Abstract
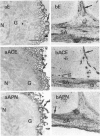
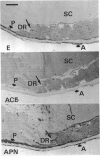
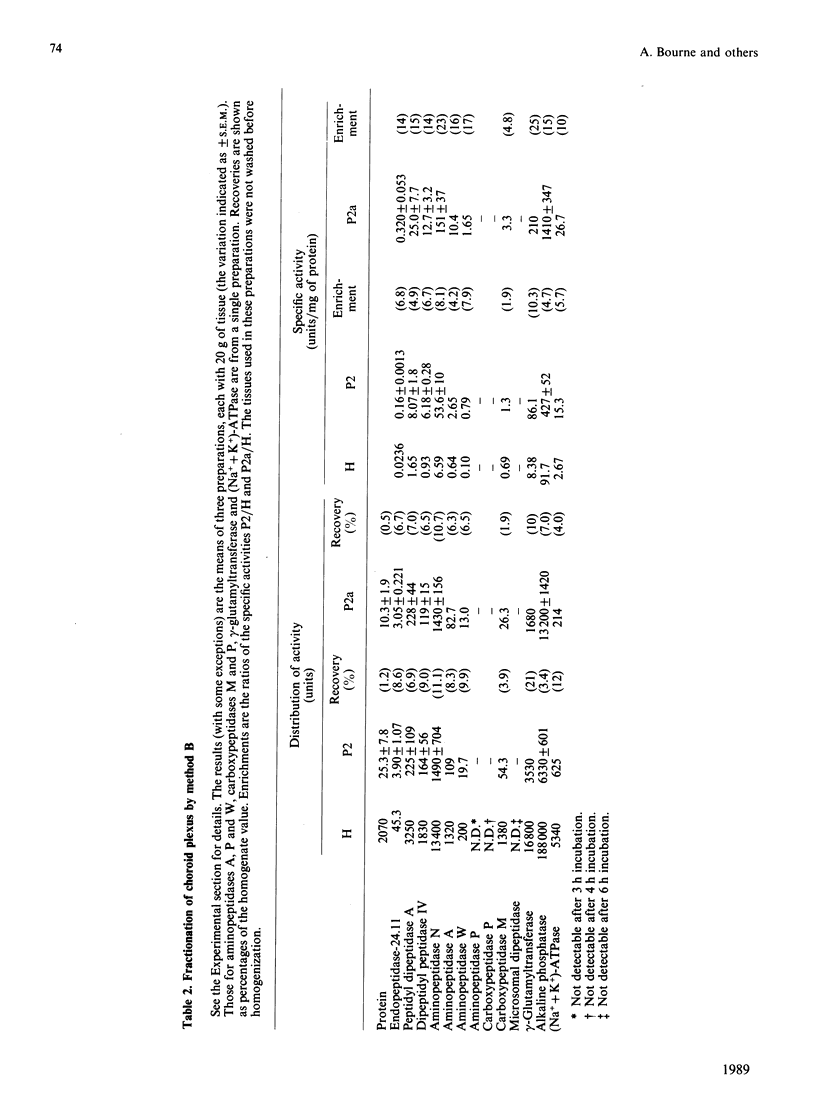
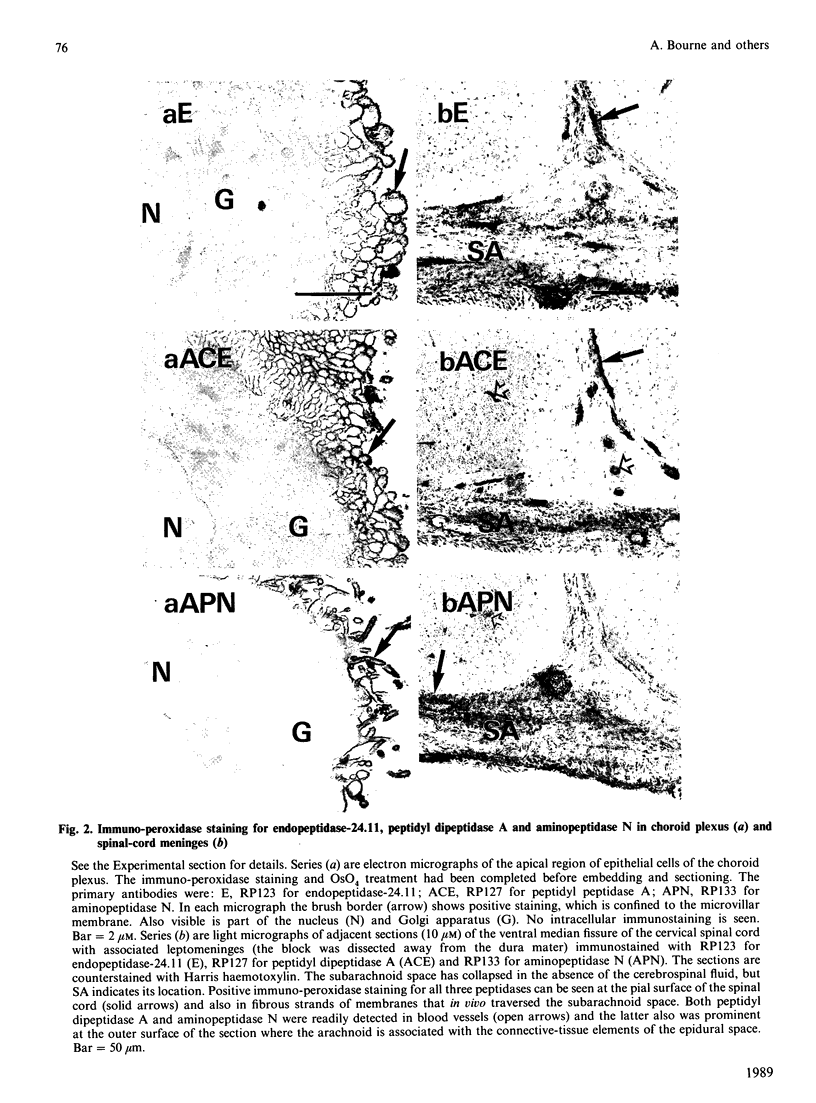
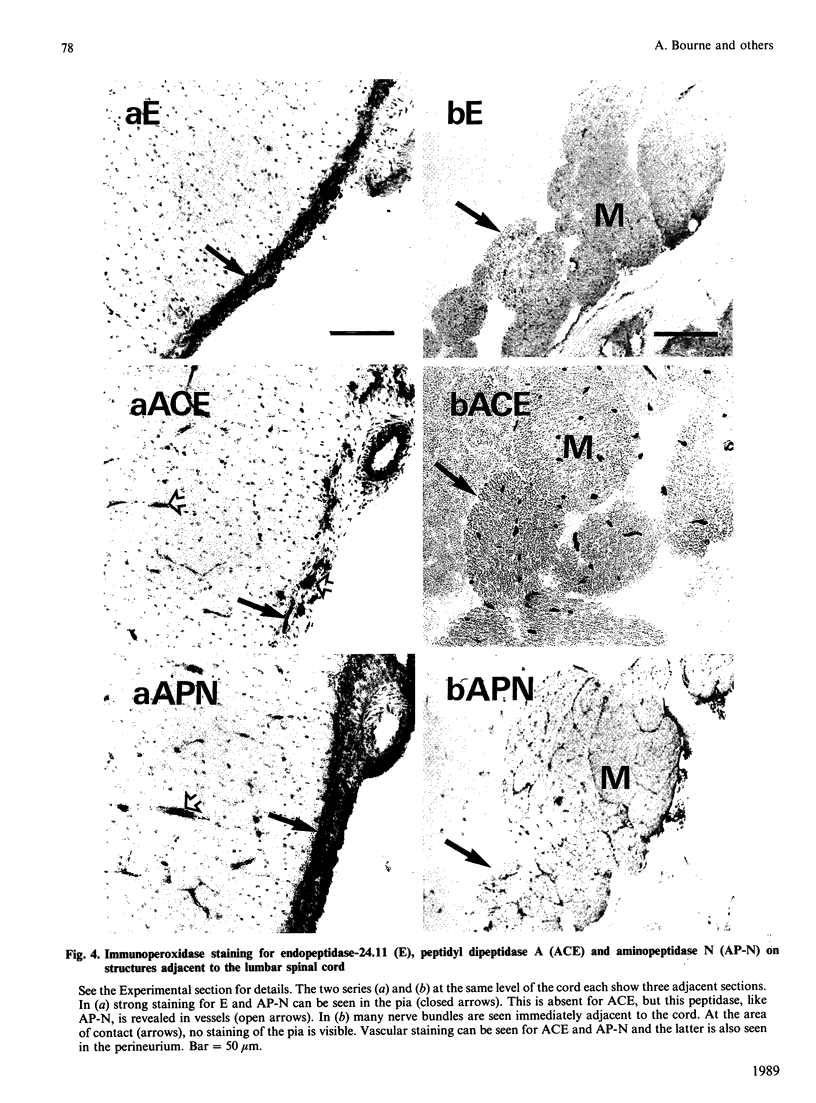
A comprehensive survey of 11 peptidases, all of which are markers for renal microvillar membranes, has been made in membrane fractions prepared from pig choroid plexus. Two fractionation schemes were explored, both depending on a MgCl2-precipitation step, the preferred one having advantages in speed and yield of the activities. The specific activities of the peptidases in the choroid-plexus membranes were, with the exception of carboxypeptidase M, lower than in renal microvillar membranes: those of aminopeptidase N, peptidyl dipeptidase A ('angiotensin-converting enzyme') and gamma-glutamyltransferase were 3-5-fold lower, those of aminopeptidase A and endopeptidase-24.11 were 12-15 fold lower, and those of dipeptidyl peptidase IV and aminopeptidase W were 50-70-fold lower. Carboxypeptidase M had a similar activity in both membranes. Alkaline phosphatase and (Na+ + K+)-activated ATPase were more active in the choroid-plexus membranes. No activity for microsomal dipeptidase, aminopeptidase P and carboxypeptidase P could be detected. Six of the peptidases and (Na+ + K+)-activated ATPase were also studied by immunoperoxidase histochemistry at light- and electron-microscopic levels. Endopeptidase-24.11 and (Na+ + K+)-activated ATPase were uniquely located on the brush border, and the other two peptidases appeared to be much more abundant on the endothelial lining of microvessels. Dipeptidyl peptidase IV and aminopeptidase W were also detected in microvasculature. Pial membranes associated with the brain and spinal cord also stained positively for endopeptidase-24.11, aminopeptidase N and peptidyl dipeptidase A. The immunohistochemical studies indicated the subcellular fractionation did not discriminate between membranes derived from epithelial cells (i.e. microvilli) and those from endothelial cells. The possible significance of these studies in relation to neuropeptide metabolism and the control of cerebrospinal fluid production is discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arregui A., Iversen L. L. Angiotensin-converting enzyme: presence of high activity in choroid plexus of mammalian brain. Eur J Pharmacol. 1978 Nov 1;52(1):147–150. doi: 10.1016/0014-2999(78)90035-3. [DOI] [PubMed] [Google Scholar]
- Barnes K., Matsas R., Hooper N. M., Turner A. J., Kenny A. J. Endopeptidase-24.11 is striosomally ordered in pig brain and, in contrast to aminopeptidase N and peptidyl dipeptidase A ('angiotensin converting enzyme'), is a marker for a set of striatal efferent fibres. Neuroscience. 1988 Dec;27(3):799–817. doi: 10.1016/0306-4522(88)90184-4. [DOI] [PubMed] [Google Scholar]
- Barnes K., Turner A. J., Kenny A. J. Electronmicroscopic immunocytochemistry of pig brain shows that endopeptidase-24.11 is localized in neuronal membranes. Neurosci Lett. 1988 Nov 22;94(1-2):64–69. doi: 10.1016/0304-3940(88)90271-6. [DOI] [PubMed] [Google Scholar]
- Bianchi C., Gutkowska J., Ballak M., Thibault G., Garcia R., Genest J., Cantin M. Radioautographic localization of 125I-atrial natriuretic factor binding sites in the brain. Neuroendocrinology. 1986;44(3):365–372. doi: 10.1159/000124670. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn P. J., Sanders-Bush E., Hoffman B. J., Hartig P. R. A unique serotonin receptor in choroid plexus is linked to phosphatidylinositol turnover. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4086–4088. doi: 10.1073/pnas.83.11.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook R. B., Farber M. B., Prusiner S. B. H2 histamine receptors on the epithelial cells of choroid plexus. J Neurochem. 1986 Feb;46(2):489–493. doi: 10.1111/j.1471-4159.1986.tb12994.x. [DOI] [PubMed] [Google Scholar]
- Crook R. B., Prusiner S. B. Vasoactive intestinal peptide stimulates cyclic AMP metabolism in choroid plexus epithelial cells. Brain Res. 1986 Oct 1;384(1):138–144. doi: 10.1016/0006-8993(86)91229-1. [DOI] [PubMed] [Google Scholar]
- Defendini R., Zimmerman E. A., Weare J. A., Alhenc-Gelas F., Erdös E. G. Angiotensin-converting enzyme in epithelial and neuroepithelial cells. Neuroendocrinology. 1983 Jul;37(1):32–40. doi: 10.1159/000123512. [DOI] [PubMed] [Google Scholar]
- Ernst S. A., Palacios J. R., 2nd, Siegel G. J. Immunocytochemical localization of Na+,K+-ATPase catalytic polypeptide in mouse choroid plexus. J Histochem Cytochem. 1986 Feb;34(2):189–195. doi: 10.1177/34.2.3003182. [DOI] [PubMed] [Google Scholar]
- Fulcher I. S., Kenny A. J. Proteins of the kidney microvillar membrane. The amphipathic forms of endopeptidase purified from pig kidneys. Biochem J. 1983 Jun 1;211(3):743–753. doi: 10.1042/bj2110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee N. S., Bowes M. A., Buck P., Kenny A. J. An immunoradiometric assay for endopeptidase-24.11 shows it to be a widely distributed enzyme in pig tissues. Biochem J. 1985 May 15;228(1):119–126. doi: 10.1042/bj2280119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee N. S., Kenny A. J. Proteins of the kidney microvillar membrane. The 130 kDa protein in pig kidney, recognized by monoclonal antibody GK5C1, is an ectoenzyme with aminopeptidase activity. Biochem J. 1985 Sep 15;230(3):753–764. doi: 10.1042/bj2300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeager-Sørensen S., Kenny A. J. Proteins of the kidney microvillar membrane. Purification and properties of carboxypeptidase P from pig kidneys. Biochem J. 1985 Jul 1;229(1):251–257. doi: 10.1042/bj2290251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N. M., Low M. G., Turner A. J. Renal dipeptidase is one of the membrane proteins released by phosphatidylinositol-specific phospholipase C. Biochem J. 1987 Jun 1;244(2):465–469. doi: 10.1042/bj2440465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N. M., Turner A. J. Ectoenzymes of the kidney microvillar membrane. Aminopeptidase P is anchored by a glycosyl-phosphatidylinositol moiety. FEBS Lett. 1988 Mar 14;229(2):340–344. doi: 10.1016/0014-5793(88)81152-9. [DOI] [PubMed] [Google Scholar]
- Hooper N. M., Turner A. J. Ectoenzymes of the kidney microvillar membrane. Differential solubilization by detergents can predict a glycosyl-phosphatidylinositol membrane anchor. Biochem J. 1988 Mar 15;250(3):865–869. doi: 10.1042/bj2500865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N. M., Turner A. J. Isolation of two differentially glycosylated forms of peptidyl-dipeptidase A (angiotensin converting enzyme) from pig brain: a re-evaluation of their role in neuropeptide metabolism. Biochem J. 1987 Feb 1;241(3):625–633. doi: 10.1042/bj2410625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D., Srivatsa S., Pazos A., Engel G., Palacios J. M. [125I]LSD labels 5-HT1C recognition sites in pig choroid plexus membranes. Comparison with [3H]mesulergine and [3H]5-HT binding. Neurosci Lett. 1986 Sep 12;69(3):269–274. doi: 10.1016/0304-3940(86)90492-1. [DOI] [PubMed] [Google Scholar]
- Jackson M. C., Choudry Y., Bourne A., Woodley J. F., Kenny A. J. A fluorimetric assay for aminopeptidase W. Biochem J. 1988 Jul 1;253(1):299–302. doi: 10.1042/bj2530299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen P. L., Skou J. C. Preparation of highly active (Na+ + K+)-ATPase from the outer medulla of rabbit kidney. Biochem Biophys Res Commun. 1969 Sep 24;37(1):39–46. doi: 10.1016/0006-291x(69)90877-8. [DOI] [PubMed] [Google Scholar]
- Marumo F., Masuda T., Ando K. Presence of the atrial natriuretic peptide in human cerebrospinal fluid. Biochem Biophys Res Commun. 1987 Mar 30;143(3):813–818. doi: 10.1016/0006-291x(87)90321-4. [DOI] [PubMed] [Google Scholar]
- Masuzawa T., Ohta T., Kawamura M., Nakahara N., Sato F. Immunohistochemical localization of Na+, K+-ATPase in the choroid plexus. Brain Res. 1984 Jun 8;302(2):357–362. doi: 10.1016/0006-8993(84)90250-6. [DOI] [PubMed] [Google Scholar]
- Masuzawa T., Saito T., Sato F. Cytochemical study on enzyme activity associated with cerebrospinal fluid secretion in the choroid plexus and ventricular ependyma. Brain Res. 1981 Oct 19;222(2):309–322. doi: 10.1016/0006-8993(81)91035-0. [DOI] [PubMed] [Google Scholar]
- Matsas R., Fulcher I. S., Kenny A. J., Turner A. J. Substance P and [Leu]enkephalin are hydrolyzed by an enzyme in pig caudate synaptic membranes that is identical with the endopeptidase of kidney microvilli. Proc Natl Acad Sci U S A. 1983 May;80(10):3111–3115. doi: 10.1073/pnas.80.10.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Kenny A. J., Turner A. J. An immunohistochemical study of endopeptidase-24.11 ("enkephalinase") in the pig nervous system. Neuroscience. 1986 Aug;18(4):991–1012. doi: 10.1016/0306-4522(86)90113-2. [DOI] [PubMed] [Google Scholar]
- Matsas R., Kenny A. J., Turner A. J. The metabolism of neuropeptides. The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem J. 1984 Oct 15;223(2):433–440. doi: 10.1042/bj2230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Turner A. J., Kenny A. J. Endopeptidase-24.11 and aminopeptidase activity in brain synaptic membranes are jointly responsible for the hydrolysis of cholecystokinin octapeptide (CCK-8). FEBS Lett. 1984 Sep 17;175(1):124–128. doi: 10.1016/0014-5793(84)80583-9. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A. Beta-adrenergic-sensitive adenylate cyclase in secretory cells of choroid plexus. Science. 1979 May 25;204(4395):843–844. doi: 10.1126/science.220707. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A. beta-Adrenergic-sensitive adenylate cyclase in choroid plexus: properties and cellular localization. Mol Pharmacol. 1980 Sep;18(2):199–209. [PubMed] [Google Scholar]
- Pazos A., Hoyer D., Palacios J. M. The binding of serotonergic ligands to the porcine choroid plexus: characterization of a new type of serotonin recognition site. Eur J Pharmacol. 1984 Nov 27;106(3):539–546. doi: 10.1016/0014-2999(84)90057-8. [DOI] [PubMed] [Google Scholar]
- Pernow B. Substance P. Pharmacol Rev. 1983 Jun;35(2):85–141. [PubMed] [Google Scholar]
- Rix E., Ganten D., Schüll B., Unger T., Taugner R. Converting-enzyme in the choroid plexus, brain, and kidney: immunocytochemical and biochemical studies in rats. Neurosci Lett. 1981 Mar 10;22(2):125–130. doi: 10.1016/0304-3940(81)90075-6. [DOI] [PubMed] [Google Scholar]
- Ross H. J., Wright E. M. Neutral amino acid transport by plasma membrane vesicles of the rabbit choroid plexus. Brain Res. 1984 Mar 12;295(1):155–160. doi: 10.1016/0006-8993(84)90826-6. [DOI] [PubMed] [Google Scholar]
- Schmitz J., Preiser H., Maestracci D., Ghosh B. K., Cerda J. J., Crane R. K. Purification of the human intestinal brush border membrane. Biochim Biophys Acta. 1973 Sep 27;323(1):98–112. doi: 10.1016/0005-2736(73)90434-3. [DOI] [PubMed] [Google Scholar]
- Skidgel R. A. Basic carboxypeptidases: regulators of peptide hormone activity. Trends Pharmacol Sci. 1988 Aug;9(8):299–304. doi: 10.1016/0165-6147(88)90015-6. [DOI] [PubMed] [Google Scholar]
- Solhonne B., Gros C., Pollard H., Schwartz J. C. Major localization of aminopeptidase M in rat brain microvessels. Neuroscience. 1987 Jul;22(1):225–232. doi: 10.1016/0306-4522(87)90212-0. [DOI] [PubMed] [Google Scholar]
- Steardo L., Nathanson J. A. Brain barrier tissues: end organs for atriopeptins. Science. 1987 Jan 23;235(4787):470–473. doi: 10.1126/science.2879355. [DOI] [PubMed] [Google Scholar]
- Stephenson S. L., Kenny A. J. Metabolism of neuropeptides. Hydrolysis of the angiotensins, bradykinin, substance P and oxytocin by pig kidney microvillar membranes. Biochem J. 1987 Jan 1;241(1):237–247. doi: 10.1042/bj2410237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson S. L., Kenny A. J. The hydrolysis of alpha-human atrial natriuretic peptide by pig kidney microvillar membranes is initiated by endopeptidase-24.11. Biochem J. 1987 Apr 1;243(1):183–187. doi: 10.1042/bj2430183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi K., Niwa M., Himeno A., Kurihara M., Kawano T., Ibaragi M., Ozaki M., Mori K. Alpha-atrial natriuretic peptide binding sites in the rat choroid plexus are increased in the presence of hydrocephalus. Neurosci Lett. 1988 Apr 22;87(1-2):93–98. doi: 10.1016/0304-3940(88)90151-6. [DOI] [PubMed] [Google Scholar]
- Yagaloff K. A., Hartig P. R. Solubilization and characterization of the serotonin 5-HT1c site from pig choroid plexus. Mol Pharmacol. 1986 Feb;29(2):120–125. [PubMed] [Google Scholar]