Abstract
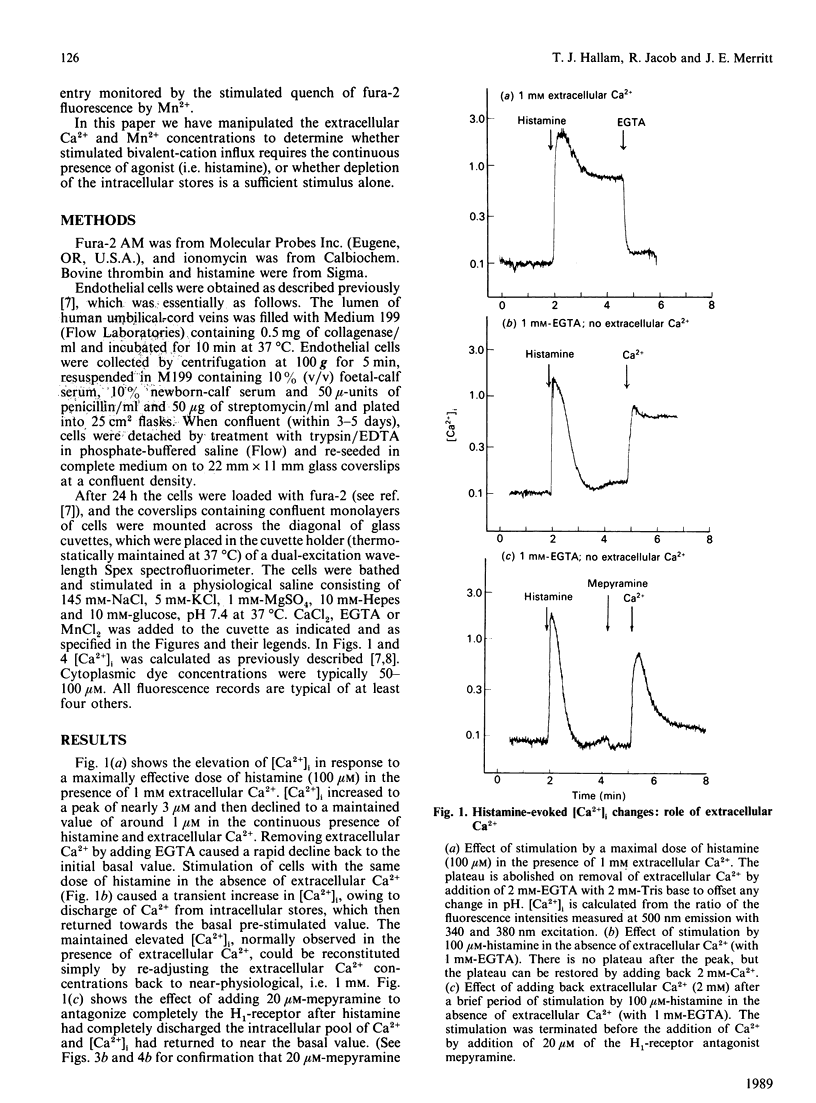
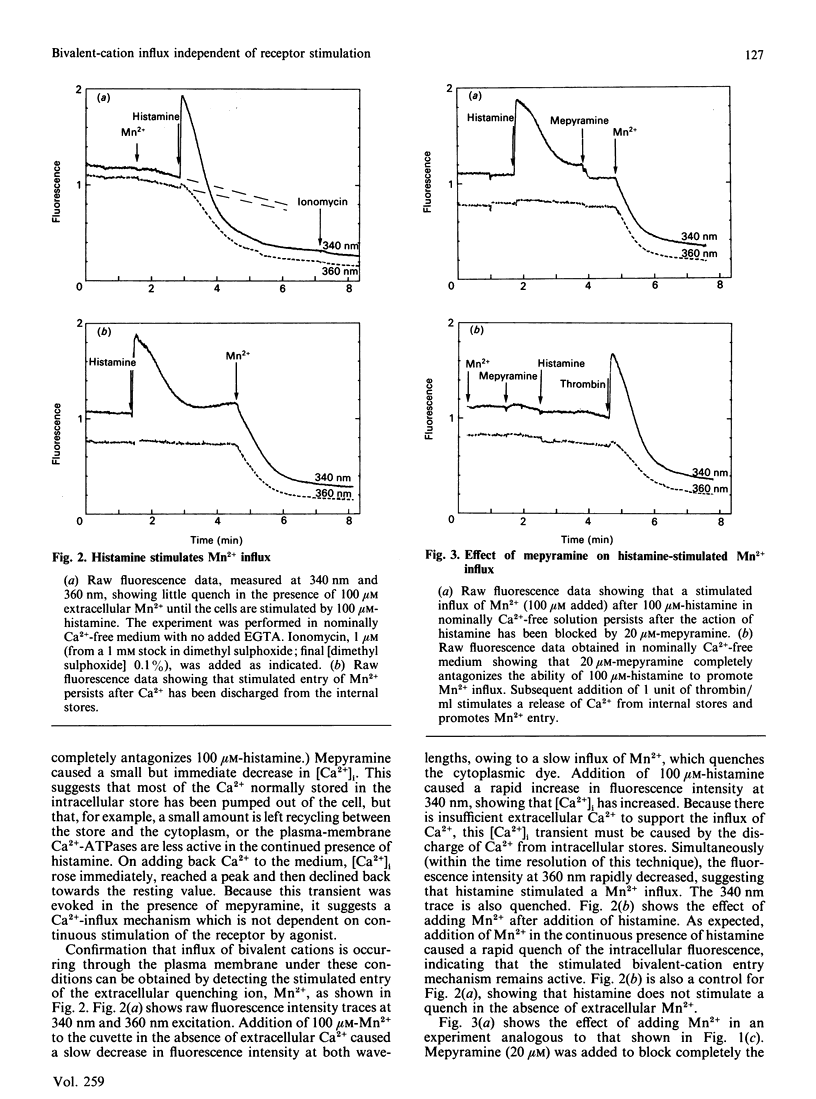
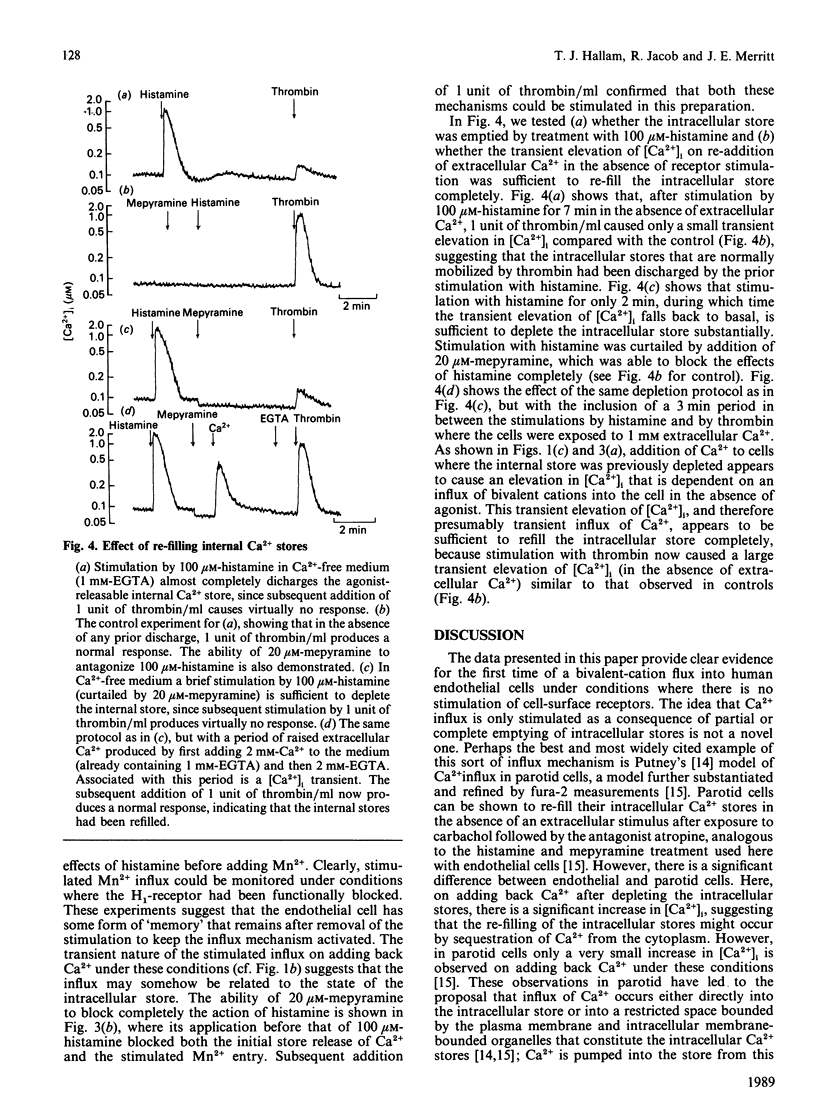
Stimulation of human umbilical-vein endothelial cells by agonists such as histamine or thrombin promotes an influx of Ca2+, causing an increase in cytoplasmic free Ca2+ ([Ca2+]i) that is dependent on the continued presence of both agonist and extracellular Ca2+. This influx can also be clearly detected by using Mn2+ as a marker for Ca2+ entry, since Mn2+ quenches fura-2 fluorescence. The internal stores can be discharged in nominally Ca2+-free solution by stimulation for a brief period by 100 microM-histamine, with the stimulation being terminated by addition of 20 microM of the H1 antagonist mepyramine. After this (i.e. in the continuous presence of antagonist) a stimulated bivalent-cation influx can still be detected, as evidenced by the following observations: (a) addition of Mn2+ produces a stimulated quench, (b) addition of Ca2+ produces a transient rise in [Ca2+]i, (c) addition of 1 unit of thrombin/ml produces a much attenuated response unless the cells are exposed for a short period to 1 mM extracellular Ca2+. These results imply that stimulated bivalent-cation influx may be a direct consequence of the discharge of the internal Ca2+ stores rather than a direct consequence of the presence of agonist.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Bussolino F., Aglietta M., Sanavio F., Stacchini A., Lauri D., Camussi G. Alkyl-ether phosphoglycerides influence calcium fluxes into human endothelial cells. J Immunol. 1985 Oct;135(4):2748–2753. [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Evidence that agonists stimulate bivalent-cation influx into human endothelial cells. Biochem J. 1988 Oct 1;255(1):179–184. doi: 10.1042/bj2550179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D. Exogenous ATP raises cytoplasmic free calcium in fura-2 loaded piglet aortic endothelial cells. FEBS Lett. 1986 Oct 20;207(1):95–99. doi: 10.1016/0014-5793(86)80019-9. [DOI] [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D., Needham L. A. Thrombin-stimulated elevation of human endothelial-cell cytoplasmic free calcium concentration causes prostacyclin production. Biochem J. 1988 Apr 1;251(1):243–249. doi: 10.1042/bj2510243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Rink T. J. Agonists stimulate divalent cation channels in the plasma membrane of human platelets. FEBS Lett. 1985 Jul 8;186(2):175–179. doi: 10.1016/0014-5793(85)80703-1. [DOI] [PubMed] [Google Scholar]
- Jacob R., Merritt J. E., Hallam T. J., Rink T. J. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988 Sep 1;335(6185):40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- Johns A., Lategan T. W., Lodge N. J., Ryan U. S., Van Breemen C., Adams D. J. Calcium entry through receptor-operated channels in bovine pulmonary artery endothelial cells. Tissue Cell. 1987;19(6):733–745. doi: 10.1016/0040-8166(87)90015-2. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Hallam T. J. Platelets and parotid acinar cells have different mechanisms for agonist-stimulated divalent cation entry. J Biol Chem. 1988 May 5;263(13):6161–6164. [PubMed] [Google Scholar]
- Merritt J. E., Rink T. J. Rapid increases in cytosolic free calcium in response to muscarinic stimulation of rat parotid acinar cells. J Biol Chem. 1987 Apr 15;262(11):4958–4960. [PubMed] [Google Scholar]
- Morgan-Boyd R., Stewart J. M., Vavrek R. J., Hassid A. Effects of bradykinin and angiotensin II on intracellular Ca2+ dynamics in endothelial cells. Am J Physiol. 1987 Oct;253(4 Pt 1):C588–C598. doi: 10.1152/ajpcell.1987.253.4.C588. [DOI] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Gallin J. I. Histamine type I receptor occupancy increases endothelial cytosolic calcium, reduces F-actin, and promotes albumin diffusion across cultured endothelial monolayers. J Cell Biol. 1986 Dec;103(6 Pt 1):2379–2387. doi: 10.1083/jcb.103.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage S. O., Rink T. J. The kinetics of changes in intracellular calcium concentration in fura-2-loaded human platelets. J Biol Chem. 1987 Dec 5;262(34):16364–16369. [PubMed] [Google Scholar]
- Whorton A. R., Young S. L., Data J. L., Barchowsky A., Kent R. S. Mechanism of bradykinin-stimulated prostacyclin synthesis in porcine aortic endothelial cells. Biochim Biophys Acta. 1982 Jul 20;712(1):79–87. doi: 10.1016/0005-2760(82)90087-x. [DOI] [PubMed] [Google Scholar]


