Abstract
We investigated associations of glycemic measures, and insulin resistance and secretion measures with hippocampal and subfield volumes. In this cross-sectional study, 7400 community-dwelling participants underwent brain MRI and health checkups between 2016 and 2018. Hemoglobin A1c (HbA1c), glycated albumin (GA), homeostasis model assessment for insulin resistance (HOMA-IR), and HOMA of percent β-cell function (HOMA-β) were evaluated. The associations of each measure with a smaller volume of the hippocampus and twelve hippocampal subfields were investigated. As a result, higher HbA1c or GA and lower HOMA-β levels were significantly associated with smaller volumes in multiple hippocampal subfields. Furthermore, even when we analyzed non-diabetic individuals, substantial associations remained between higher GA or lower HOMA-β levels and smaller volumes of the whole hippocampus or the fimbria. Our findings indicate that postprandial glucose fluctuations, postprandial hyperglycemia, and low insulin secretion have a specific effect on the development of smaller hippocampal volume, suggesting that primary prevention of diabetes and/or sufficient glucose control are important for the prevention of dementia.
Subject terms: Risk factors, Dementia
Introduction
Alzheimer’s disease (AD) and cognitive impairments are costly medical problems worldwide, and diabetes mellitus (DM) is a major risk factor for AD and cognitive impairments1–8. It has been reported that patients with type 2 DM and animal models with DM have impaired cognitive functions such as memory, particularly episodic and spatial memory, learning, cognitive flexibility, executive function, processing speed, and attention5,6,9.
Several studies have shown considerable associations between type 2 DM and smaller volume of the hippocampus and its subfields1–4,10–12, and found that abnormalities of some glycemic measures, insulin resistance, and secretion measures were associated with smaller volume of the hippocampus and its subfields2,10–12. Hyperglycemia, impaired insulin secretion, and insulin resistance have been considered to impair the hippocampus to cause oxidative stress13,14, inflammation, and vascular damage11, decreased neurogenesis2,12 and excessive production of amyloid-β (Aβ)11, and phosphorylation of tau11. However, there were inconsistent findings on which subfields had been smaller in patients with DM or abnormalities of glycemic or insulin resistance/secretion measures. There have been no reports of simultaneous studies of glycemic measures, insulin resistance, and secretion in the same participant group, and few papers have excluded patients with dementia and mild cognitive impairment. Large-scale studies examining the associations between glucose metabolism markers and the hippocampus in people without dementia are essential to understanding these relationships.
The Japan Prospective Studies Collaboration for Aging and Dementia (JPSC-AD) is an ongoing community-based observational study for dementia in eight research sites in Japan, in which ~10,000 older participants have undergone a brain MRI examination and the following glycemic measures at the baseline survey15. Hemoglobin A1c (HbA1c) is a commonly used glycemic measure in clinical fields, and glycated albumin (GA) is an alternative glycemic measure of postprandial glucose fluctuations16. Because the glycation speed of albumin is faster than that of hemoglobin17, therefore, the GA levels rise faster than the HbA1c levels in response to the rapid increase in blood glucose levels16. Furthermore, the homeostasis model assessment for insulin resistance (HOMA-IR) is utilized as a marker of insulin resistance18, and the homeostasis model assessment of percent beta cell function (HOMA-β) is considered to be the marker of insulin secretion. This study aimed to clarify which hippocampal subfields are vulnerable to glucose dysmetabolism. We investigated the associations between glycemic measures (HbA1c and GA) and insulin resistance (HOMA-IR) and secretion (HOMA-β) measures with hippocampal and subfield volumes in an older Japanese population with normal cognition. By targeting only cognitively normal individuals, we were able to exclude some individuals with moderate or greater AD pathology, and further clarify the association between glycemic measures or insulin resistance/ secretion and hippocampus.
Results
Characteristics of participants
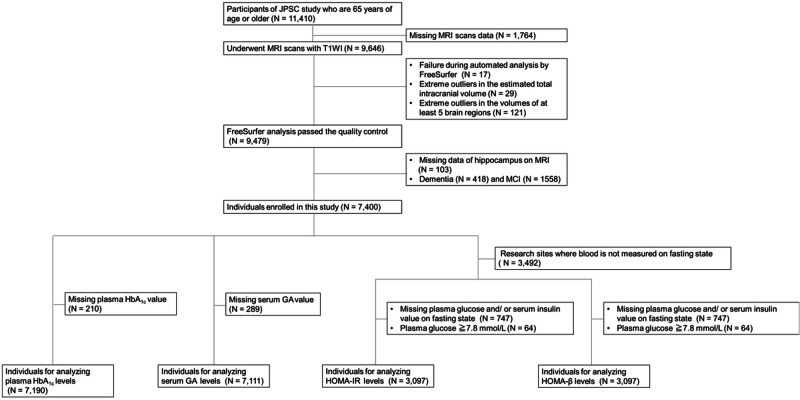
The baseline survey was conducted from 2016 to 2018. A total of 11,957 community residents in 8 research sites consented to participate in the study, of which 11,410 individuals were aged 65 years or older. Full community surveys were conducted at 6 rural sites; at each of these sites, all residents aged 65 years or older were recruited based on the basic resident registers at the initial year of the baseline survey and were encouraged to participate in the surveys. As a consequence, 8030 individuals aged 65 years or older (participation rate, 67% of the total residents of this age group across the 6 sites) consented to participate in these surveys: Yahaba Town (n = 962), Nakajima Town (n = 2128), Ama Town (n = 722), Nakayama Town (n = 927), Hisayama Town (n = 1714), and Arao City (n = 1577). The remaining 3380 individuals were selected by a simple random sampling (Arakawa Ward, n = 1099) and a voluntary response sampling (Hirosaki City, n = 2281) at the 2 sites with larger populations. The recruited residents visited the research facilities (e.g., the health centers, clinics, or hospitals) for the baseline survey, MRI scans, and blood samplings. We enrolled 7400 participants with normal cognitive function (3030 men and 4370 women) in this study. Among them, 210 and 289 individuals without available measurements of HbA1c and GA, respectively, were excluded. Therefore, we analyzed 7,190 and 7,111 individuals for HbA1c and GA, respectively (Fig. 1).
Fig. 1. A flow chart of enrolled individuals for each analysis of the associations between the hippocampus volumes and each diabetes status, HbA1c levels, GA levels, HOMA-IR, and HOMA-β.
GA glycated albumin, HbA1c hemoglobin A1c, HOMA-β homeostasis model assessment of percent β-cell function, HOMA-IR homeostasis model assessment for insulin resistance, JPSC-AD the Japan Prospective Studies Collaboration for Aging and Dementia, MRI magnetic resonance imaging, T1WI T1-weighted imaging.
Additionally, 3492 individuals in the sites where blood is not measured on fasting state, 747 individuals without available measurement of fasting glucose, 64 individuals with a fasting glucose level ≥7.8 mmol/L in the analysis of the HOMA-IR and the analysis of the HOMA-β were excluded. The remaining 3097 individuals were enrolled for HOMA-IR and HOMA-β levels, respectively (Fig. 1). The median values of HbA1c, GA, HOMA-IR, and HOMA-β in the study population were 5.7% [interquartile range (IQR): 5.5–6.0%], 14.8% (IQR: 13.9–16.0%), 1.08% (IQR:0.78–1.58%), and 67.0% (IQR: 47.7–93.6%), respectively. The clinical characteristics are summarized according to the levels of HbA1c, GA, HOMA-β, and HOMA-IR (Table 1 and supplementary Table 1). Table 1 shows the clinical characteristics of the participants according to HbA1c, GA and HOMA-β levels. The frequencies of hypertension, smoking habits, the mean values of age and BMI all increased significantly with higher HbA1c levels. Conversely, the frequencies of female sex, APOE ε4, the mean values of serum LDL cholesterol, and serum HDL cholesterol all decreased significantly with higher HbA1c levels. Similar findings were obtained for GA, except for the presence of APOE ε4, BMI, educational levels, and smoking habits. For HOMA-β, similar findings were obtained except for age, the presence of APOE ε4, hypertension, BMI, and drinking habits (Table 1). Similar findings were obtained for GA, except for the presence of APOE ε4, BMI, educational levels, and smoking habits. For HOMA-β, except for age, the presence of APOE ε4, hypertension, BMI, drinking habits, and estimated total intracranial volume (eTIV) (Table 1). Similar findings were obtained for serum HOMA-IR, except for age, sex, presence of APOE ε4, smoking habits, regular exercise, and eTIV (Supplementary Table 1). We also show the clinical characteristics of 7400 participants according to the status of each risk factor as supplementary Table 2.
Table 1.
Clinical characteristics according to hemoglobinA1c, serum glycated albumin, and homeostasis model assessment of β cell function (HOMA-β) levels
| Variables | Hemoglobin A1c levels (%) | Serum glycated albumin levels (%) | HOMA-β levels | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <5.7 | 5.7–6.4 | ≥6.5 | <13.9 | 13.9–14.8 | 14.9–16.0 | ≥16.1 | ≤30 | >30 | |
| Number of participants | 3406 | 2989 | 795 | 1736 | 1854 | 1815 | 1706 | 257 | 2840 |
| Age, y | 72.0 (5.7) | 72.2 (5.6) | 72.4 (5.4)* | 70.6 (4.8) | 71.5 (5.1) | 72.7 (5.7) | 73.7 (6.2)* | 71.3 (4.9) | 71.1 (5.1) |
| Women, % | 60.1 | 61.1 | 48.0* | 54.7 | 62.7 | 64.2 | 54.5* | 31.9* | 62.3 |
| ApoE Ɛ4, present, % | 18.1 | 16.5 | 15.0* | 16.1 | 17.8 | 17.6 | 16.7 | 19.8 | 17.6 |
| Hypertension, % | 48.6 | 56.6 | 64.8* | 52.1 | 49.5 | 51.7 | 61.6* | 70.3 | 69.6 |
| BMI, kg/m2 | 23.3 (3.3) | 23.7 (3.3) | 24.6 (3.5)* | 24.1 (3.2) | 23.3 (3.1) | 22.8 (3.2) | 23.1 (3.4)* | 21.6 (2.6)* | 23.1 (3.1) |
| Serum LDL-chol, mg/dL | 118.7 (30.3) | 118.7 (30.3) | 113.3 (30.6)* | 121.1 (31.6) | 120.3 (29.5) | 117.9 (29.4) | 113.1 (29.8)* | 113.8 (31.5)* | 123.1 (30.6) |
| Serum HDL-chol, mg/dL | 65.4 (16.9) | 61.9 (16.5) | 56.2 (15.3)* | 60.6 (15.8) | 64.0 (16.9) | 65.0 (17.2) | 62.3 (17.6)* | 70.0 (18.3)* | 64.4 (16.7) |
| Education ≤ 9 y, % | 25.1 | 25.1 | 25.1 | 22.1 | 23.9 | 23.9 | 27.7* | 23.3 | 20.4 |
| Current smoking habits, % | 8.5 | 7.8 | 9.8* | 11.8 | 7.4 | 6.9 | 7.3* | 15.2* | 7.0 |
| Current alcohol intakes, % | 47.5 | 41.1 | 43.2 | 52.9 | 43.9 | 40.7 | 40 | 62.3* | 44.5 |
| Regular exercise, % | 43.9 | 44.2 | 43.5 | 42.9 | 44.9 | 42.7 | 45.6 | 48.6 | 44.0 |
| Estimated intracranial volume, ×103 mm3 | 1421.3 (155.8) | 1409.6 (150.7) | 1433.1 (156.1) | 1423.8 (155.2) | 1410.4 (153.8) | 1408.0 (150.5) | 1430.3 (155.0) | 1449.6* (140.8) | 1375.6 (146.7) |
APOE apolipoprotein E, BMI body mass index, eTIV estimated intracranial volume, HDL-chol high-density lipoprotein cholesterol, HOMA-β homeostasis model assessment of beta cell function, LDL chol low-density lipoprotein cholesterol. Values were shown as mean values (standard deviations or frequencies). *p for trend < 0.05.
Associations of hippocampal and subfield volumes with diabetic status
We analyzed the hippocampal volume (HV) and volume of 12 hippocampal subfields [hippocampus amygdala transition area (HATA), fimbria, hippocampal fissure, molecular layer, granule cell and molecular cell layer of the dentate gyrus (GC ML DG), Cornu Ammonis (CA) 1, CA3, CA4, subiculum, presubiculum, parasubiculum, and hippocampal tail] (Fig. 2A). When we checked the normality, the distributions of outcome variables such as HV and the volume of 12 hippocampus subfields were found to be normal. Figure 3A–C show the whole hippocampus with a significantly lower volume in the higher levels of HbA1c or GA, or the lower levels of HOMA-β in the Models 1 and 2 analysis (Fig. 3A–C). Figure 2B–D show the hippocampal subfields with significantly lower volumes in the higher levels of HbA1c or GA, or the lower levels of HOMA-β in the Model 2 analysis (Fig. 2B–D). Regarding the levels of HbA1c, substantial differences were observed in the whole hippocampus and following subfields: fimbria, hippocampal fissure, subiculum, presubiculum, and hippocampal tail after adjusting for age, sex, research site, educational levels, and eTIV (Model 1), and fimbria, subiculum, presubiculum, and hippocampal tail after adjusting for age, sex, research site, educational levels, eTIV, presence of APOE ε4, hypertension, BMI levels, serum LDL and HDL cholesterol levels, regular exercise and smoking and drinking habits (Model 2) (Table 2, Fig. 2B). Higher HbA1c levels showed smaller hippocampal subfield volume (HSV) in the fimbria subiculum, presubiculum, and hippocampal tail (Table 2, Fig. 2B). No subfields showed significant differences in the non-DM participants (Supplementary Table 3).
Fig. 2. Hippocampal subfields and the relationships between hippocampal subfields and glycemic measures or insulin secretion.
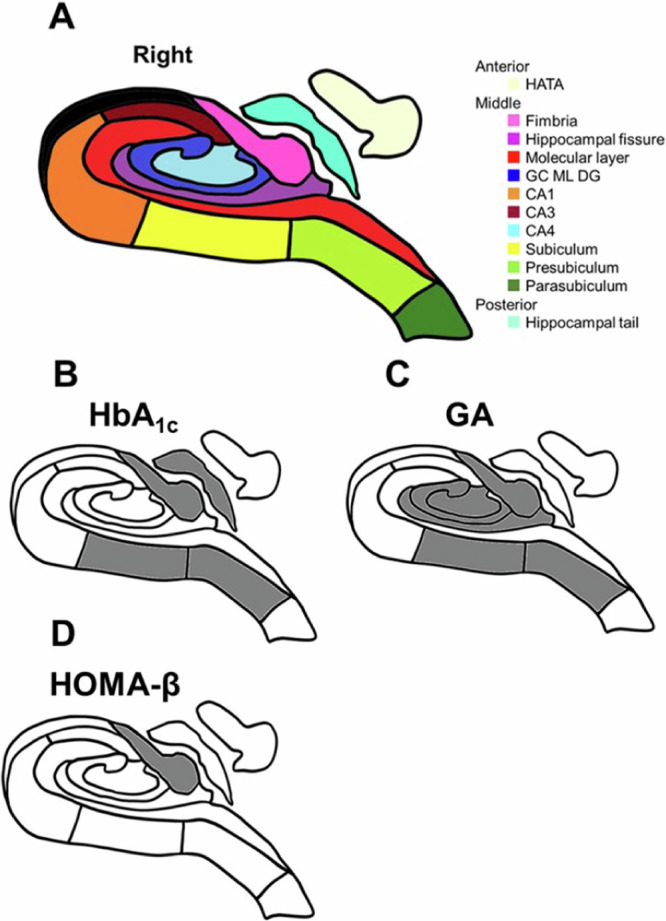
A A schematic representation of the right hippocampus in a coronal section. Hippocampal subfields are represented in different colors. HATA Hippocampus-amygdala-transition-area, GC, DG, ML, Granule cell and molecular cell layer of the dentate gyrus, CA Cornu Ammonis. Notice that the subfield HATA is transposed from an anterior coronal section, while the tail is transposed from a posterior coronal section. B–D A schematic representation displaying the substantial associations between hippocampal subregions and glycemic measures or insulin secretion measures in the analysis of the multivariable-adjusted values (Model 2): HbA1c (B), GA (C), and HOMA-β value (D). Gray color represents a substantially smaller volume in the subfields.
Fig. 3. The mean values of the volumes of the whole hippocampus according to HbA1c, GA, and HOMA-β value.
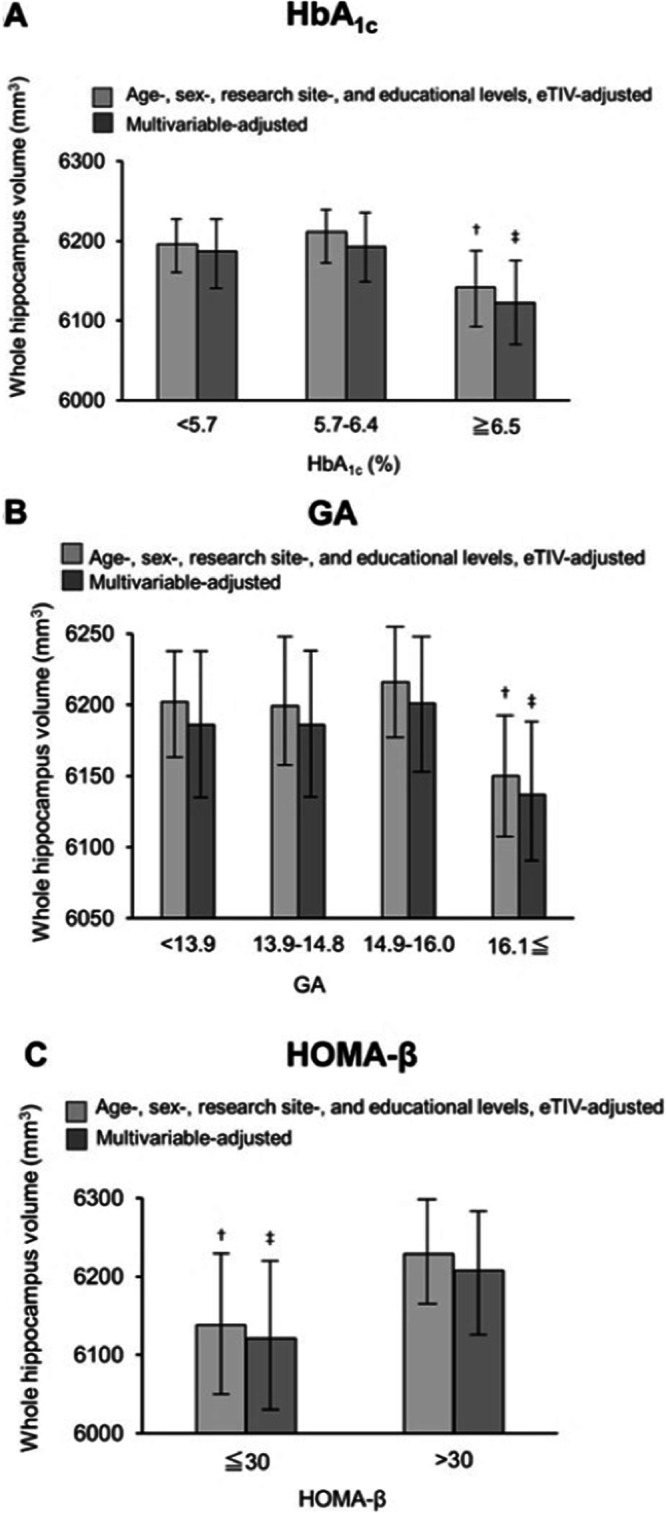
A–C The mean values of the volumes of the whole hippocampus according to HbA1c (A), GA (B), and HOMA-β value (C), with analysis of the age-, sex-, research site-, and educational levels-, estimated intracranial volume (eTIV)- adjusted values (Model 1) and multivariable-adjusted values (Model 2). †<0.05. ‡<0.001.
Table 2.
Multivariable-adjusted mean values of the volumes of the hippocampus and hippocampal subregions according to hemoglobinA1c levels
| Hemoglobin A1c levels, % | p for trend | q value of FDR correction | ||||
|---|---|---|---|---|---|---|
| <5.7 (n = 3406) | 5.7–6.4 (n = 2989) | ≥6.5 (n = 795) | ||||
| Whole hippocampus, HV, mm3 | 6187.727 (6144.512–6230.942) | 6193.960 (6149.737–6238.183) | 6122.181 (6067.664–6176.698) | 0.003* | – | |
| Hippocampal subregions | ||||||
| HATA, HV, mm3 | 97.152 (95.889–98.416) | 96.858 (95.565–98.152) | 95.597 (94.003–97.191) | 0.046* | 0.069 | |
| Fimbria, HV, mm3 | 102.353 (99.642–105.064) | 99.652 (96.878–102.427) | 93.592 (90.172–97.012) | <0.001* | <0.001† | |
| Hippocampal fissure, HV, mm3 | 346.103 (342.022–350.184) | 348.721 (344.545–352.898) | 350.870 (345.721–356.019) | 0.025* | 0.060 | |
| Molecular_layer, HV, mm3 | 803.394 (796.486–810.303) | 805.025 (797.955–812.094) | 796.674 (787.959–805.389) | 0.049* | 0.065 | |
| GC ML DG, HV, mm3 | 540.797 (536.343–545.251) | 540.954 (536.396–545.511) | 535.250 (529.632–540.869) | 0.026* | 0.052 | |
| CA1, HV, mm3 | 1211.672 (1201.436–1221.908) | 1213.804 (1203.328–1224.279) | 1203.977 (1191.063–1216.890) | 0.150 | 0.180 | |
| CA3, HV, mm3 | 397.392 (393.178–401.607) | 399.835 (395.523–404.148) | 395.177 (389.860–400.494) | 0.038* | 0.065 | |
| CA4, HV, mm3 | 478.310 (474.420–482.201) | 479.303 (475.322–483.284) | 475.788 (470.880–480.696) | 0.180 | 0.196 | |
| Subiculum, HV, mm3 | 838.303 (831.211–845.395) | 836.156 (828.898–843.413) | 824.620 (815.673–833.567) | <0.001* | 0.002† | |
| Presubiculum, HV, mm3 | 560.557 (554.904–566.210) | 557.340 (551.555–563.125) | 550.431 (543.300–557.562) | 0.001* | 0.004† | |
| Parasubiculum, HV, mm3 | 109.487 (107.416–111.557) | 108.293 (106.174–110.412) | 108.706 (106.094–111.318) | 0.180 | 0.196 | |
| Hippocampal tail, HV, mm3 | 1048.310 (1037.476–1059.144) | 1056.740 (1045.653–1067.827) | 1042.368 (1028.701–1056.036) | 0.006* | 0.018† | |
CA Cornu Ammonis, eTIV estimated intracranial volume, FDR false discovery rate, GC ML DG granule cell and molecular cell layer of the dentate gyrus, HATA hippocampus amygdala transition area, HV hippocampal volume. Values are shown as multivariable-adjusted mean values (95% confidence intervals) where values are calculated as follows: (left + right) volumes of hippocampus or hippocampal subregions. Adjusted for age, sex, educational level, research site, apolipoprotein E Ɛ4, body mass index, hypertension, serum low-density lipoprotein cholesterol, serum high-density lipoprotein cholesterol, current smoking habits, current alcohol intakes, regular exercise, eTIV. *p for trend <0.05. †q value of FDR correction <0.05.
Significant associations were observed between the GA levels and HV and HSV. Substantial differences were found in the whole hippocampus and the following subfields: HATA, fimbria, hippocampal fissure, GC ML DG, CA4, subiculum, and presubiculum in Model 1, and fimbria, hippocampal fissure, GC ML DG, CA4, subiculum, and presubiculum in Model 2 (Table 3, Figs. 2C and 3B). The group with higher GA levels showed smaller HSV in the fimbria, GC ML DG, CA4, subiculum, and presubiculum, and larger HSV in the hippocampal fissure. A significant difference was found only in the fimbria in the analysis of the non-DM participants (Supplementary Table 4).
Table 3.
Multivariable-adjusted mean values of the volumes of the hippocampus and hippocampal subregions according to serum glycated albumin levels
| Serum glycated albumin levels, % | p for trend | q value of FDR correction | ||||
|---|---|---|---|---|---|---|
| <13.9 (n = 1736) | 13.9–14.8 (n = 1854) | 14.9–16.0 (n = 1815) | ≥16.1 (n = 1706) | |||
| Whole hippocampus, HV, mm3 | 6186.512 (6139.262−6233.762) | 6186.906 (6139.777−6234.035) | 6201.668 (6154.382−6248.945) | 6137.553 (6090.263−6184.843) | 0.003* | − |
| Hippocampal subregions | ||||||
| HATA, HV, mm3 | 97.551 (96.134−98.967) | 97.781 (96.396−99.193) | 98.047 (96.631−99.463) | 96.525 (95.109−97.942) | 0.031* | 0.053 |
| Fimbria, HV, mm3 | 103.165 (100.207−106.122) | 102.379 (99.429−105.330) | 101.703 (98.743−104.663) | 94.823 (91.863−97.783) | <0.001* | <0.001† |
| Hippocampal fissure, HV, mm3 | 344.556 (340.118−348.994) | 344.140 (339.714−348.567) | 347.012 (342.570−351.453) | 349.329 (344.888−353.771) | 0.009* | 0.027† |
| Molecular_layer, HV, mm3 | 805.593 (798.038−813.147) | 805.133 (797.598−812.668) | 802.392 (794.832−809.952) | 798.359 (790.799−805.920) | 0.059 | 0.078 |
| GC ML DG, HV, mm3 | 541.073 (536.208−545.938) | 540.880 (536.027−545.733) | 542.606 (537.737−547.475) | 534.958 (530.088−539.827) | <0.001* | 0.001† |
| CA1, HV, mm3 | 1212.591 (1201.390−1223.792) | 1209.935 (1198.763−1221.108) | 1214.922 (1203.712−1226.131) | 1204.180 (1192.969−1215.390) | 0.075 | 0.112 |
| CA3, HV, mm3 | 396.994 (392.388−401.600) | 398.176 (393.581−402.770) | 399.892 (395.282−404.502) | 395.893 (391.283−400.503) | 0.125 | 0.136 |
| CA4, HV, mm3 | 478.029 (473.777−482.281) | 478.330 (474.089−482.571) | 480.370 (476.115−484.625) | 475.274 (471.019−479.530) | 0.019* | 0.038† |
| Subiculum, HV, mm3 | 838.538 (830.778−846.299) | 836.945 (829.205−844.686) | 838.422 (830.656−846.188) | 828.178 (820.411−835.945) | 0.001* | 0.004† |
| Presubiculum, HV, mm3 | 560.138 (553.959−564.161) | 557.998 (551.835−564.161) | 559.778 (553.595−565.961) | 552.829 (546.645−559.013) | 0.009* | 0.027† |
| Parasubiculum, HV, mm3 | 109.262 (107.003−111.521) | 108.682 (106.429−110.935) | 108.994 (106.734−111.255) | 108.092 (105.831−110.353) | 0.585 | 0.585 |
| Hippocampal tail, HV, mm3 | 1043.944 (1032.095−1055.793) | 1051.311 (1039.492−1063.129) | 1055.309 (1043.450−1067.167) | 1049.210 (1037.350−1061.069) | 0.092 | 0.110 |
CA Cornu Ammonis, eTIV estimated intracranial volume, FDR false discovery rate, GC ML DG granule cell and molecular cell layer of the dentate gyrus, HATA hippocampus amygdala transition area, HV hippocampal volume. Values are shown as multivariable-adjusted mean values (95% confidence intervals), where values are calculated as follows: (left + right) volumes of hippocampus or hippocampal subregions. Adjusted for age, sex, educational level, research site, apolipoprotein E Ɛ4, body mass index, hypertension, serum low-density lipoprotein cholesterol, serum high-density lipoprotein cholesterol, current smoking habits, current alcohol intakes, regular exercise, eTIV. * p for trend <0.05. †q value of FDR correction <0.05.
There were substantial associations between HOMA-β levels and the HV and HSV. Significant differences were found in the whole hippocampus and the fimbria in both Model 1 and Model 2 (Table 4, Figs. 2D and 3C). The HV and HSV in these areas were smaller in the group with a lower HOMA-β. In the analysis of participants without DM, substantial differences were found in the whole hippocampus and the fimbria (Supplementary Table 5).
Table 4.
Multivariable-adjusted mean values of the volumes of the hippocampus and hippocampal subregions according to homeostasis model assessment of β cell function (HOMA-β) levels
| HOMA-β levels | p value | q value of FDR correction | ||
|---|---|---|---|---|
| ≤30 (n = 257) | >30 (n = 2840) | |||
| Whole hippocampus, HV, mm3 | 6121.331 (6024.162–6218.500) | 6208.870 (6132.305–6285.434) | 0.013* | – |
| Hippocampal subregions | ||||
| HATA, HV, mm3 | 93.744 (90.970–96.518) | 95.459 (93.273–97.645) | 0.087 | 0.130 |
| Fimbria, HV, mm3 | 101.041 (94.801–107.281) | 108.221 (103.304–113.138) | 0.001* | 0.012† |
| Hippocampal fissure, HV, mm3 | 341.212 (0332.141–350.284) | 337.296 (330.148–344.444) | 0.232 | 0.253 |
| Molecular_layer, HV, mm3 | 870.085 (854.298–885.872) | 880.248 (867.808–892.687) | 0.075 | 0.150 |
| GC ML DG, HV, mm3 | 526.831 (517.148–536.515) | 535.342 (527.712–542.972) | 0.015* | 0.09 |
| CA1, HV, mm3 | 1183.722 (1160.750–1206.694) | 1198.105 (1180.004–1216.205) | 0.083 | 0.142 |
| CA3, HV, mm3 | 390.998 (381.655–400.342) | 395.505 (388.142–402.867) | 0.182 | 0.242 |
| CA4, HV, mm3 | 465.290 (456.866–473.713) | 471.411 (464.773–478.048) | 0.044* | 0.132 |
| Subiculum, HV, mm3 | 813.709 (798.120–829.297) | 825.507 (813.224–837.790) | 0.036* | 0.144 |
| Presubiculum, HV, mm3 | 540.658 (528.446–552.870) | 545.982 (536.360–555.605) | 0.227 | 0.272 |
| Parasubiculum, HV, mm3 | 109.341 (104.803–113.879) | 109.044 (105.469–112.620) | 0.856 | 0.856 |
| Hippocampal tail, HV, mm3 | 1025.912 (1001.013–1050.811) | 1044.046 (1024.427–1063.665) | 0.044* | 0.132 |
CA Cornu Ammonis, eTIV estimated intracranial volume, FDR false discovery rate, GC ML DG granule cell and molecular cell layer of the dentate gyrus, HATA hippocampus amygdala transition area, HOMA-β homeostasis model assessment of beta cell function, HV hippocampal volume. Values are shown as multivariable-adjusted mean values (95% confidence intervals), where values are calculated as follows: (left + right) volumes of hippocampus or hippocampal subregions. Adjusted for age, sex, educational level, research site, apolipoprotein E Ɛ4, body mass index, hypertension, serum low-density lipoprotein cholesterol, serum high-density lipoprotein cholesterol, current smoking habits, current alcohol intakes, regular exercise, eTIV. *p for trend <0.05. †q value of FDR correction <0.05.
We did not find any significant associations between the HOMA-IR level and either the HV or HSV (Supplementary Table 6). Analyses of non-DM participants showed similar results (Supplementary Table 7).
The results of multiple linear-regressions analysis for sensitivity analysis, showed that HbA1c and GA were similar to the original analysis, and log-transformed HOMA-β was significantly related to fimbria, GC ML DG, and CA1, and FDR correction also showed an association with fimbria (supplementary Tables 8–11).
Regarding the stratified analysis by age, only the group under 75 years of age showed significant differences and the p value for the interaction effect was less than 0.05 in fimbria and hippocampal fissure for GA (Supplementary Tables 12–15). About the results of stratified analysis by presence/absence of ApoE Ɛ4, the group without ApoE Ɛ4 showed significant differences and the p value for the interaction effect was <0.05 in fimbria for HbA1c and hippocampal tail for HOMA-β (Supplementary Tables 16–19).
When we analyzed including people with dementia or MCI, we observed substantially the same results except for the number of subfields of hippocampus showing significant association increased from 4 to 5 for HbA1c and 6 to 11 for GA and disappeared the differences of HV for HOMA-β (Supplementary Tables 20–23).
Discussion
This cross-sectional study showed substantial associations between a higher blood hyperglycemic state or lower insulin secretion and extensive smaller hippocampal volume in some subregions. Particularly, it was found that higher levels of HbA1c caused a smaller volume of the hippocampal tail, fimbria, subiculum, and presubiculum, that higher levels of GA caused a smaller volume of fimbria, dentate gyrus (GC ML DG), CA4, subiculum, presubiculum, and that lower levels of the HOMA-β caused the smaller volume of fimbria. In particular, the higher levels of GA, the more subfields had smaller volumes.
The significant interaction effects between GA and age were observed in fimbria, and hippocampal fissure. We considered that this result reflected more AD pathology, vascular disease, florid changes, and other pathologies added in older participants, and the relationships between diabetes markers and hippocampal subregion volume changes with age. The mechanism of the interaction effect between HbA1c or HOMA-β and the presence/absence of ApoE Ɛ4 in the fimbria and hippocampal tail of the group without ApoE Ɛ4 is not known.
For HOMA-β, when we added people with cognitively impaired, the significant differences of HV were disappeared. We speculate that it was because people with cognitively impaired had different reasons that associated for hippocampal atrophy other than decreased insulin secretions.
The hippocampal subfields are primarily composed of the ammon horn (CA1-4), dentate gyrus that includes the molecular layer and granule cells of the molecular layer, subiculum, presubiculum, and parasubiculum. Additionally, the hippocampus includes the fimbria and HATA. The hippocampus sends major outputs from the CA1 and subiculum to the cortical regions. The CA1 and subiculum are considered the most important regions for the formation of memories such as episodic memory, spatial memory, and autonoetic consciousness19–23. Another output from the hippocampus is sent through the fimbria, which is composed of myelinated axons and comprises the Papez circuit and polysynaptic pathway. Fimbria is important for memory and learning24,25. The hippocampus receives major inputs through the dentate gyrus. The dentate gyrus is thought to contribute to the formation of novelty detection, pattern separation, pattern completion, spatial working memory, information encoding, and memory consolidation and retention26–28. The fact that higher HbA1c or GA or lower HOMA-β impairs these areas might be related to the various cognitive dysfunctions, including episodic memory, spatial memory, and learning in patients with DM. However, the present study did not assess detailed cognitive functions such as episodic memory, spatial memory, and learning, therefore the mediation of the association between the hippocampal subfields where showed lower volume in the present results and cognitive function, could not be established.
Regarding the hyperglycemia index, this study demonstrated more widespread smaller hippocampal volume, particularly with elevated GA, than with HbA1c. HbA1c reflects the mean glucose level over the preceding 3 months29, and GA has been reported to reflect the state of glucose level over the preceding 2-3 weeks30. A disadvantage of HbA1c is that HbA1c levels fluctuates with anemia31, and more than 10% of community-dwelling adults aged 65 years and older have been reported to have WHO-defined anemia32. Therefore, it is possible that HbA1c may have been less effective in indicating blood glucose levels compared to GA. Furthermore, GA has been reported to reflect short-term average glucose and fluctuations in glucose33. Past studies have reported that smaller hippocampal volume might be preceded by not only chronic hyperglycemia but also glycemic variability11, and this result is consistent with that of a previous study. Possible mechanisms underlying the association between hyperglycemia or glycemic variability and smaller hippocampal volume is known to cause oxidative stress, inflammation, and vascular damage. Hyperglycemia is believed to promote the production of advanced glycation end products (AGEs), and the blood receptor of AGEs (i.e., RAGE levels) is altered in patients with prediabetes associated with higher oxidative stress levels13,14. In animal studies, prediabetes rat models with cognitive decline showed neuroinflammation and neuronal apoptosis in the hippocampus34. Glucose toxicity causes microvascular ischemic damage, and chronic hypoxia causes microglia activation, phosphorylation of tau, and cell death, all of which could lead to smaller volume of the hippocampus11.
This study showed that the low insulin secretory capacity results in smaller volume of the whole hippocampus and numerous hippocampal subregions. Furthermore, this study showed that even in people without DM, reduced levels of HOMA-β were associated with smaller volume of the whole hippocampus and fimbria, which are important for memory. The possible mechanisms of smaller hippocampal volume induced by decreased insulin levels are decreased neurogenesis and excessive production of amyloid-β (Aβ), and phosphorylation of tau2,12. Insulin and its receptors are widely expressed in the brain and play important roles in neuronal proliferation and differentiation2. The subgranular zone, which is located in the dentate gyrus in the hippocampus, is one of the two major neural stem cell regions of adults, and insulin and insulin-like growth factors are crucial in neurogenesis and neural stem cell self-renewal through different ligand-receptor interactions2. Furthermore, insulin has previously been reported to promote dendritic spines formation in rat hippocampal neurons35. Therefore, decreased insulin might weaken neurogenesis and decrease volumes of hippocampus or hippocampal subfields. Another possible mechanism is Aβ overproduction and hyperphosphorylation of the tau protein11. Insulin has been reported to be involved in the regulation of Aβ fibrillization, and to protect neurons against Aβ-induced cell-death36, and Aβ overproduction has been reported in the brain of mice that were administered streptozotocin37,38. Furthermore, the deficiency in insulin signaling promotes the tau phosphorylation mediated by glycogen synthase kinase 3β, which is one of the tau phosphorylases39.
In this study, no substantial association was observed between HOMA-IR values and hippocampal volume. Insulin resistance was hypothesized to reduce the insulin concentration in the cerebrospinal fluid40, and result in Aβ overproduction and excessive amount of hyperphosphorylated tau protein41. Some studies reported that HOMA-IR levels were significantly associated with smaller volume of hippocampus and its subfields in non-elderly patients with type 2 DM and postmenopausal women undergoing hormone therapy2,42. Further large-scaled studies are required to clarify the association between HOMA-IR levels and atrophies of hippocampus and its subregions in community-dwelling elderly.
This study had several limitations. First, we did not obtain data on the duration of DM. Second, this study did not exclude patients with preclinical AD because amyloid PET and/or cerebrospinal fluid tests were not performed. Thus, smaller hippocampal volume may also be associated with AD pathogenesis. Third, because the findings of this study were derived from cross-sectional data, the temporal trends of glycemic measures, insulin secretion and atrophy of the hippocampus and its subfields were unknown. Fourth, some study areas used 1.5 T MRIs, and others used 3 T MRIs. Although we standardized MRI images, and we tried to adjust for errors in advance by including the survey sites as an adjustment variable, the MRI conditions might not be exactly the same. Finally, the data in this study are for the Japanese only; therefore, generalizability to other ethnicities, and races has not been examined. The strengths of this study are that it was a large study that could adjust for many factors. In conclusion, this study revealed that higher plasma HbA1c levels, higher serum GA levels, and lower HOMA-β levels were related to smaller hippocampal volume including multiple subfields. Particularly higher serum GA levels or low levels of HOMA-β can be associated with smaller hippocampal volume even in patients with non-DM. Our findings indicate that sufficient glucose control or primary prevention of diabetes is important for the prevention of dementia, and that postprandial hyperglycemia, postprandial glucose fluctuations, and low insulin secretion have a more specific effect on the development of smaller hippocampal volume. Further basic research and prospective longitudinal studies are required to verify these findings.
Methods
Study population
Among the 11,410 participants in the JPSC-AD study aged 65 years or older, 9646 underwent MRI scans with 3-dimensional T1-weighted images. After excluding 167 participants who did not pass the quality control of the FreeSurfer analysis, 103 participants who did not have hippocampal measurement data, and 1976 participants who were diagnosed with dementia or mild cognitive impairment, 7400 participants with normal cognitive function (3030 men and 4370 women) were enrolled in this study (Fig. 1).
Standard protocol approvals, registrations, and patient consents
The study was approved by the Kyushu University Institutional Review Board for clinical research (approval number 686-10) and by each of the local ethics committee of the eight research institutes: Kanazawa University (approval number 2185), Hirosaki University (approval number 2019-064-3), Iwate Medical University (approval number HG2020-017), Keio University School of Medicine (approval number 20160214), Matsue Medical Center (approval number H28-14), and Ehime University (approval number 1610004 and 2210016), Kumamoto University (approval number 333), and Tohoku University (approval number 2021-1-245). The all participants gave their informed consent.
MRI analysis
Structural MRI studies were performed using 1.5 T MRI (four Philips, one Hitachi, and one General-Electric [GE]) and 3 T MRI (one GE and one Siemens) machines. The two sites, Arakawa Ward and Hirosaki City used 3 T MRI, and the other sites used 1.5 T MRI. 3D volumetric acquisition of T1-weighted turbo field echo images was conducted according to the brain MRI protocol for the Alzheimer’s Disease Neuroimaging Initiative (ADNI) study43. In addition, the brain MRI data were standardized by using the MRI Phantom, Human Phantom, and ADNI Phantom to correct geometric distortions among the different pieces of equipment. All T1-structural images were analyzed using FreeSurfer version 7.0 (FreeSurfer version 7.0; http://surfer.nmr.mgh.harvard.edu)44 in Tohoku University and preprocessed according to the standard method. The volumes of the area of interest were created using FreeSurfer 7.045, in particular, the HV and volume of 12 HSV (Fig. 2A) and eTIV were calculated automatically. The hippocampal and subfield volumes were calculated as the sum of the right and left volumes, respectively.
Measurements of HbA1c, GA, HOMA-IR, and HOMA-β and diagnosis of DM
Plasma HbA1c levels were measured using the National Glycohemoglobin Standardization Program (NGSP), and serum GA levels were determined using the enzymatic method. Additionally, serum insulin levels were measured using a chemiluminescence immunoassay. Detailed measurement methods of blood chemistry have been previously reported15. For analyses of the association between the HbA1c category and hippocampal volumes, we categorized HbA1c using standard clinical cutoff points: normal, ≤5.7%; prediabetes, 5.7–6.5%; and diabetes, ≤6.5%. For the analyses, the GA value was divided into quartiles. We calculated the HOMA-IR using the following formula: HOMA-IR = fasting serum insulin (μU/mL)/fasting plasma glucose (mmol/L)/22.546. Subsequently, we calculated the HOMA-β using the following equation: HOMA-β = fasting serum insulin (μU/mL) × 20/[fasting plasma glucose (mmol/L)–3.5]46. For analyses, the HOMA-IR was categorized using standard clinical cutoff points: ≤1.6%, 1.6–2.5%, ≥2.5%, and HOMA-β was also categorized using standard clinical cutoff points: ≤30%, and >30%. For the HOMA-IR and HOMA-β analysis, we excluded individuals who administered insulin injections to remove the effects of exogenous insulin46. DM was determined by the 2010 American Diabetes Association (ADA) criteria47.
Other risk factor and confounding factors measurements
We identified gender, ApoE Ɛ4 status, alcohol consumption, low education, and exercise habits as confounding factors associated with reduced hippocampal volume. Furthermore, hypertension, obesity, hyper-LDL-cholesterolemia, hypo-HDL-cholesterolemia, and smoking, identified as risk factors for vascular disease that could serve as modifiers. Each participant completed a self-administered questionnaire containing questions on sociodemographic data (age, sex, and educational level), medical history (hypertension), regular exercise, and smoking and drinking habits. Regular exercise was defined as physical activity specifically undertaken for exercise or sports performed for at least 30 min twice per week over the most recent year or longer. Completed questionnaires were reviewed by trained researchers to identify unanswered or inconsistent items. The body mass index (BMI) (kg/m2) was used as an indicator of obesity. Serum HDL and LDL cholesterol were enzymatically measured15. To determine Apolipoprotein E (APOE) polymorphisms, two single nucleotide polymorphisms (rs429358 and rs7412) were genotyped using a multiplex PCR-based targeted sequencing method, as previously reported48.
Statistical analyses
Clinical characteristics were evaluated using the t-test or Jonckheere-Tsrpsta test and chi-squared test or Mantel-Haenszel test for continuous and categorical variables, respectively. Analysis of covariance was used to estimate and compare multivariable-adjusted values and their 95% confidence intervals for HV and each HSV. Model 1 was adjusted for age, sex, research site, educational levels and eTIV. Model 2 was further adjusted for the presence of APOE ε4 allele, hypertension, BMI levels, serum LDL and HDL cholesterol levels, regular exercise, and smoking and drinking habits. We performed additional analyses on participants without DM. The problem of multiple comparisons was addressed by controlling Benjamini–Hochberg false discovery rate (FDR)49. The FDR-adjusted p-values (i.e., q values) below 0.05 were considered statistically significant. SPSS software package (version 29; SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. For sensitivity analysis, multiple linear-regressions analysis was performed using the HV and each HSV and the real values of HbA1c, GA. Since HOMA-IR and HOMA-β were not normally distributed, a log transformation was performed to confirm that they were normally distributed, and the log-transformed HOMA-IR and HOMA-β values were used. The covariates were which of the above model 2 covariates.
Furthermore, to confirm the interaction between the diabetes markers and other risk factors for hippocampal atrophy, a stratified analysis of age and the presence of ApoE Ɛ4 as a representative was also performed and confirmed the interaction effect. The stratified analysis about age was analyzed separately for those under 75 and over 75 years of age.
We also performed a sensitivity analysis that included individuals with dementia and MCI.
Supplementary information
Acknowledgements
We would like to thank the participants for contributing their time to the JPSC-AD study and gratefully acknowledge the diligent work and contributions of all researchers and investigators in the JPSC-AD Study Group. This study was supported by the Japan Agency for Medical Research and Development (JP23dk0207053) and Suntory Holdings Limited (Osaka, Japan). The funders had no role in the design of the study, the collection, analysis, and interpretation of data, or the writing of the manuscript.
Author contributions
Research idea and study design: A.S., M.N.-S., T.N., K.O.; data acquisition: A.S., M.N.-S., S.S., Y.U., Y.T., B.T., J.H., T.O., T.H., Y.T., S.N,. T.M., M.M., K.N,. J.I, M.T.; data analysis/interpretation and supervision or mentorship: M.N.-S., H.N., T.N., K.O.; statistical analysis: A.S., M.N.-S. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Data availability
The datasets used in the present study are not publicly available, because they contain confidential clinical data on the study participants. However, the data are available upon reasonable request and with the permission of the Principal Investigator of this study, Toshiharu Ninomiya.
Code availability
No custom code or mathematical algorithm was developed for this study. Details regarding the specific codes used can be found in the references cited. As indicated in the references, any access restrictions or licensing information associated with the codes used can be obtained from the respective sources.
Competing interests
A.S., M.N.-S., S.S., Y.U., Y.T., B.T., J.H., T.O., T.H., Y.T., S.N., T.M., M.M., K.N., J.-i.I., M.T., H.N., and K.O. declares no competing financial or non-financial interests. T.N. declares no competing non-financial interests but the following competing financial interests: Grants from Suntory Holdings Limited.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Moeko Noguchi-Shinohara, Email: m-nohara@med.kanazawa-u.ac.jp.
Kenjiro Ono, Email: onoken@med.kanazawa-u.ac.jp.
the Japan Prospective Studies Collaboration for Aging and Dementia (JPSC-AD) study group:
Toshiharu Ninomiya, Jun Hata, Mao Shibata, Takanori Honda, Tomoyuki Ohara, Masato Akiyama, Shigeyuki Nakaji, Koichi Murashita, Tatsuya Mikami, Songee Jung, Mina Misawa, Tetsuya Maeda, Naoki Ishizuka, Hiroshi Akasaka, Yasuo Terayama, Hisashi Yonezawa, Junko Takahashi, Kenjiro Ono, Moeko Noguchi-Shinohara, Kazuo Iwasa, Sohshi Yuki-Nozaki, Masahito Yamada, Masaru Mimura, Shogyoku Bun, Hidehito Niimura, Ryo Shikimoto, Hisashi Kida, Kenji Nakashima, Yasuyo Fukada, Hisanori Kowa, Toshiya Nakano, Kenji Wada, Masafumi Kishi, Tomoki Ozaki, Ayumi Tachibana, Yuta Yoshino, Jun-ichi Iga, Shu-ichi Ueno, Minoru Takebayashi, Tomohisa Ishikawa, Seiji Yuki, Ryuji Fukuhara, Asuka Koyama, Mamoru Hashimoto, Manabu Ikeda, Yoshihiro Kokubo, Kazuhiro Uchida, Midori Esaki, Yasuyuki Taki, Yasuko Tatewaki, Benjamin Thyreau, Koji Yonemoto, Hisako Yoshida, Kaori Muto, Yusuke Inoue, Izen Ri, Yukihide Momozawa, Chikashi Terao, Michiaki Kubo, and Yutaka Kiyohara
Supplementary information
The online version contains supplementary material available at 10.1038/s41514-024-00164-2.
References
- 1.Zhang, W. et al. Hippocampal subfields atrophy contribute more to cognitive impairment in middle-aged patients with type 2 diabetes rather than microvascular lesions. Acta Diabetol.58, 1023–1033 (2021). 10.1007/s00592-020-01670-x [DOI] [PubMed] [Google Scholar]
- 2.Li, M. et al. Altered hippocampal subfields volumes is associated with memory function in type 2 diabetes mellitus. Front. Neurol.12, 756500 (2021). 10.3389/fneur.2021.756500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee, S. et al. Hippocampal subregional alterations and verbal fluency in the early stage of type 2 diabetes mellitus. Eur. J. Neurosci.54, 7550–7559 (2021). 10.1111/ejn.15505 [DOI] [PubMed] [Google Scholar]
- 4.Li, M. et al. Atrophy patterns of hippocampal subfields in T2DM patients with cognitive impairment. Endocrine68, 536–548 (2020). 10.1007/s12020-020-02249-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadanand, S. et al. Memory and executive functions in persons with type 2 diabetes: a meta-analysis. Diabetes Metab. Res. Rev.32, 132–142 (2016). 10.1002/dmrr.2664 [DOI] [PubMed] [Google Scholar]
- 6.Palta, P. et al. Magnitude of cognitive dysfunction in adults with type 2 diabetes: a meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J. Int. Neuropsychol. Soc.20, 278–291 (2014). 10.1017/S1355617713001483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohara, T. et al. Glucose tolerance status and risk of dementia in the community: the Hisayama study. Neurology77, 1126–1134 (2011). 10.1212/WNL.0b013e31822f0435 [DOI] [PubMed] [Google Scholar]
- 8.Noguchi-Shinohara, M. et al. Diabetes mellitus, elevated hemoglobin A1c, and glycated albumin are associated with the presence of all-cause dementia and Alzheimer’s disease: the JPSC-AD Study. J. Alzheimers Dis.85, 235–247 (2022). 10.3233/JAD-215153 [DOI] [PubMed] [Google Scholar]
- 9.Hu, Y. et al. Resveratrol improves diabetes-induced cognitive dysfunction in part through the miR-146a-5p/TXNIP axis. Kaohsiung J. Med. Sci.39, 404–415 (2023). 10.1002/kjm2.12643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monereo-Sánchez, J. et al. The association of prediabetes and type 2 diabetes with hippocampal subfields volume: the Maastricht study. NeuroImage Clin.12, 1908–1913 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohara, T. et al. Elevated serum glycated albumin and glycated albumin: hemoglobin A 1c ratio were associated with hippocampal atrophy in a general elderly population of Japanese: the Hirayama Study. J. Diabetes Investig.11, 971–979 (2020). 10.1111/jdi.13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adachi, Y., Ota, K., Minami, I., Yamada, T. & Watanabe, T. Lower insulin secretion is associated with hippocampal and parahippocampal gyrus atrophy in elderly patients with type 2 diabetes mellitus. J. Diabetes Investig.12, 1908–1913 (2021). 10.1111/jdi.13554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vlassara, H. & Palace, M. R. Diabetes and advanced glycation endproducts. J. Intern. Med.251, 87–101 (2002). 10.1046/j.1365-2796.2002.00932.x [DOI] [PubMed] [Google Scholar]
- 14.Huang, M., Que, Y. & Shen, X. Correlation of the plasma levels of soluble RAGE and endogenous secretory RAGE with oxidative stress in pre-diabetic patients. J. Diabetes Complicat.29, 422–426 (2015). 10.1016/j.jdiacomp.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 15.Ninomiya, T. et al. Study design and baseline characteristics of a population-based prospective cohort study of dementia in Japan: the Japan Prospective Studies Collaboration for Aging and Dementia (JPSC-AD). Environ. Health Prev. Med.25, 64 (2020). 10.1186/s12199-020-00903-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, K. J. & Lee, B. W. The roles of glycated albumin as intermediate glycation index and pathogenic protein. Diabetes Metab. J.36, 98–107 (2012). 10.4093/dmj.2012.36.2.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iberg, N. & Flückiger, R. Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites. J. Biol. Chem.261, 13542–13545 (1986). 10.1016/S0021-9258(18)67052-8 [DOI] [PubMed] [Google Scholar]
- 18.Conwell, L. S., Trost, S. G., Brown, W. J. & Batch, J. A. Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care27, 314–319 (2004). 10.2337/diacare.27.2.314 [DOI] [PubMed] [Google Scholar]
- 19.Jeong, Y. et al. Role of the hippocampal CA1 region in incremental value learning. Sci. Rep.29, 9870 (2018). 10.1038/s41598-018-28176-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamura, R., Nakada, Y., Nishijo, H., Miyake, N. & Ono, T. Ameliorative effects of tamolarizine on place learning impairment induced by transient forebrain ischemia in rats. Brain Res.853, 81–92 (2000). 10.1016/S0006-8993(99)02247-7 [DOI] [PubMed] [Google Scholar]
- 21.Bartsch, T., Döhring, J., Rohr, A., Jansen, O. & Deuschl, G. CA1 neurons in the human hippocampus are critical for autobiographical memory, mental time travel, and autonoetic consciousness. Proc. Natl. Acad. Sci. USA108, 17562–17567 (2011). 10.1073/pnas.1110266108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumoto, N., Kitanishi, T. & Mizuseki, K. The subiculum: unique hippocampal hub and more. Neurosci. Res.143, 1–12 (2019). 10.1016/j.neures.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 23.Morris, R. G. M., Schenk, F., Tweedie, F. & Jarrard, L. E. Ibotenate lesions of hippocampus and/or subiculum: dissociating components of allocentric spatial learning. Eur. J. Neurosci.2, 1016–1028 (1990). 10.1111/j.1460-9568.1990.tb00014.x [DOI] [PubMed] [Google Scholar]
- 24.Sutherland, R. J. & Rodriguez, A. J. The role of fornix/fimbria and some related subcortical structures in place learning and memory. Behav. Brain Res.32, 265–277 (1989). 10.1016/S0166-4328(89)80059-2 [DOI] [PubMed] [Google Scholar]
- 25.Dahmani, L. et al. Fimbria-fornix volumes is associated with spatial memory and olfactory identification in humans. Front. Syst. Neurosci.13, 87 (2019). 10.3389/fnsys.2019.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitchigina V. F., Shubina L. V., Popova Y. The role of the dentate gyrus in mediating hippocampal functions: the healthy brain. Neurosci. Behav. Phys. 52, 1401–1417 (2022).
- 27.Sasaki, T. et al. Dentate network activity is necessary for spatial working memory by supporting CA3 sharp-wave ripple generation and prospective firing of CA3 neurons. Nat. Neurosci.21, 258–269 (2018). 10.1038/s41593-017-0061-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Dijk, M. T. V. & Fenton, A. A. On how the dentate gyrus contributes to memory discrimination. Neuron98, 832–845 (2018). 10.1016/j.neuron.2018.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rohlfing, C. L. et al. Defining the relationship between plasma glucose and HbA (1c): analysis of glucose profiles and HbA (1c) in the Diabetes Control and Complications Trial. Diabetes Care25, 275–278 (2002). 10.2337/diacare.25.2.275 [DOI] [PubMed] [Google Scholar]
- 30.Yoshiuchi, K. et al. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr. J.55, 503–507 (2008). 10.1507/endocrj.K07E-089 [DOI] [PubMed] [Google Scholar]
- 31.Katwal, P. C., Jirjees, S., Htun, Z. M., Aldawudi, I. & Khan, S. The effect of anemia and the goal of optimal HbA1c control in diabetes and non-diabetes. Cureus12, 6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel, K. V. Epidemiology of anemia in older adults. Semin. Hematol.45, 210–217 (2008). 10.1053/j.seminhematol.2008.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koga, M. et al. Serum glycated albumin to haemoglobin A(1C) ratio can distinguish fulminant type 1 diabetes mellitus from type 2 diabetes mellitus. Ann. Clin. Biochem.47, 313–317 (2010). 10.1258/acb.2010.009234 [DOI] [PubMed] [Google Scholar]
- 34.Fakih, W. et al. Dysfunctional cerebrovascular tone contributes to cognitive impairment in a non-obese rat model of prediabetic challenge: role of suppression of autophagy and modulation by anti-diabetic drugs. Biochem. Pharm.178, 114041 (2020). 10.1016/j.bcp.2020.114041 [DOI] [PubMed] [Google Scholar]
- 35.Lee, C. C., Huang, C. C. & Hsu, K. S. Insulin promotes dendritic spine and synapse formation by the PI3K/AKT/mTOR and Rac1 signaling pathways. Neuropharmacology61, 867–879 (2011). 10.1016/j.neuropharm.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 36.Rensink, A. A. M. et al. Insulin inhibits amyloid beta-induced cell death in cultured human brain pericytes. Neurobiol. Aging25, 93–103 (2004). 10.1016/S0197-4580(03)00039-3 [DOI] [PubMed] [Google Scholar]
- 37.Devi, L., Alldred, M. J., Ginsberg, S. D. & Ohno, M. Mechanisms underlying insulin deficiency-induced acceleration of β-amyloidosis in a mouse model of Alzheimer’s disease. PLoS One7, e32792 (2012). 10.1371/journal.pone.0032792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lannert, H. & Hoyer, S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav. Neurosci.112, 1199–1208 (1998). 10.1037/0735-7044.112.5.1199 [DOI] [PubMed] [Google Scholar]
- 39.Dozza B., Smith M. A., Perry G., Tabaton M. & Strocchi P. Regulation of glycogen synthase kinase-3beta by products of lipid peroxidation in human neuroblastoma cells. J Neurochem. 89, 1224–1232 l (2004). [DOI] [PubMed]
- 40.Arnold, S. E. et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat. Rev. Neurol.14, 168–181 (2018). 10.1038/nrneurol.2017.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verdile, G., Fuller, S. J. & Martins, R. N. The role of type 2 diabetes in neurodegeneration. Neurobiol. Dis.84, 22–38 (2015). 10.1016/j.nbd.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 42.Rasgon, N. L. et al. Insulin resistance and hippocampal volume in women at risk for Alzheimer’s disease. Neurobiol. Aging32, 1942–1948 (2011). 10.1016/j.neurobiolaging.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jack, C. R. et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J. Magn. Reson Imaging27, 685–691 (2008). 10.1002/jmri.21049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischl, B. et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron33, 341–355 (2002). 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- 45.Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage31, 968–980 (2006). 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 46.Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia28, 412–419 (1985). 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 47.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care33, S62–S69 (2010). 10.2337/dc10-S062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Momozawa, Y. et al. Low-frequency coding variants in CETP and CFB are associated with susceptibility of exudative age-related macular degeneration in the Japanese population. Hum. Mol. Genet.25, 5027–5034 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc.57, 289–300 (1995). 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used in the present study are not publicly available, because they contain confidential clinical data on the study participants. However, the data are available upon reasonable request and with the permission of the Principal Investigator of this study, Toshiharu Ninomiya.
No custom code or mathematical algorithm was developed for this study. Details regarding the specific codes used can be found in the references cited. As indicated in the references, any access restrictions or licensing information associated with the codes used can be obtained from the respective sources.