Abstract
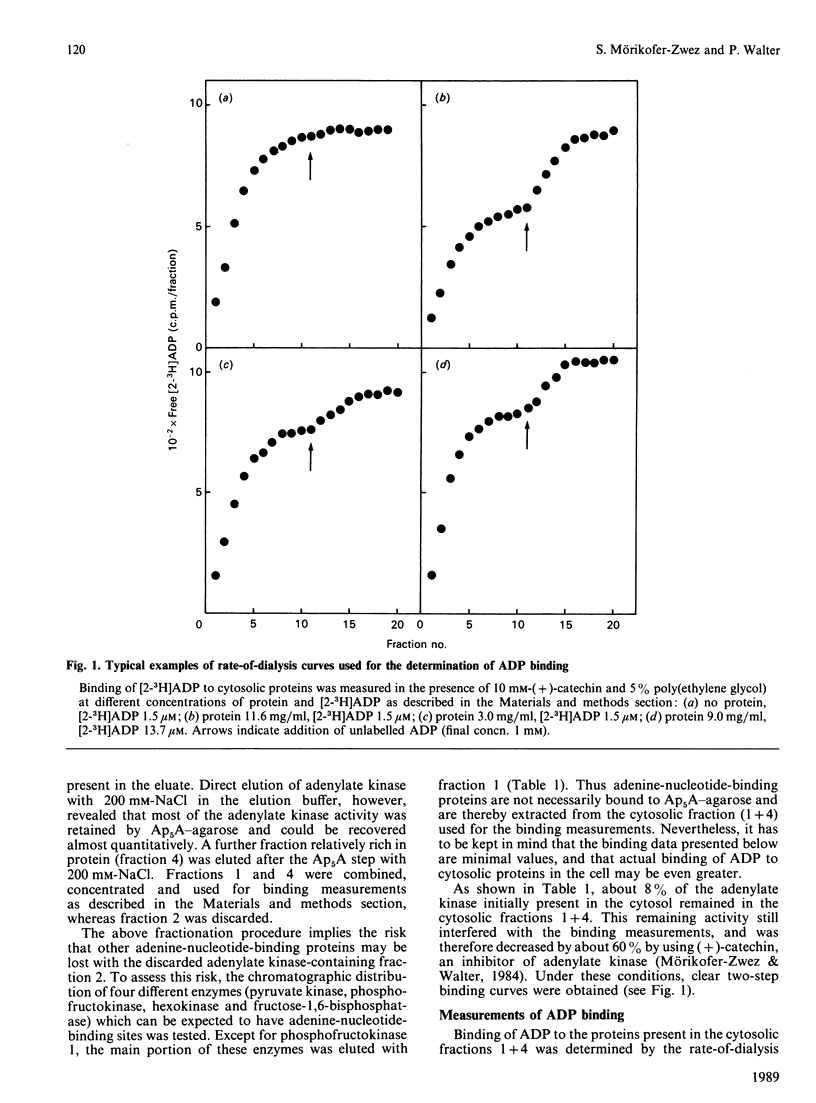
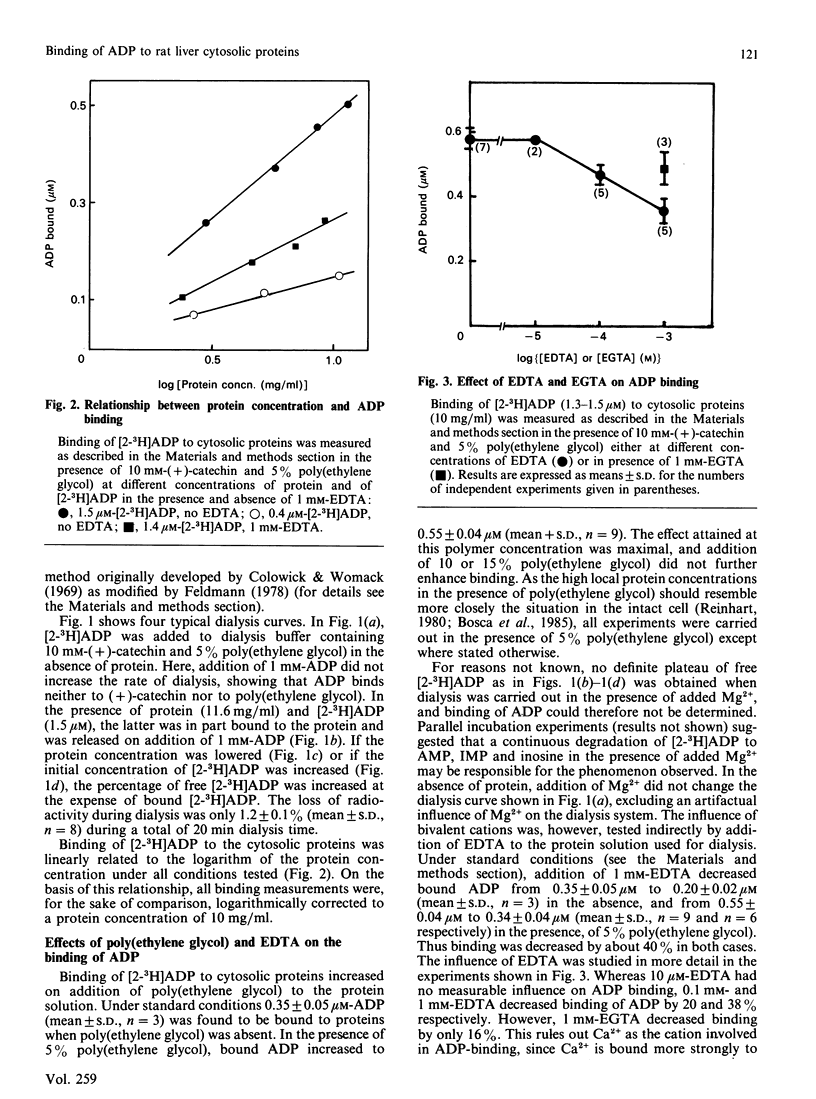
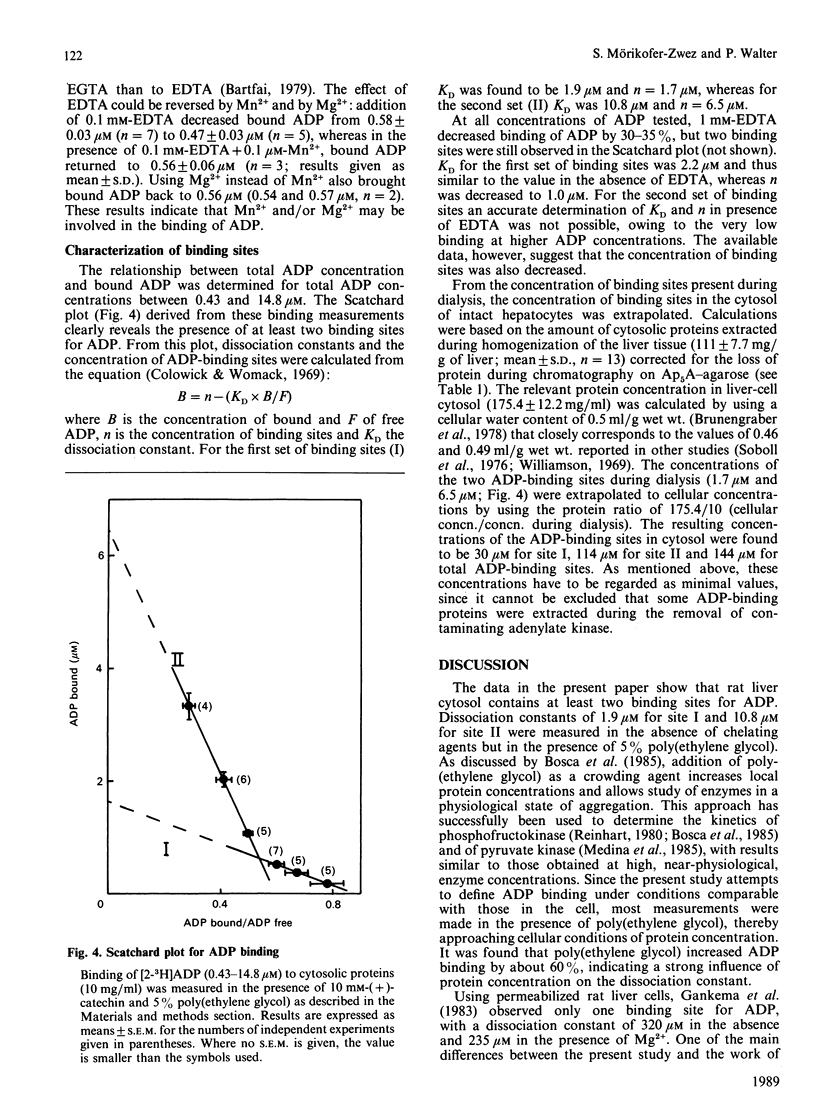
In a cytosolic extract from rat liver, the number and the concentration of ADP-binding sites as well as their dissociation constants were determined by using the rate-of-dialysis technique. Interfering cytosolic adenylate kinase was extracted from the cytosol by affinity chromatography on Ap5A-agarose, and remaining traces of enzyme activity were inhibited with (+)-catechin. Binding of ADP to cytosolic proteins was increased by poly(ethylene glycol) and decreased by EDTA. The effect of 0.1 mM-EDTA could be reversed by addition of equimolar concentrations of Mn2+ or Mg2+. In presence of 5% poly(ethylene glycol), added to increase local protein concentration, two binding sites for ADP were observed, with KD values of 1.9 microM (site I) and 10.8 microM (site II). The concentration of these binding sites, when extrapolated to cellular protein concentrations, were 30 microM (site I) and 114 microM (site II). It is concluded that a minimum of about 50% of total cytosolic ADP is bound to proteins, and that the ratio of free ATP/free ADP is at least twice that of total ATP/total ADP.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Bookelman H., Zuurendonk P. F., van der Meer R., Tager J. M. Intramitochondrial and extramitochondrial concentrations of adenine nucleotides and inorganic phosphate in isolated hepatocytes from fasted rats. Eur J Biochem. 1978 Mar 15;84(2):413–420. doi: 10.1111/j.1432-1033.1978.tb12182.x. [DOI] [PubMed] [Google Scholar]
- Bartfai T. Preparation of metal-chelate complexes and the design of steady-state kinetic experiments involving metal nucleotide complexes. Adv Cyclic Nucleotide Res. 1979;10:219–242. [PubMed] [Google Scholar]
- Boscá L., Aragón J. J., Sols A. Modulation of muscle phosphofructokinase at physiological concentration of enzyme. J Biol Chem. 1985 Feb 25;260(4):2100–2107. [PubMed] [Google Scholar]
- Brunengraber H., Boutry M., Lowenstein J. M. Fatty acid, 3-beta-hydroxysterol, and ketone synthesis in the perfused rat liver. Effects of (--)-hydroxycitrate and oleate. Eur J Biochem. 1978 Jan 16;82(2):373–384. doi: 10.1111/j.1432-1033.1978.tb12032.x. [DOI] [PubMed] [Google Scholar]
- Castaño J. G., Nieto A., Felíu J. E. Inactivation of phosphofructokinase by glucagon in rat hepatocytes. J Biol Chem. 1979 Jul 10;254(13):5576–5579. [PubMed] [Google Scholar]
- Cohen S. M. Simultaneous 13C and 31P NMR studies of perfused rat liver. Effects of insulin and glucagon and a 13C NMR assay of free Mg2+. J Biol Chem. 1983 Dec 10;258(23):14294–14308. [PubMed] [Google Scholar]
- Colman R. F. Determination by gel filtration of association constants for metal-nucleotide interaction. Anal Biochem. 1972 Mar;46(1):358–363. doi: 10.1016/0003-2697(72)90430-7. [DOI] [PubMed] [Google Scholar]
- Colowick S. P., Womack F. C. Binding of diffusible molecules by macromolecules: rapid measurement by rate of dialysis. J Biol Chem. 1969 Feb 25;244(4):774–777. [PubMed] [Google Scholar]
- Criss W. E. Rat liver adenosine triphosphate: adenosine monophosphate phosphotransferase activity. II. Subcellular localization of adenylate kinase isozymes. J Biol Chem. 1970 Dec 10;245(23):6352–6356. [PubMed] [Google Scholar]
- Cunningham C. C., Malloy C. R., Radda G. K. Effect of fasting and acute ethanol administration on the energy state of in vivo liver as measured by 31P-NMR spectroscopy. Biochim Biophys Acta. 1986 Jan 23;885(1):12–22. doi: 10.1016/0167-4889(86)90033-9. [DOI] [PubMed] [Google Scholar]
- Ekman P., Dahlqvist U., Humble E., Engström L. Comparative kinetic studies on the L-type pyruvate kinase from rat liver and the enzyme phosphorylated by cyclic 3', 5'-AMP-stimulated protein kinase. Biochim Biophys Acta. 1976 Apr 8;429(2):374–382. doi: 10.1016/0005-2744(76)90285-0. [DOI] [PubMed] [Google Scholar]
- Faupel R. P., Seitz H. J., Tarnowski W., Thiemann V., Weiss C. The problem of tissue sampling from experimental animals with respect to freezing technique, anoxia, stress and narcosis. A new method for sampling rat liver tissue and the physiological values of glycolytic intermediates and related compounds. Arch Biochem Biophys. 1972 Feb;148(2):509–522. doi: 10.1016/0003-9861(72)90170-1. [DOI] [PubMed] [Google Scholar]
- Feldhau P., Fröhlich T., Goody R. S., Isakov M., Schirmer R. H. Synthetic inhibitors of adenylate kinases in the assays for ATPases and phosphokinases. Eur J Biochem. 1975 Sep 1;57(1):197–204. doi: 10.1111/j.1432-1033.1975.tb02291.x. [DOI] [PubMed] [Google Scholar]
- Feldmann K. New devices for flow dialysis and ultrafiltration for the study of protein--ligand interactions. Anal Biochem. 1978 Jul 15;88(1):225–235. doi: 10.1016/0003-2697(78)90414-1. [DOI] [PubMed] [Google Scholar]
- Gankema H. S., Groen A. K., Wanders R. J., Tager J. M. Measurement of binding of adenine nucleotides and phosphate to cytosolic proteins in permeabilized rat-liver cells. Eur J Biochem. 1983 Mar 15;131(2):447–451. doi: 10.1111/j.1432-1033.1983.tb07283.x. [DOI] [PubMed] [Google Scholar]
- Groen A. K., Vervoorn R. C., Wanders R. J., Van der Meer R., Tager J. M. An evaluation of the metabolite indicator method for determining the cytosolic phosphate potential in rat liver cells. Biochim Biophys Acta. 1982 Oct 11;721(2):172–177. doi: 10.1016/0167-4889(82)90065-9. [DOI] [PubMed] [Google Scholar]
- Hammes G. G., Hurst J. K. Relaxation spectra of adenosine triphosphate-creatine phosphotransferase. Biochemistry. 1969 Mar;8(3):1083–1094. doi: 10.1021/bi00831a040. [DOI] [PubMed] [Google Scholar]
- Iles R. A., Stevens A. N., Griffiths J. R., Morris P. G. Phosphorylation status of liver by 31P-n.m.r. spectroscopy, and its implications for metabolic control. A comparison of 31P-n.m.r. spectroscopy (in vivo and in vitro) with chemical and enzymic determinations of ATP, ADP and Pi. Biochem J. 1985 Jul 1;229(1):141–151. doi: 10.1042/bj2290141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz G. W., Eber S., Kessler W., Krietsch H., Krietsch W. K. Isolation of phosphoglycerate kinases by affinity chromatography. Eur J Biochem. 1978 Apr 17;85(2):493–501. doi: 10.1111/j.1432-1033.1978.tb12265.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Medina R., Aragón J. J., Sols A. Effect of polyethylene glycol on the kinetic behaviour of pyruvate kinase and other potentially regulatory liver enzymes. FEBS Lett. 1985 Jan 21;180(1):77–80. doi: 10.1016/0014-5793(85)80235-0. [DOI] [PubMed] [Google Scholar]
- Mörikofer-Zwez S., Stoecklin F. B., Walter P. Gluconeogenesis in vitro. Formation of glucose 6-phosphate from malate by a cell-free rat-liver system consisting of cytosol and mitochondria. Eur J Biochem. 1982 Jun 15;125(1):27–33. doi: 10.1111/j.1432-1033.1982.tb06646.x. [DOI] [PubMed] [Google Scholar]
- Mörikofer-Zwez S., Stoecklin F. B., Walter P. In vitro formation of glucose 6-phosphate from 3-phosphoglycerate by rat liver cytosol. Hoppe Seylers Z Physiol Chem. 1981 Jan;362(1):47–57. doi: 10.1515/bchm2.1981.362.1.47. [DOI] [PubMed] [Google Scholar]
- Mörikofer-Zwez S., Walter P. In vitro formation of glucose-6-phosphate from glyceraldehyde-3-phosphate by liver cytosol from fed and starved rats. Effect of divalent cations on the conversion rate. Biochem Biophys Res Commun. 1979 Dec 14;91(3):1182–1189. doi: 10.1016/0006-291x(79)92004-7. [DOI] [PubMed] [Google Scholar]
- O'SULLIVAN W. J., PERRIN D. D. THE STABILITY CONSTANTS OF METAL-ADENINE NUCLEOTIDE COMPLEXES. Biochemistry. 1964 Jan;3:18–26. doi: 10.1021/bi00889a005. [DOI] [PubMed] [Google Scholar]
- Pegoraro B., Lee C. Y. Purification and characterization of two isozymes of 3-phosphoglycerate kinase from the mouse. Biochim Biophys Acta. 1978 Feb 10;522(2):423–433. doi: 10.1016/0005-2744(78)90075-x. [DOI] [PubMed] [Google Scholar]
- Reinhart G. D. Influence of polyethylene glycols on the kinetics of rat liver phosphofructokinase. J Biol Chem. 1980 Nov 25;255(22):10576–10578. [PubMed] [Google Scholar]
- Sakakibara R., Uyeda K. Differences in the allosteric properties of pure low and high phosphate forms of phosphofructokinase from rat liver. J Biol Chem. 1983 Jul 25;258(14):8656–8662. [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Phosphorylation state of cytosolic and mitochondrial adenine nucleotides and of pyruvate dehydrogenase in isolated rat liver cells. Biochem J. 1976 Apr 15;156(1):91–102. doi: 10.1042/bj1560091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboll S., Scholz R., Heldt H. W. Subcellular metabolite concentrations. Dependence of mitochondrial and cytosolic ATP systems on the metabolic state of perfused rat liver. Eur J Biochem. 1978 Jun 15;87(2):377–390. doi: 10.1111/j.1432-1033.1978.tb12387.x. [DOI] [PubMed] [Google Scholar]
- Stoecklin F. B., Mörikofer-Zwez S., Walter P. Formation of hexose 6-phosphates from lactate + pyruvate + glutamate by a cell-free system from rat liver. Biochem J. 1986 May 15;236(1):61–70. doi: 10.1042/bj2360061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler M. E., Hecht P., Williamson J. R. Determination of mitochondrial/cytosolic metabolite gradients in isolated rat liver cells by cell disruption. Arch Biochem Biophys. 1977 May;181(1):278–293. doi: 10.1016/0003-9861(77)90506-9. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538–6547. [PubMed] [Google Scholar]
- Wilson D. F., Erecińska M., Schramm V. L. Evaluation of the relationship between the intra- and extramitochondrial [ATP]/[ADP] ratios using phosphoenolpyruvate carboxykinase. J Biol Chem. 1983 Sep 10;258(17):10464–10473. [PubMed] [Google Scholar]
- Wilson D. F., Nelson D., Erecińska M. Binding of the intramitochondrial ADP and its relationship to adenine nucleotide translocation. FEBS Lett. 1982 Jul 5;143(2):228–232. doi: 10.1016/0014-5793(82)80105-1. [DOI] [PubMed] [Google Scholar]


