remission of tumor immunosuppression. Taken together, PTPRT can reverse CD8+ TEX by affecting the expression of receptors on CD8+ T cells, thus playing a role in suppressing tumor growth.
Fig. 8.
Over-expression of PTPRT inhibits CD8+ TEX within tumor tissues. (A) Percentage of total CD8+ T cells in tumor cell suspensions from mice. Percentages of PD-1 positive (B) and Tim-3 positive (C) cells among CD8+ T cells from tumor-bearing mice. *P < 0.05; **P < 0.01.
Discussion
Gastric cancer ranks among the most prevalent malignancies globally22. Despite the significant role of TEX in the progression of various cancers, including gastric cancer, there remains a lack of systematic studies on TEX specifically in gastric cancer. Consequently, we constructed a TEX-based prognostic model, with aim of assessing the prognosis and tumor microenvironment of gastric cancer patients, this study seeks to provide a promising foundation for novel treatments.
In the present work, we identified four TEX-related subtypes in TCGA by unsupervised cluster analysis and a total of 286 TEX-related genes were identified through WGCNA. Furthermore, PTPRT, CAV2, PPIH, PRDM2, and FGF1 were found was core TEX-related genes by RF and lasso algorithms, which were applied to generate a risk-score model. This model exhibited promising performance in prognostic prediction for gastric cancer. Additionally, an expanding body of research has substantiated the close association between TEX and the activation of immune responses. Consequently, we further explored the association between TEX genes and infiltrating immune cells. A total of 14 immune cells were differentially expressed in between different clusters. MAF analysis showed that PTPRT gene possessed the largest number of SNP sites. Subsequently, we observed differential expression of CAV2 and PTPRT between gastric cancer tissues and normal samples in validation of single-cell RNA sequencing. CAV2, belonging to the caveolin family, serves pivotal functions in intracellular cellular transport and signal transduction. Increased expression of CAV2 has been associated with the progression of diverse tumor types, including head and neck squamous cell carcinoma and kidney carcinoma23, and pancreatic cancer24. In this study, the correlation between CAV2 and CD8 T cells is remote, so CAV2 is not considered for subsequent experiments. PTPRT, a protein tyrosine phosphatase receptor type T, is among the most frequently mutated tyrosine phosphatases in human cancers. Research has demonstrated an enrichment of PTPRT mutations in distant metastatic colon cancer, and their mutations enhance STAT3 activation and modulate the expression of inflammatory genes in head and neck cancer25. There are few studies on PTPRT gene and gastric cancer, studies have solely demonstrated a correlation between PTPRT mutation and a positive response to immune checkpoint inhibitors in gastric cancer.26. And it was found that PTPRT had the highest correlation with CD8 T cells, so PTPRT gene was selected for follow-up experiments. In vivo experiment showed that over-expression of TEX-related gene PTPRT inhibited GC progression and cell apoptosis. Moreover, over-expression of PTPRT could regulate immune system and evaluated the CD8 expression and decrease the PD-1 level. CD8+ T cells are an important type of immune cell that recognizes and kills cancer cells. The increase of CD8 indicated an enhanced immune response to cancer. PD-1 is an immune checkpoint molecule, and its overexpression inhibits immune cell attack on cancer cells, thereby promoting tumor escape and growth.
Immunotherapy has emerged as a crucial therapeutic strategy for diverse solid tumors, leveraging the host immune system to stimulate anti-tumor responses27. Immune checkpoint inhibitors, by binding to PD-1/PD-L1, empower the host immune system to recognize and eliminate cancer cells, thereby thwarting tumor immune evasion28. Several studies highlight the pivotal role of PTPRT as phosphatases in the JAK-STAT pathway, particularly in T cell immunity, and their association with response to immune checkpoint blockade (ICB) therapies29. While the TEX signature we developed demonstrates remarkable capabilities in delineating the immune landscape and predicting prognosis in gastric cancer patients, further data from gastric cancer patients are essential to validate the model's utility and the accuracy of immunotherapy prediction.
In this work, we performed a series of bioinformatics and machine learning approaches to establish a TEX-related gene signature for gastric cancer prognosis, and then utilized an animal model to explore the potential role of the key gene PTPRT in regulating CD8+ TEX in gastric cancer. However, this study still has several limitations. First, real-world data were lacking to validate the predictive performance of the model. Besides, the biological effects of PTPRT on the tumor immune microenvironment landscape require further in vivo and in vitro functional research. Therefore, in future studies, we will continue to explore the mechanism TEX-related genes in modulating immunity in gastric cancer.
In summary, clustering gastric cancer patients based on TEX genes proved to have prognostic significance. The risk score model, incorporating PTPRT, CAV2, PPIH, PRDM2, and FGF1, was developed for gastric cancer prognosis. Notably, TEX gene PTPRT exhibited a strong anti-tumor performance owning to its remission of CD8+ TEX and may be used in clinical applications in the future, thereby offering novel insights into prognostic evaluation and immunotherapeutic response among patients with gastric cancer.
Abbreviations
- TCGA
The Cancer Genome Atlas
- GSVA
Gene set variance analysis
- WGCNA
Weighted gene correlation network analysis
- LASSO
Least absolute shrinkage and selection operator
- MAF
Mutation annotation format analysis
- TEX
T cell exhaustion
- TIME
Tumor immune microenvironment
- PD-1
Programmed death receptor 1
- STAD
Stomach adenocarcinoma
- ROC
Receiver operating characteristic
- PCA
Principal component analysis
- UMAP
Uniform manifold approximation and projection
- NC
Negative control
- ANOVA
Analysis of variance
- SD
Standard deviation
Author contributions
Conception and design of the research: Zhenyun Cheng; Acquisition and analysis of data: Le Li; Statistical analysis and experiments: Jianli Wu; Drafting the manuscript: Zhenyun Cheng; Revision of manuscript for important intellectual content: Zhenyun Cheng.
Funding
Online open course in medical chemistry (ZLG202029), Henan Provincial Department of Education.
Data availability
The data that support the findings of this study are available from [TCGA database] but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of [TCGA database]. GSCA database (http://bioinfo.life.hust.edu.cn/GSCA/#/mutation).
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The animal experiments were performed in accordance with ARRIVE guidelines and approved by the Experimental Animal Ethics Committee of Medical School of Huanghe S&T University (Approval number 2023-006). We confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kankeu Fonkoua, L. & Yee, N. S. Molecular characterization of gastric carcinoma: Therapeutic implications for biomarkers and targets. Biomedicines6(1), 32 (2018). 10.3390/biomedicines6010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joshi, S. S. & Badgwell, B. D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin.71(3), 264–279 (2021). 10.3322/caac.21657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang, R.-Y. et al. Outcomes of conversion surgery for metastatic gastric cancer compared with in-front surgery plus palliative chemotherapy or in-front surgery alone. J. Pers. Med.12(4), 555 (2022). 10.3390/jpm12040555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwai, N. et al. High-risk comorbidity influences prognosis in early gastric cancer after noncurative endoscopic submucosal dissection: A retrospective study. Dig. Dis.39(2), 96–105 (2021). 10.1159/000510115 [DOI] [PubMed] [Google Scholar]
- 5.Philip, M. & Schietinger, A. CD8(+) T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol.22(4), 209–223 (2022). 10.1038/s41577-021-00574-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wherry, E. J. T cell exhaustion. Nat. Immunol.12(6), 492–499 (2011). 10.1038/ni.2035 [DOI] [PubMed] [Google Scholar]
- 7.Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol.15(8), 486–499 (2015). 10.1038/nri3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao, S. et al. Expression of hub genes of endothelial cells in glioblastoma-A prognostic model for GBM patients integrating single-cell RNA sequencing and bulk RNA sequencing. BMC Cancer22(1), 1274 (2022). 10.1186/s12885-022-10305-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLane, L. M., Abdel-Hakeem, M. S. & Wherry, E. J. CD8 T cell exhaustion during chronic viral infection and cancer. Annu. Rev. Immunol.37, 457–495 (2019). 10.1146/annurev-immunol-041015-055318 [DOI] [PubMed] [Google Scholar]
- 10.Di Federico, A. et al. Hacking pancreatic cancer: Present and future of personalized medicine. Pharmaceuticals (Basel)14(7), 677 (2021). 10.3390/ph14070677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding, J. T. et al. Landscapes and mechanisms of CD8(+) T cell exhaustion in gastrointestinal cancer. Front. Immunol.14, 1149622 (2023). 10.3389/fimmu.2023.1149622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow, A. et al. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat. Rev. Clin. Oncol.19(12), 775–790 (2022). 10.1038/s41571-022-00689-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkerson, M. D. & Hayes, D. N. ConsensusClusterPlus: A class discovery tool with confidence assessments and item tracking. Bioinformatics26(12), 1572–1573 (2010). 10.1093/bioinformatics/btq170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu, Z. & Hübschmann, D. Make interactive complex Heatmaps in R. Bioinformatics38(5), 1460–1462 (2022). 10.1093/bioinformatics/btab806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langfelder, P. & Horvath, S. WGCNA: An R package for weighted correlation network analysis. Bmc Bioinform.9, 559 (2008). 10.1186/1471-2105-9-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alderden, J. et al. Predicting pressure injury in critical care patients: A machine-learning model. Am. J. Crit. Care27(6), 461–468 (2018). 10.4037/ajcc2018525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin, Y. et al. Identification of novel subtypes based on ssGSEA in immune-related prognostic signature for tongue squamous cell carcinoma. Cancer Med.10(23), 8693–8707 (2021). 10.1002/cam4.4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunson, J. C. Ggalluvial: Layered grammar for alluvial plots. J. Open Source Softw.5(49), 2017 (2020). 10.21105/joss.02017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar, V. et al. Single-cell atlas of lineage states, tumor microenvironment, and subtype-specific expression programs in gastric cancer. Cancer Discov.12(3), 670–691 (2022). 10.1158/2159-8290.CD-21-0683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takano, S., Saito, H. & Ikeguchi, M. An increased number of PD-1+ and Tim-3+ CD8+ T cells is involved in immune evasion in gastric cancer. Surg. Today46(11), 1341–1347 (2016). 10.1007/s00595-016-1305-9 [DOI] [PubMed] [Google Scholar]
- 21.Wang, Q., Qin, Y. & Li, B. CD8+ T cell exhaustion and cancer immunotherapy. Cancer Lett.559, 216043 (2023). 10.1016/j.canlet.2022.216043 [DOI] [PubMed] [Google Scholar]
- 22.Siegel, R. L. et al. Cancer statistics, 2023. CA Cancer J. Clin.73(1), 17–48 (2023). 10.3322/caac.21763 [DOI] [PubMed] [Google Scholar]
- 23.Liu, F., Shangli, Z. & Hu, Z. CAV2 promotes the growth of renal cell carcinoma through the EGFR/PI3K/Akt pathway. Onco Targets Ther.11, 6209–6216 (2018). 10.2147/OTT.S172803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, D. et al. CAV2 regulates Mir-4723/Wnt7A signalling axis through endocytosis and epithelial–mesenchymal transition to promote proliferation, invasion, and metastasis of pancreatic cancer cells. J. Cancer13(7), 2200–2212 (2022). 10.7150/jca.69617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lui, V. W. et al. Frequent mutation of receptor protein tyrosine phosphatases provides a mechanism for STAT3 hyperactivation in head and neck cancer. Proc. Natl. Acad. Sci. USA111(3), 1114–1119 (2014). 10.1073/pnas.1319551111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin, M. et al. Impact of programmed death-ligand 1 (PD-L1) positivity on clinical and molecular features of patients with metastatic gastric cancer. Cancer Med.12(18), 18633–18642 (2023). 10.1002/cam4.6472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coutzac, C. et al. Immunotherapy in advanced gastric cancer, is it the future?. Crit. Rev. Oncol./Hematol.133, 25–32 (2019). 10.1016/j.critrevonc.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 28.Yang, L., Wang, Y. & Wang, H. Use of immunotherapy in the treatment of gastric cancer. Oncol. Lett.18(6), 5681–5690 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, X. et al. Association of PTPRD/PTPRT mutation with better clinical outcomes in NSCLC patients treated with immune checkpoint blockades. Front. Oncol.11, 650122 (2021). 10.3389/fonc.2021.650122 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from [TCGA database] but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of [TCGA database]. GSCA database (http://bioinfo.life.hust.edu.cn/GSCA/#/mutation).