Abstract
Over the last decades, silica nanoparticles (SiNPs) have been studied for their applications in biomedicine as an alternative used for conventional diagnostics and treatments. Since their properties can be modified and adjusted for the desired use, they have many different potential applications in medicine: they can be used in diagnosis because of their ability to be loaded with dyes and their increased selectivity and sensitivity, which can improve the quality of the diagnostic process. SiNPs can be functionalized by targeting ligands or molecules to detect certain cellular processes or biomarkers with better precision. Targeted delivery is another fundamental use of SiNPs. They could be used as drug delivery systems (DDS) since their structure allows the loading of therapeutic agents or other compounds, and studies have demonstrated their biocompatibility. When SiNPs are used as DDS, the drug’s toxicity and the off-target effects are reduced significantly, and they can be used to treat conditions like cancer and neurological diseases and even aid in regenerative processes, such as wound healing or bone repair. However, safety concerns must be considered before SiNPs can be used extensively in clinical practice because NPs can cause toxicity in certain conditions and accumulate at undesired locations. Therefore, an overview of the potential applications that SiNPs could have in medicine, as well as their safety concerns, will be covered in this review paper.
Keywords: silica nanoparticles , drug delivery , imaging , regenerative medicine , wound healing , biosensors
Introduction
Nanotechnology has been involved in many fields since it was first discovered, including medicine, electronics, and environmental applications [1]. It involves the fabrication of nano-sized materials, with sizes ranging from 1 to 100 nm, named nanomaterials. These nanomaterials have unique properties that are different from their bulk parts, such as an increased surface area, improved conductivity, and better magnetic and mechanical properties. They can be made from different substances, including metals, carbon, ceramics, and polymers, and they offer alternatives to applications in medicine for diagnosis and treatment [2, 3, 4]. Silica nanoparticles (SiNPs) are part of the nanomaterials that are currently being explored for their potential medical applications. These have unique properties and multi-functionality, characteristics which have made them attract more interest recently [5].
Silica is made of silicon dioxide (SiO2), which is a silicate mineral that can be found in Earth’s crust, in sand, soil, and stones, but it can also be found in some plants. SiNPs are inorganic materials that have specific properties: biocompatibility, high surface area-to-volume ratio, and the possibility of surface modification, which make them useful in biomedical applications. These properties give SiNPs superior stability in acidic environments and temperature changes [6, 7]. SiNPs can be mesoporous (MSN) and non-porous (Figure 1).
Figure 1.
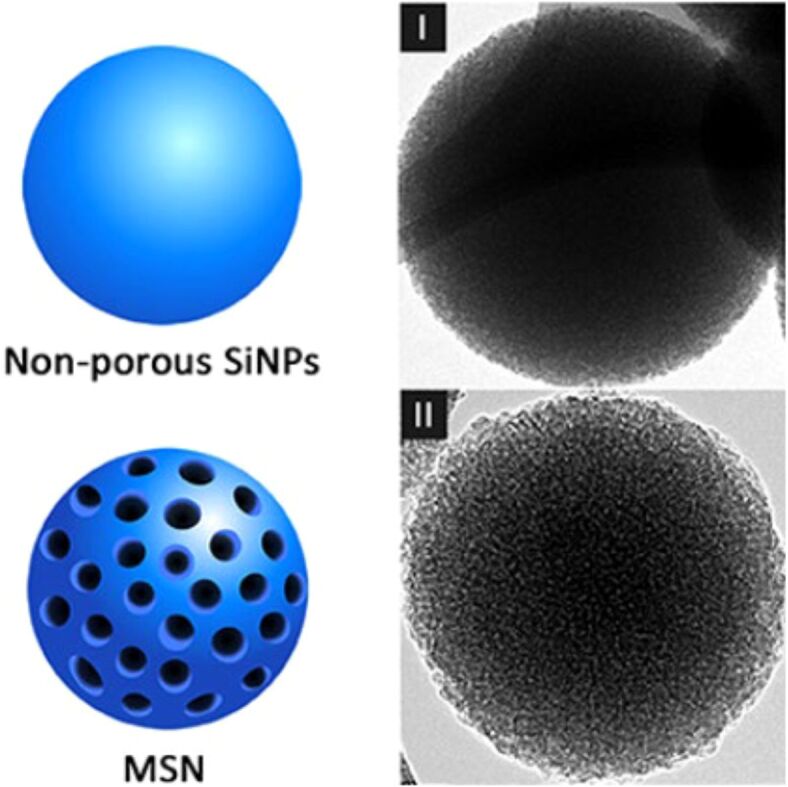
Nonporous (I) and mesoporous (II) SiNPs. Adapted from an open-access source [8]. MSN: Mesoporous silica nanoparticle; SiNPs: Silica nanoparticles
Nonporous SiNPs are currently used in a few areas like cosmetics, packaging, printer toners, and imaging. In medicine, this type of NPs needs surface modification to transport drugs or other substances [9, 10]. The cargo can be released from the NPs through processes like the degradation of the silica matrix or the use of chemical linkers. MSNs were first reported in scientific literature in the late 1990s by Yanagisawa et al. [11]. Since then, numerous research has studied the potential of SiNPs in medicine. MSN can be used for water purification, indoor air purification, and in biomedicine. MSNs have mesopores (2–50 nm), in which the drug or therapeutic substance can be encapsulated, and this protects it from degradation and allows the controlled release [9, 12]. The comparison between nonporous SiNPs and MSN is presented in Table 1 .
Table 1.
|
Property |
MSN |
Nonporous SiNPs |
|
Pore structure |
Ordered mesopores with diameters typically 2–50 nm |
Lack of pores |
|
Surface area |
High surface area due to porosity |
Lower surface area (compared to MSN) |
|
Drug delivery |
Controlled release due to pore structure |
Release kinetics depend on surface chemistry |
|
Imaging applications |
Diverse applications for imaging |
Suitable for fluorescence imaging, MRI contrast |
|
Biocompatibility |
Generally biocompatible |
Generally biocompatible |
MRI: Magnetic resonance imaging; MSN: Mesoporous silica nanoparticle; SiNPs: Silica nanoparticles
Besides drug delivery applications, SiNPs can improve imaging techniques for diagnostic purposes. Fluorescence dye or imaging contrast agents can be added to the SiNPs, so they could be used as contrast probes for computed tomography (CT), magnetic resonance imaging (MRI) and fluorescence imaging [14, 15]. These imaging methods allow for the detection of illness earlier and the creation of individualized therapies by visualizing biological structures and processes in high detail. In addition, the development of multifunctional SiNPs that combine imaging and therapeutic properties is promising for theranostics applications [16]. Besides, SiNPs have potential applications in photothermal therapy, gene delivery, regenerative medicine, drug delivery, and diagnosis. Their natural biocompatibility and tunable characteristics make them attractive for creating or improving therapeutics [17, 18, 19].
It has been established that SiNPs are a versatile nanomaterials class with promising applications in medicine. But there is also a concern about their possible toxicity. Although SiO2 is considered biocompatible in general, excessive exposure or accumulation of SiNPs in the body can cause unwanted adverse effects, leading to inflammation and oxidative stress. Thus, it is required to evaluate the toxicity profile of these NPs for their safe application in biomedicine [20, 21].
Owing to their attractive properties, the present review highlights the possible applications of SiNPs in medicine which can revolutionize healthcare practices and, ultimately, patient outcomes, while also considering the toxicity aspect. Specifically, the advances in SiNP-based drug delivery systems (DDS), SiNPs as diagnostic imaging tools, their involvement in regenerative medicine, and their applications as biosensors are further described.
Biomedical applications
SiNPs could be used in many areas of medicine, which are illustrated in Figure 2.
Figure 2.
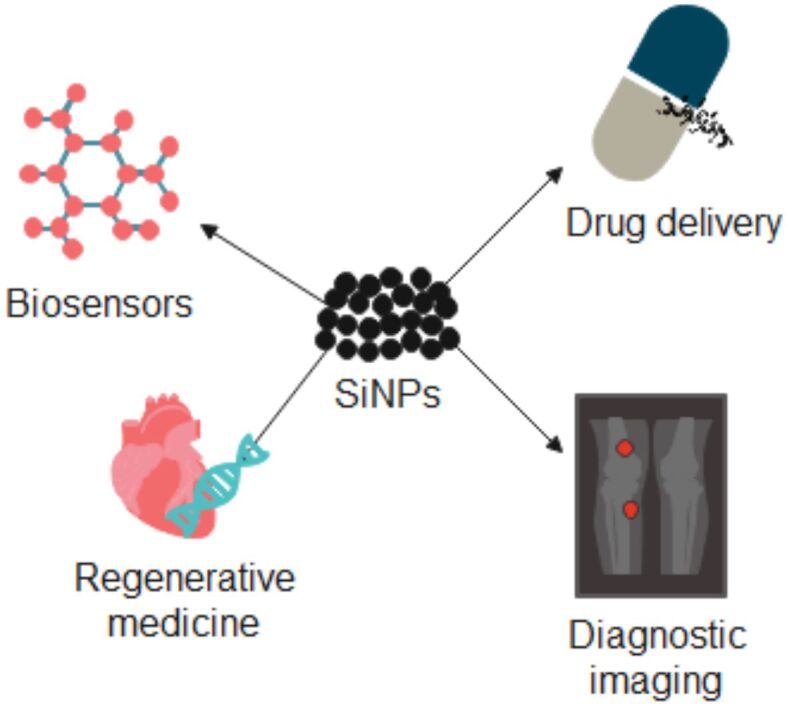
SiNPs’ applications in medicine. Created based on information from [10, 22, 24, 25]. SiNPs: Silica nanoparticles.
SiNPs are generally biocompatible, and they have low toxicity, which makes them suitable for biomedical applications. For example, they could be used in regenerative medicine, tissue engineering, cancer treatment, and as biosensors. They are an optimal nanomaterial for targeted drug delivery because of their high surface area-to-volume ratio, which allows for effective drug loading and distribution. The targeted delivery can be obtained through the functionalization of the NPs with specific compounds, in order to have a minimal negative effect on healthy tissues. SiNPs can also be used as contrast agents in imaging techniques, such as MRI and CT scans, because they can provide higher resolution and better specificity. Due to ongoing research and development, SiNPs continuously aim to improve medical diagnostic techniques and treatments [8, 10, 22, 23].
Drug delivery systems: innovations in controlled and targeted delivery
Drug delivery assumes the administration of therapeutic agents to target tissues or cells in a controlled and targeted manner. It is essential to modern medicine because it increases the safety and effectiveness of treatments while reducing systemic side effects. To achieve targeted therapeutic effects, DDS aim to enhance drug pharmacokinetics and overcome biological barriers. For drug delivery, a few delivery methods have been discovered so far, for example, implantable devices and NP-based delivery systems [26, 27]. NP DDS can deliver the therapeutic drug to the target site and release it in a controlled manner. The SiNPs properties, especially the large surface area and their small size, make them ideal candidates for DDS. These DDS can encapsulate and deliver different therapeutic substances, such as proteins, nucleic acids, and molecules, to particular locations within the body [28, 29, 30]. Many administration routes are available for the DDS, such as oral administration, transdermal, intra-nasal, and parenteral, which are illustrated in Figure 3.
Figure 3.
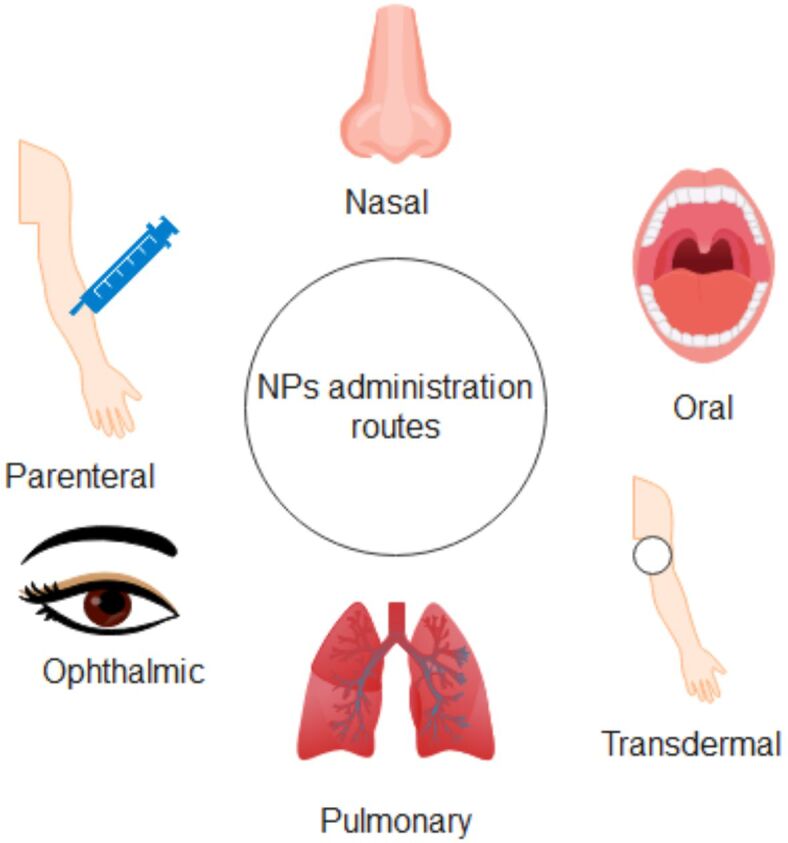
Routes of NPs administration. Created based on information from [31, 32]. NPs: Nanoparticles
SiNPs are excellent vehicles for therapeutic drugs because of their large surface area, nanoscale size, and biocompatibility. Because of their organized pore architectures, MSNs allow for precise control over the kinetics of drug release. Drugs enclosed in MSNs are administered and then released gradually through either stimulus-responsive processes or diffusion, providing long-lasting therapeutic effects. Site-specific delivery is possible by surface modifications, such as the attachment of biomolecules or targeting ligands, which guide the compounds to ill cells or tissues while reducing off-target effects [33, 34, 35].
There are many diseases that DDS can target, but much research has focused on cancer since it is one of the leading causes of death worldwide [36]. Igaz et al. [37] developed a fluorescently labeled MSN-based DDS using Rhodamine B (RhoB) for multidrug-resistant cancer cells. The results showed that the system had no toxicity on healthy cells, proving MSN’s biocompatibility. The NPs were internalized by the cancer cells, and the drug was released. The anticancer activity was increased when the drug was administered with MSN, compared to the free drug. This research highlights the potential of SiNPs in drug delivery for anticancer treatments. Tiburcius et al. [38] also evaluated the anticancer potential of SiNPs against two prostate cancer cell lines. An egg-yolk core-shell MSN loaded with Doxorubicin (Dox) was created and investigated. The findings highlight the importance of surface functionalization: while all the MSN-based DDS showed cytotoxicity against the cancer cell lines, the nitrogen-functionalized MSN showed even higher cytotoxicity. Furthermore, the SiNPs-based systems showed a controlled release of Dox. Ghaferi et al. [39] aimed to enhance an anticancer drug’s effect, Albendazole, by using SiNPs. This in vitro study was conducted on liver cell lines, and the SiNPs-loaded systems showed significantly enhanced cytotoxic effects of Albendazole against the liver cancer cells, increasing potency by 2.6 times.
Besides being used in cancer treatment, SiNPs have been investigated in drug delivery to the brain for treating neurological disorders [40]. The blood–brain barrier (BBB) is a highly selective semipermeable membrane that divides the brain’s extracellular fluid from the blood. It is very important for human health to protect the brain from harmful substances that do not have to reach the brain (for example, pathogens or bacteria) but still let necessary molecules and nutrients pass. However, this selective permeability makes it difficult for certain drugs to pass through and treat a neurological disorder [41, 42]. SiNPs can be synthetized and functionalized in a way that overcomes BBB and delivers therapeutic agents to the brain. By doing so, their small size and surface properties allow them to cross the BBB through various mechanisms, for example passive diffusion [43]. Studies have demonstrated that SiNPs can pass the BBB; for example, Janjua et al. [44] developed ultra-small, large pore SiNPs (USLP) to deliver Temozolomide to the brain. The authors pointed out the significance of surface modification; in this case, the USLPs were PEGylated (polyethylene glycol, PEG) and modified with a targeting protein to aid the SiNPs in crossing the BBB. In vitro and in vivo experiments demonstrated that USLP can successfully pass through the BBB in a three-dimensional (3D) spheroidal model and on mice models and release the drug to the brain. Nevertheless, further studies are required to thoroughly understand the long-term impact of SiNPs on the brain. Ribeiro et al. [45] studied Curcumin-loaded SiNPs for the treatment of Alzheimer’s disease. They found that the SiNPs formulations were biocompatible and could reverse the cognitive deficit when studied on mice, thus highlighting the potential benefit of SiNPs in neurological disorders.
Infections are very concerning currently, mainly because people are facing antibiotic resistance, and therefore, other treatment methods are being investigated. Because of this, researchers investigated the effect of SiNPs in combating infections. In a recent study, Salmonella typhimurium infection was aimed to be treated with Ciprofloxacin-loaded MSN. The findings showed that the drug was released in a controlled manner, so it had a prolonged antibacterial effect and prevented biofilm formation. The dose of antibiotics was lower, and the possible adverse effects they could have on the body were reduced. Moreover, the created DDS demonstrated a reduction of oxidative stress and inflammation [46]. In another study, the researchers aimed to target fungal ocular infections with silica/chitosan NPs loaded with Terconazole, which were tested on rabbits. The formulation was biocompatible since it did not cause any adverse effects. Due to the controlled release, the treatment had a prolonged effect, although this can be attributed to the system’s mucoadhesive properties as well. The loaded NPs improved ocular bioavailability and drug penetration [47]. It was proven that SiNPs may also be used in treating encephalitic alphavirus infection (Venezuelan equine encephalitis virus, VEEV), as observed during a study conducted by LaBauve et al. [48]. ML336 is a chemical that can treat VEEV, but it has limited solubility and stability. The researchers have created lipid-coated MSNs to aid VEEV treatment and loaded them with ML336. The findings showed that the system improved the drug’s stability and ensured its controlled release. The DDS was non-toxic and reduced the viral load in the brain, making it promising for treating VEEV and possibly other viral infections.
Other studies investigated the beneficial effects of SiNPs as DDS, most of them having promising outcomes, as presented in Table 2 .
Table 2.
Studies for SiNPs as DDS and their findings
|
SiNPs DDS |
Targeted disease |
Findings |
Ref. |
|
Radially MSN loaded with Dexamethasone |
Rheumatoid arthritis |
Efficient drug loading Decreased inflammation in rat models |
[49] |
|
Hyaluronic acid-functionalized MSN loaded with Simvastatin |
Atherosclerosis |
Reduced the secretion of proinflammatory cytokines Prolonged circulation in the blood |
[50] |
|
Leptin and Pioglitazone-loaded MSN |
Amyotrophic lateral sclerosis |
Prevented drug degradation Improved motor function in mice over time |
[51] |
|
5-Fluorouracil-loaded MSN |
Ophthalmic drug delivery/Glaucoma |
Enhanced ocular bioavailability of the drug Increased ocular retention Light irritation of the eye |
[52] |
|
MSN arctigenin/CAQK composite |
Spinal cord injury |
Efficient blood–spinal cord barrier crossing Improved nerve function Decreased inflammation Astrocyte’s function regulation |
[53] |
|
Gentamicin-loaded SiNPs |
Skin bacterial infections |
Good dispersion Controlled release Increased drug availability on the skin Antibacterial effect |
[54] |
|
L-arginine MSN |
Cariogenic bacteria |
Bacteria growth inhibition Prevented biofilm formation Prolonged drug release |
[55] |
|
Curcumin-loaded SiNPs |
Periodontal disease |
Controlled release of drug Bacteria inhibition Prevented biofilm formation |
[56] |
|
Silver/SiNPs |
Fungal infections |
Non-toxic Physical barrier against mycelial invasion Fungal growth inhibition |
[57] |
CAQK: Cysteine–alanine–lysine glutamine; DDS: Drug delivery systems; MSN: Mesoporous silica nanoparticle; SiNPs: Silica nanoparticles
Many studies have concluded that SiNPs could be used as DDS, in order to have a targeted and controlled release of therapeutic drug. As nanotechnology advances, SiNPs could improve therapeutic efficacy, reduce adverse effects, cross biological barriers like the BBB, and aid in difficult-to-treat diseases, such as cancer or neurological disorders. The potential of SiNPs as drug carriers looks promising for future medical applications, although more research is needed to understand their long-term effects on the body.
Diagnostic imaging: advances in contrast agents and fluorescent markers
In medicine, imaging techniques are essential to diagnosing, planning, and monitoring numerous diseases. These methods provide many insights into biological tissues’ morphology, physiology, and disease. Radiography and CT are two forms of X-ray imaging that are commonly used to diagnose cancer, manage lung conditions, and offer comprehensive anatomical information. To create high-resolution images of soft tissues, organs, and the brain, MRI uses strong magnetic fields and radio waves. This makes MRI useful for cardiovascular, musculoskeletal, and neurological examinations [58, 59, 60, 61]. Based on sound waves, ultrasound imaging is a non-invasive and flexible diagnostic tool for heart assessments, abdomen examinations, and pregnancy screening. Utilizing radioactive tracers to monitor metabolic processes and molecular interactions within the body, nuclear medicine techniques like positron emission tomography (PET) and single-photon emission computed tomography (SPECT) aid in cancer staging, cardiac assessments, and neurology research. Endoscopy and fluorescence imaging are two examples of optical imaging techniques that provide real-time visualization of tissues at the cellular level, enabling minimally invasive procedures, gastrointestinal evaluations, and cancer diagnosis. As imaging technologies progress, they provide medical professionals with valuable resources for early disease identification, precise diagnosis, and individualized treatment plans [58, 62, 63, 64]. While imaging techniques in medicine offer valuable insights into disease diagnosis and treatment, they also have certain drawbacks, such as limited accuracy, lack of sensitivity, and patient exposure to ionizing radiation [65, 66].
The application of nanotechnology, particularly the use of SiNPs, has the potential to overcome these challenges and improve medical imaging. Targeting and imaging capabilities can be enhanced by precisely controlling the size, shape, and surface characteristics of SiNPs. Clinicians can precisely locate and visualize ill tissues or cells by functionalizing NPs with targeting ligands or contrast agents. SiNPs can increase the accuracy and precision of diagnostic methods and eliminate the need for invasive procedures [67].
A few studies observed that SiNPs could improve diagnostic accuracy. In a recent study, researchers synthesized pH-responsive upconversion MSNs doped with lanthanides and encapsulated with Chlorin e6 and used it for cancer diagnosis. The system had no significant degradation over time, and the NPs were successfully internalized into the cancer cells. After, they showed excitation by near-infrared (NIR) light and NIR laser light-mediated thermal imaging and fluorescence imaging effects. These modified SiNPs improved the therapeutic effect of phototherapy, meaning that they could be successfully used as alternative cancer therapies. Even though this study was successful, more research is needed to understand the long-term effects of NPs [68]. SiNPs can also be used in breast cancer (BC) theranostics, as demonstrated by Laranjeira et al. [69], by using magnetic MSN. The focus of the study was to create iron oxide MSN to be used in MRI for BC and carry Exemestane, which is an anticancer drug. The magnetic response of the created NPs was investigated in this study. The NPs showed superparamagnetic behavior and high saturation magnetization, which are crucial for an accurate MRI. Additionally, the NPs showed no cytotoxicity, making them a great candidate for future applications in cancer detection. Bock et al. [70] used gold NP-assembled SiNPs (SiO2@Au@Au NPs) microscopic nanogaps to generate highly sensitive nanoprobes for imaging. Researchers noticed that detection sensitivity was improved, as these nanoprobes enabled single-molecule detection when excited by NIR lasers. Using HCT116 cells, the nanoprobes were examined for in vitro imaging and also for surface-enhanced Raman scattering (SERS). At higher concentrations of Au, more absorbance was observed, especially in the NIR spectrum. These NPs generated recognizable signals even in deep tissues, and they were able to image HCT116 cancer cells. Considering this, the SiO2@Au@Au system shows potential for highly sensitive imaging.
In summary, using SiNPs in diagnostic imaging, especially as fluorescent markers and contrast agents, indicates a significant step in medical diagnostics. SiNPs’ unique qualities allow for more precision, improved contrast, and better imaging results overall. These findings could aid medicine to obtain earlier and more accurate disease detection, but still more studies need to be performed in order to fully understand their effects.
Therapeutics: SiNPs in regenerative medicine
Regenerative medicine aims to develop new treatments and strategies to heal damaged tissues and organs and restore/improve their functions. It combines medicine with engineering to create treatments for specific diseases and wounds; for example, it can be applied in diabetes, chronic wounds, and other chronic conditions. It also focuses on repairing injured or damaged tissues caused by aging, injury, or disease [71, 72, 73].
SiNPs could be used to accelerate the healing of wounds because of their properties. They can be used in wound dressings to release therapeutic agents, such as growth factors, antimicrobial agents, and anti-inflammatory drugs. This way the therapeutic agents are released for longer, leading to tissue regeneration and lowering the chance of infection. Even more, SiNPs can improve the mechanical properties of wound dressings [73, 74, 75, 76]. In a recent study, MSNs were loaded with Salicylic Acid (SA) and Ketoconazole (KCZ) to treat fungal infections and aid in wound healing. The designed system showed better efficacy in eliminating fungal infection and less cytotoxicity when compared to pure medications. The healing was faster, which further led to a faster recovery. The healing effect of MSNs was further demonstrated by their larger zone of inhibition and reduced skin irritation. This effect was attributed to the controlled drug release and bioadhesive properties of MSN. The histopathological examination in this study showed that MSN therapy improved wound healing [77]. The antifungal and wound healing effect was further evaluated by another study, this time by using Econazole and Triamcinolone as drugs. This research also proved that the encapsulated drugs showed less cytotoxicity than the pure drugs. The SiNPs led to faster healing, which could happen because of the occlusive and bioadhesive properties of MSN [78]. An in vivo study on rats evaluated the potential of Curcumin-loaded SiNPs for wound healing. The healing effect was much higher when the SiNPs were involved compared to the control group. It reduced inflammation, improved fibroblast proliferation, and showed an increase in collagen production [79]. These findings suggest that SiNPs can be an alternative treatment for wound healing applications, although more research is needed to fully understand their effects.
In addition to these applications, SiNPs could be incorporated into different biomaterials and used in regenerative medicine. In a study, MSN loaded with Artemisia argyi extract was incorporated into a hydrogel to be used in chronic wound healing. The loaded hydrogel helped to reduce inflammation and increase collagen production. Furthermore, it protected the wound from external factors and bacteria, resulting in faster and more linear healing [80]. Another novel study examined coated cotton fabrics with drug-loaded SiNPs for wound healing. The cotton fabrics were successfully created, and by adding Ibuprofen and Norfloxacin into the NPs, it is expected that they could have great effect in wound healing, although they have not been studied yet for this purpose [81].
SiNPs were investigated in bone regeneration, too. Their large surface area allows for improved adsorption of biomolecules involved in bone growth. Additionally, SiNPs can be loaded with therapeutic agents, such as growth factors, to help in the regeneration of the bone [82, 83]. Researchers have created mesoporous silica rods with cone-shaped pores to aid in bone regeneration in a study by Xu et al. [84]. The advantage of cone-shaped pores is that they can hold larger molecules, which helps reduce inflammation and support bone growth. Also, they were able to carry and release a protein called bone morphogenetic protein-2 (BMP-2), which aided in osteogenetic differentiation and promoted bone formation. Shen et al. [85] investigated the potential of MSN in transporting basic fibroblast growth factor (bFGF) for bone regeneration. The bFGF-loaded MSN promoted cell proliferation, adhesion, and regulation. Furthermore, by triggering the Wnt/β-catenin signaling pathway, bFGF@MSNs promoted osteogenesis. The capacity of bFGF-loaded MSN to stimulate bone regeneration in distal femur defects has been confirmed in vivo tests, and this indicated its beneficial effect for bone regeneration. Another study by Zhao et al. [86] confirmed the regenerative effect of SiNPs in bone defects. In this case, magnetic NPs were coated with cobalt-doped MSN (Co-MMSNs) and tested in vitro and in vivo on rats. The angiogenesis and osteogenesis processes were improved because the MMSNs’ surface was doped with Co2+ ions, which sped up the regeneration process. Additionally, the doped MSNs promoted the angiogenic activity of osteoblasts and improved MSCs’ osteogenic differentiation. Considering these, Co-MMSNs can have the capacity for bone repair.
Because of their properties and biological effects, like controlled drug release and bioadhesive characteristics, SiNPs could be used in regenerative medicine in the future. SiNPs may be able to address some of the current medical challenges because of their capacity to improve some processes for regeneration in the body. The recent increase in SiNP studies indicates a huge interest in using SiNPs in regenerative medicine; however, more investigation is needed to understand their modes of action fully and the possible long-term effects.
SiNPs as biosensors
A biosensor is a small, analytical device with high sensitivity that can detect a biological substance or analytes. A biosensor uses a physicochemical transducer in combination with a biological sensing element (which could be an enzyme, antibodies, or nucleic acids), to transform a biological signal into an electrical one. They are used in medicine applications for diagnostics, disease monitoring, and therapy. They are able to identify biomarkers linked to conditions such as cancer, diabetes, and infectious diseases, which makes early diagnosis and treatment easier [87, 88, 89, 90]. Recently, research has focused on creating new biosensors at the nanoscale level, more exactly, based on NPs. This is happening thanks to their unique physicochemical properties and versatile surface chemistry [91]. Among these NPs, SiNPs have become promising biosensors since their surface can be altered to amplify the signal to improve sensitivity and detection [92].
SiNPs can be used to detect pathogens, as demonstrated by research. Chitra & Annadurai [93] synthesized fluorescent SiNPs, for the detection of Escherichia coli. Their surface was modified with specific monoclonal antibodies to accurately target the pathogen. The findings showed the successful identification of E. coli when many NPs were bound with the bacterial cell wall, and moreover, they demonstrated an antibacterial effect. The NPs showed great contrast and brightness, thanks to the efficient loading with dye. It has also been found that the SiNPs had no toxic effects. Another study investigated fluorescent SiNPs (SNP-RhoB) derived from natural amorphous silica and modified with the RhoB dye, serving as a biosensor for E. coli. The presence of E. coli is shown by a decrease in the fluorescence intensity of the RhoB dye in the samples. The fluorescence of the SNP-RhoB samples decreased when they were combined with E. coli bacteria and subjected to light at a particular wavelength (553 nm). Furthermore, the NPs’ maximum fluorescence intensity was detected at a wavelength of 580 nm. In essence, the maximum fluorescence emission wavelength and the fluorescence quenching impact serve as markers of the interaction between the bacteria and the NPs. They responded quickly in just 15 minutes, detecting a wide range of bacteria. Even more, they only reacted to E. coli, not to other substances, making SiNPs excellent biosensors for pathogens [94]. SiNPs have also been studied for Brucella abortus identification in a study where the researchers synthesized blue-colored SiNPs and paramagnetic NPs and modified their surface with a polyclonal antibody. This was done in order to overcome challenges for this pathogen’s identification, as testing laboratories require a long time to detect it. The analysis showed that the formulation detected B. abortus and B. melitensis, but also had a small reaction to other bacteria, like E. coli, although it has not been considered significant. This immunosensor can be employed as an effective and fast biosensor for pathogen detection, especially given its quick reaction time (i.e., it could detect the bacteria within 90 minutes) [95].
SiNPs could also be used for the immobilization of biomolecules in order to stabilize them and enable their interaction with other molecules for various applications, including biosensors and biocatalysis. In a recent study, SiNPs were functionalized with amine groups, which could “grab” onto other molecules. Itaconic acid was further used to cross-link these amine groups together toward creating a stable surface to which enzymes were attached firmly. The SiNPs worked as a support structure, keeping the enzymes in place during the enzyme-linked immunosorbent assay (ELISA) process. SiNPs utilization augmented the sensitivity of ELISA for detecting antirabies viruses, making it more accurate than conventional ELISA. This makes using SiNPs for enzyme immobilization a significant advancement, especially for the early detection of illnesses with abnormally low biomarker concentrations [96].
Research has demonstrated that SiNPs can be used to create sensitive and selective biosensors because of their distinctive characteristics since they are compatible with various recognition elements. As discussed in this section, if SiNPs are functionalized with different compounds, they can lead to excellent sensitivity in detecting the desired substances. This could improve medical procedures, for example, earlier disease diagnosis, faster detection, and improved accuracy.
Biocompatibility and toxicity of SiNPs
When creating nanomaterials for medical applications, one of the most important things to consider is their biocompatibility. This means that the nanomaterial should be compatible with the human body without causing harm or producing any undesirable effects. SiNPs are ideal for various biomedical uses, such as diagnostics, imaging, tissue engineering, and drug delivery, because of their biocompatibility. Their biocompatibility could be attributed to their non-toxicity and chemical inertness. SiNPs, in contrast to other NPs, are made of SiO2, which is a naturally occurring substance on Earth and frequently seen in everyday life, as in stones, soil, and plants. The NPs’ surface can be modified to adapt to how they interact with biological molecules and tissues. The biocompatibility, stability, and solubility of SiNPs can be improved by surface modifications through adding functional groups or biocompatible coatings [10, 97, 98, 99].
Xiao et al. [100] studied the effect, biocompatibility and safety of SiNPs in their work. The aim of the study was to coat SiNPs with red blood cell membrane (RBM) and load them with Dox and Indocyanine green (ICG) for lung cancer treatment. The RBM coating improved the stability and biocompatibility of the NPs since it made them closely resemble the body’s own cells. This prolonged the circulation time in the body and increased the accumulation of NPs at the tumor site, meaning that it had an anticancer effect without harming the healthy cells. In addition, the coating facilitated superior cell internalization of the drugs. Santino et al. [101] have synthesized peptide-coated Si/PEG NPs and evaluated their cytotoxicity. Here, the peptides’ role was to improve the stability. PEG made the NPs more hydrophilic and prevented them from being cleared out too fast by the circulatory system. At low concentrations, the coated SiNPs showed no toxicity. However, cell damage was observed at concentrations of 10 μm. Particle agglomeration was observed, too, indicating a possible limitation in using SiNPs in medical applications.
The safety and toxicology of SiNPs have been investigated in a recent study by using bare, PEG-ylated, and galactooligosaccharide MSN (MSN-GAL). At 48 hours exposure of cells to all three types of MSN at different concentrations, the survival rate was still high (above 80%). Although, the highest survival rate was seen at concentrations less than 10–20 μg/mL. In long-time exposures, MSN-PEG and MSN-GAL formed colonies compared to both non-exposed controls and those exposed to bare MSNs. This suggests that these NPs might potentially cause cells to change in a way that could lead to cancer [102]. These findings highlight the importance of continuing the research on SiNPs to find an optimal concentration and better understand the long-term effects.
It was observed that SiNPs injected in some vital organs caused a slight worsening of existing damage in a study. The SiNPs were coated with chitosan and PEG and then injected into mice. PEG-coated MSN, particularly those with low molecular weight, exacerbated pre-existing vascular problems, but chitosan-coated MSN did not cause any adverse effects. Based on these findings, it appears that PEG coatings could exacerbate pre-existing diseases. Although it demonstrated efficient drug delivery, PEG-coated MSN caused mice’s vascular injuries to aggravate, suggesting a possible adverse effect on individuals who already have vascular risks. Furthermore, in several mice, low molecular weight PEG resulted in kidney dysfunction [102].
Several studies have discovered that SiNPs can induce inflammation under certain conditions, which are presented in Table 3 .
Table 3.
Studies on SiNPs and their inflammatory effect
|
Studied NPs |
Findings |
Effect |
Ref. |
|
SiNPs |
Generated proinflammatory mediators ROS generation, lysosomal dysfunction |
Pulmonary inflammation in mice |
[103] |
|
SiNPs |
Weight loss Presence of ulcers Cellular infiltration No effect on microbial composition |
Exacerbated intestinal inflammation |
[104] |
|
SiNPs |
Myocardial tissue rupture Myocyte apoptosis Activation of systemic and heart inflammation Oxidative damage |
Myocardial injury and inflammation |
[105] |
|
SiNPs |
Inflammatory cytokines activation ROS generation |
Pulmonary inflammation |
[106] |
|
MSN |
Slight inflammation Changes in gut microbiota |
Potential colon inflammation |
[107] |
|
Nano-SiO 2 |
Mast cell activation Increased mucus production Airway inflammation |
Exacerbating allergic inflammation |
[108] |
MSN: Mesoporous silica nanoparticle; NPs: Nanoparticles; ROS: Reactive oxygen species; SiNPs: Silica nanoparticles; SiO2: Silicon dioxide
More studies are needed to reduce the negative effects of SiNPs, as their application in industry and medicine, among other disciplines, keeps growing. A domain that requires substantial research is the thorough evaluation of SiNP toxicity at various exposure routes and times. Through multiple studies that have been done, it has been demonstrated that the toxicity of SiNPs can be influenced by many parameters: particle size, shape, surface functionalization, and method of administration. So, in order to fully understand how these factors increase or reduce SiNP’s toxicity, there is still a need for research. In this way, clinicians could establish standard procedures and methods of synthesis to obtain safe and effective SiNPs.
Furthermore, it is important to gain a deeper understanding of how the SiNPs’ mechanisms cause cytotoxicity in order to obtain safe SiNPs. This means investigating how they interact with cellular processes and organelles, as well as intracellular communication. Understanding these mechanisms is very important so it can be possible to anticipate and lower the toxicity of SiNPs and create safer NPs compositions. More studies are needed to understand the long-term effects of SiNP exposure on the human body. Besides that, more research should be done for SiNPs’ surface functionalizations which can improve biocompatibility without impacting the desired functions. These coatings could improve the SiNPs’ safety and reduce the possibility of undesired biological interactions.
Conclusions
As medical research advances, SiNPs could be incorporated into medical practice because they provide many promising opportunities in drug delivery, diagnostics, and therapeutics and thus represent an important step in clinical research. The functions of SiNPs and how they could revolutionize modern-day medicine were discussed throughout this review paper. Firstly, SiNPs can be used in diagnostics because they facilitate early disease detection and monitoring of therapy. These NPs have a high surface area-to-volume ratio and are compatible with many imaging techniques, like fluorescence. Because of these, they have more sensitivity when detecting certain biomarkers or other molecules. Researchers can create very accurate diagnostic systems for diseases by functionalizing SiNPs with targeting compounds or biomolecule sensors. Another important aspect of SiNPs is their use in drug delivery since they act as DDS, meaning that they can deliver therapeutic agents to a desired site. Their properties, such as size, shape, pores’ structure, and surface properties, can be modified to achieve efficient drug loading, controlled release, and biodistribution. Loading drugs into the SiNPs or functionalizing their surface with certain ligands is beneficial because in this way the drug is protected from degradation and premature elimination from the body, the off-target effects are reduced, and the therapeutic effect is improved. Targeted delivery has great promise for situations where traditional medications or treatments may not be very effective and cause negative effects, such as cancer treatment, infectious diseases, or neurological diseases. SiNPs can be used as alternative treatments for some diseases and facilitate regenerative medicine. Their biocompatibility and controlled release abilities make them attractive candidates for targeted delivery, which could aid in tissue engineering and wound repair. SiNPs can be loaded with growth factors, anti-inflammatory drugs, and other medicines to treat wounds or promote bone repair. Beyond their current applications, the future of SiNPs in the medical field seems to be very promising. One such new possibility is the development of intelligent SiNP-based devices that can selectively release drugs in response to specific biological targets or environmental stimuli. To overcome off-target toxicity and enhance the therapeutic effect, researchers aspire to develop SiNPs that may release their therapeutic agents at disease sites or respond to specific physiological changes by incorporating stimuli-responsive materials or molecular triggers into their structures [109, 110]. Additionally, SiNPs could be combined with advanced technologies such as gene editing, nanomedicine, and regenerative medicine to create better and more advanced treatments for diseases with limitations. SiNPs could be used as carriers for delivering clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) and other gene-editing tools to genes that are related to disease [111, 112]. Even though SiNPs hold great potential, some concerns must be addressed before these NPs can be used safely in real-life medical applications. Their toxicity and release profiles are the most important limitations that need to be overcome. To do so, more research is needed to accomplish the in vivo safety and efficacy of SiNPs and establish standard treatment procedures.
Conflict of interests
The authors declare that they have no conflict of interests.
References
- 1.Malik S, Muhammad K, Waheed Y. Emerging applications of nanotechnology in healthcare and medicine. Molecules. 2023;28(18):6624–6624. doi: 10.3390/molecules28186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baig N, Kammakakam I, Falath W. Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges. Mater Adv. 2021;2(6):1821–1871. [Google Scholar]
- 3.Albalawi F, Hussein MZ, Fakurazi S, Masarudin MJ. Engineered nanomaterials: the challenges and opportunities for nanomedicines. Int J Nanomedicine. 2021;16:161–184. doi: 10.2147/IJN.S288236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dippong T. Innovative nanomaterial properties and applications in chemistry, physics, medicine, or environment. Nanomaterials (Basel) 2024;14(2):145–145. doi: 10.3390/nano14020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janjua TI, Cao Y, Kleitz F, Linden M, Yu C, Popat A. Silica nanoparticles: a review of their safety and current strategies to overcome biological barriers. Adv Drug Deliv Rev. 2023;203:115115–115115. doi: 10.1016/j.addr.2023.115115. [DOI] [PubMed] [Google Scholar]
- 6.Bruckmann FdS, Nunes FB, Salles TdR, Franco C, Cadoná FC, Bohn Rhoden. Biological applications of silica-based nanoparticles. Magnetochemistry. 2022;8(10):131–131. [Google Scholar]
- 7.Nandanwar R, Singh P, Haque FZ. Synthesis and properties of silica nanoparticles by sol-gel method for the application in green chemistry. Mater Sci Res India. 2013;10(1):85–92. [Google Scholar]
- 8.Selvarajan V, Obuobi S, Ee PLR. Silica nanoparticles - a versatile tool for the treatment of bacterial infections. Front Chem. 2020;8:602–602. doi: 10.3389/fchem.2020.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang L, Cheng J. Nonporous silica nanoparticles for nanomedicine application. Nano Today. 2013;8(3):290–312. doi: 10.1016/j.nantod.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Li P, Zhao R, Zhao L, Liu J, Peng S, Fu X, Wang X, Luo R, Wang R, Zhang Z. Silica nanoparticles: biomedical applications and toxicity. Biomed Pharmacother. 2022;151:113053–113053. doi: 10.1016/j.biopha.2022.113053. [DOI] [PubMed] [Google Scholar]
- 11.Yanagisawa T, Shimizu T, Kuroda K, Kato C. The preparation of alkyltrimethylammonium-kanemite complexes and their conversion to microporous materials. Bull Chem Soc Jpn. 1990;63(4):988–992. [Google Scholar]
- 12.Pal N, Lee JH, Cho EB. Recent trends in morphology-controlled synthesis and application of mesoporous silica nanoparticles. Nanomaterials (Basel) 2020;10(11):2122–2122. doi: 10.3390/nano10112122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehman SE, Mudunkotuwa IA, Grassian VH, Larsen SC. Nano-bio interactions of porous and nonporous silica nanoparticles of varied surface chemistry: a structural, kinetic, and thermodynamic study of protein adsorption from RPMI culture medium. Langmuir. 2016;32(3):731–742. doi: 10.1021/acs.langmuir.5b03997. [DOI] [PubMed] [Google Scholar]
- 14.Mochizuki C, Nakamura J, Nakamura M. Development of non-porous silica nanoparticles towards cancer photo-theranostics. Biomedicines. 2021;9(1):73–73. doi: 10.3390/biomedicines9010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karaman DŞ, Sarparanta MP, Rosenholm JM, Airaksinen AJ. Multimodality imaging of silica and silicon materials in vivo. Adv Mater. 2018;30(24):e1703651–e1703651. doi: 10.1002/adma.201703651. [DOI] [PubMed] [Google Scholar]
- 16.Yuan D, Ellis CM, Davis JJ. Mesoporous silica nanoparticles in bioimaging. Materials (Basel) 2020;13(17):3795–3795. doi: 10.3390/ma13173795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalho AM, Cordeiro RA, Faneca H. Silica-based gene delivery systems: from design to therapeutic applications. Pharmaceutics. 2020;12(7):649–649. doi: 10.3390/pharmaceutics12070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei W, Sun C, Jiang T, Gao Y, Yang Y, Zhao Q, Wang S. Polydopamine-coated mesoporous silica nanoparticles for multi-responsive drug delivery and combined chemo-photothermal therapy. Mater Sci Eng C Mater Biol Appl. 2019;105:110103–110103. doi: 10.1016/j.msec.2019.110103. [DOI] [PubMed] [Google Scholar]
- 19.Rosenholm JM, Zhang J, Linden M, Sahlgren C. Mesoporous silica nanoparticles in tissue engineering - a perspective. Nanomedicine (Lond) 2016;11(4):391–402. doi: 10.2217/nnm.15.212. [DOI] [PubMed] [Google Scholar]
- 20.Sharma N, Kurmi BD, Singh D, Mehan S, Khanna K, Karwasra R, Kumar S, Chaudhary A, Jakhmola V, Sharma A, Singh SK, Dua K, Kakkar D. Nanoparticles toxicity: an overview of its mechanism and plausible mitigation strategies. J Drug Target. 2024;19:1–13. doi: 10.1080/1061186X.2024.2316785. [DOI] [PubMed] [Google Scholar]
- 21.Pavan C, Delle Piane, Gullo M, Filippi F, Fubini B, Hoet P, Horwell CJ, Huaux F, Lison D, Lo Giudice, Martra G, Montfort E, Schins R, Sulpizi M, Wegner K, Wyart-Remy M, Ziemann C, Turci F. The puzzling issue of silica toxicity: are silanols bridging the gaps between surface states and pathogenicity. Part Fibre Toxicol. 2019;16(1):32–32. doi: 10.1186/s12989-019-0315-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen F, Hableel G, Zhao ER, Jokerst JV. Multifunctional nanomedicine with silica: role of silica in nanoparticles for theranostic, imaging, and drug monitoring. J Colloid Interface Sci. 2018;521:261–279. doi: 10.1016/j.jcis.2018.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walcarius A. Silica-based electrochemical sensors and biosensors: recent trends. Curr Opin Electrochem. 2018;10:88–97. [Google Scholar]
- 24.Xu B, Li S, Shi R, Liu H. Multifunctional mesoporous silica nanoparticles for biomedical applications. Signal Transduct Target Ther. 2023;8(1):435–435. doi: 10.1038/s41392-023-01654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji X, Wang H, Song B, Chu B, He Y. Silicon nanomaterials for biosensing and bioimaging analysis. Front Chem. 2018;6:38–38. doi: 10.3389/fchem.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P, Bannerjee SK. Drug delivery systems: an updated review. Int J Pharm Investig. 2012;2(1):2–11. doi: 10.4103/2230-973X.96920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ezike TC, Okpala US, Onoja UL, Nwike CP, Ezeako EC, Okpara OJ, Okoroafor CC, Eze SC, Kalu OL, Odoh EC, Nwadike UG, Ogbodo JO, Umeh BU, Ossai EC, Nwanguma BC. Advances in drug delivery systems, challenges and future directions. Heliyon. 2023;9(6):e17488–e17488. doi: 10.1016/j.heliyon.2023.e17488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Jia C, Yang J. Designing nanoparticle-based drug delivery systems for precision medicine. Int J Med Sci. 2021;18(13):2943–2949. doi: 10.7150/ijms.60874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y, Gao D, Shen J, Wang Q. A review of mesoporous silica nanoparticle delivery systems in chemo-based combination cancer therapies. Front Chem. 2020;8:598722–598722. doi: 10.3389/fchem.2020.598722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Afzal O, Altamimi ASA, Nadeem MS, Alzarea SI, Almalki WH, Tariq A, Mubeen B, Murtaza BN, Iftikhar S, Riaz N, Kazmi I. Nanoparticles in drug delivery: from history to therapeutic applications. Nanomaterials (Basel) 2022;12(24):4494–4494. doi: 10.3390/nano12244494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gopu B , Pandian R , Sevvel A , Shukla S . In: Biomedical applications and toxicity of nanomaterials . Mohanan PV , et al., editors. Singapore : Springer ; 2023 . Routes of nano-drug administration and nano-based drug delivery system and toxicity ; pp. 671 – 702 . [Google Scholar]
- 33.Fang L, Zhou H, Cheng L, Wang Y, Liu F, Wang S. The application of mesoporous silica nanoparticles as a drug delivery vehicle in oral disease treatment. Front Cell Infect Microbiol. 2023;13:1124411–1124411. doi: 10.3389/fcimb.2023.1124411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bharti C, Nagaich U, Pal AK, Gulati N. Mesoporous silica nanoparticles in target drug delivery system: a review. Int J Pharm Investig. 2015;5(3):124–133. doi: 10.4103/2230-973X.160844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallet-Regí M, Schüth F, Lozano D, Colilla M, Manzano M. Engineering mesoporous silica nanoparticles for drug delivery: where are we after two decades. Chem Soc Rev. 2022;51(13):5365–5451. doi: 10.1039/d1cs00659b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. doi: 10.3322/caac.21820. [DOI] [PubMed] [Google Scholar]
- 37.Igaz N, Bélteky P, Kovács D, Papp C, Rónavári A, Szabó D, Gácser A, Kónya Z, Kiricsi M. Functionalized mesoporous silica nanoparticles for drug-delivery to multidrug-resistant cancer cells. Int J Nanomedicine. 2022;17:3079–3096. doi: 10.2147/IJN.S363952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiburcius S, Krishnan K, Jose L, Patel V, Ghosh A, Sathish CI, Weidenhofer J, Yang JH, Verrills NM, Karakoti A, Vinu A. Egg-yolk core-shell mesoporous silica nanoparticles for high Doxorubicin loading and delivery to prostate cancer cells. Nanoscale. 2022;14(18):6830–6845. doi: 10.1039/d2nr00783e. [DOI] [PubMed] [Google Scholar]
- 39.Ghaferi M, Zahra W, Akbarzadeh A, Ebrahimi Shahmabadi, Alavi SE. Enhancing the efficacy of Albendazole for liver cancer treatment using mesoporous silica nanoparticles: an in vitro study. EXCLI J. 2022;21:236–249. doi: 10.17179/excli2021-4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Attia MS, Yahya A, Monaem NA, Sabry SA. Mesoporous silica nanoparticles: their potential as drug delivery carriers and nanoscavengers in Alzheimer’s and Parkinson’s diseases. Saudi Pharm J. 2023;31(3):417–432. doi: 10.1016/j.jsps.2023.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kadry H, Noorani B, Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020;17(1):69–69. doi: 10.1186/s12987-020-00230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu D, Chen Q, Chen X, Han F, Chen Z, Wang Y. The blood-brain barrier: structure, regulation, and drug delivery. Signal Transduct Target Ther. 2023;8(1):217–217. doi: 10.1038/s41392-023-01481-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen YP, Chou CM, Chang TY, Ting H, Dembélé J, Chu YT, Liu TP, Changou CA, Liu CW, Chen CT. Bridging size and charge effects of mesoporous silica nanoparticles for crossing the blood-brain barrier. Front Chem. 2022;10:931584–931584. doi: 10.3389/fchem.2022.931584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janjua TI, Cao Y, Ahmed-Cox A, Raza A, Moniruzzaman M, Akhter DT, Fletcher NL, Kavallaris M, Thurecht KJ, Popat A. Efficient delivery of Temozolomide using ultrasmall large-pore silica nanoparticles for glioblastoma. J Control Release. 2023;357:161–174. doi: 10.1016/j.jconrel.2023.03.040. [DOI] [PubMed] [Google Scholar]
- 45.Ribeiro TC, Sábio RM, Luiz MT, de Souza, Fonseca-Santos B, Cides da, Fantini MCA, Planeta CDS, Chorilli M. Curcumin-loaded mesoporous silica nanoparticles dispersed in thermo-responsive hydrogel as potential Alzheimer disease therapy. Pharmaceutics. 2022;14(9):1976–1976. doi: 10.3390/pharmaceutics14091976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alandiyjany MN, Abdelaziz AS, Abdelfattah-Hassan A, Hegazy WAH, Hassan AA, Elazab ST, Mohamed EAA, El-Shetry ES, Saleh AA, ElSawy NA, Ibrahim D. Novel in vivo assessment of antimicrobial efficacy of Ciprofloxacin loaded mesoporous silica nanoparticles against Salmonella typhimurium infection. Pharmaceuticals (Basel) 2022;15(3):357–357. doi: 10.3390/ph15030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaghloul N, El Hoffy, Mahmoud AA, Elkasabgy NA. Cyclodextrin stabilized freeze-dried silica/chitosan nanoparticles for improved Terconazole ocular bioavailability. Pharmaceutics. 2022;14(3):470–470. doi: 10.3390/pharmaceutics14030470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LaBauve AE, Rinker TE, Noureddine A, Serda RE, Howe JY, Sherman MB, Rasley A, Brinker CJ, Sasaki DY, Negrete OA. Lipid-coated mesoporous silica nanoparticles for the delivery of the ML336 antiviral to inhibit encephalitic alphavirus infection. Sci Rep. 2018;8(1):13990–13990. doi: 10.1038/s41598-018-32033-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim SJ, Choi Y, Min KT, Hong S. Dexamethasone-loaded radially mesoporous silica nanoparticles for sustained anti-inflammatory effects in rheumatoid arthritis. Pharmaceutics. 2022;14(5):985–985. doi: 10.3390/pharmaceutics14050985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song K, Tang Z, Song Z, Meng S, Yang X, Guo H, Zhu Y, Wang X. Hyaluronic acid-functionalized mesoporous silica nanoparticles loading Simvastatin for targeted therapy of atherosclerosis. Pharmaceutics. 2022;14(6):1265–1265. doi: 10.3390/pharmaceutics14061265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Díaz-García D, Ferrer-Donato Á, Méndez-Arriaga JM, Cabrera-Pinto M, Díaz-Sánchez M, Prashar S, Fernandez-Martos CM, Gómez-Ruiz S. Design of mesoporous silica nanoparticles for the treatment of amyotrophic lateral sclerosis (ALS) with a therapeutic cocktail based on leptin and Pioglitazone. ACS Biomater Sci Eng. 2022;8(11):4838–4849. doi: 10.1021/acsbiomaterials.2c00865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alhowyan AA, Kalam MA, Iqbal M, Raish M, El-Toni AM, Alkholief M, Almomen AA, Alshamsan A. Mesoporous silica nanoparticles coated with carboxymethyl chitosan for 5-Fluorouracil ocular delivery: characterization, in vitro and in vivo studies. Molecules. 2023;28(3):1260–1260. doi: 10.3390/molecules28031260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun G, Zeng S, Liu X, Shi H, Zhang R, Wang B, Zhou C, Yu T. Synthesis and characterization of a silica-based drug delivery system for spinal cord injury therapy. Nanomicro Lett. 2019;11(1):23–23. doi: 10.1007/s40820-019-0252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Srivastava S, Singh S, Srivastava P, Kumar A, Narayan Y, Tiwari S. Gentamicin loaded mesoporous silica nano particles for topical treatment of bacterial infections. Indian J Pharm Pharmacol. 2022;9(1):35–42. [Google Scholar]
- 55.López-Ruiz M, Navas F, Fernández-García P, Martínez-Erro S, Fuentes MV, Giráldez I, Ceballos L, Ferrer-Luque CM, Ruiz-Linares M, Morales V, Sanz R, García-Muñoz RA. L-arginine-containing mesoporous silica nanoparticles embedded in dental adhesive (Arg@MSN@DAdh) for targeting cariogenic bacteria. J Nanobiotechnology. 2022;20(1):502–502. doi: 10.1186/s12951-022-01714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shirmohammadi A, Maleki Dizaj, Sharifi S, Fattahi S, Negahdari R, Ghavimi MA, Memar MY. Promising antimicrobial action of sustained released curcumin-loaded silica nanoparticles against clinically isolated Porphyromonas gingivalis. Diseases. 2023;11(1):48–48. doi: 10.3390/diseases11010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alhazmi NM. Fungicidal activity of silver and silica nanoparticles against Aspergillus sydowii isolated from the soil in western Saudi Arabia. Microorganisms. 2023;11(1):86–86. doi: 10.3390/microorganisms11010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hussain S, Mubeen I, Ullah N, Shah SSUD, Khan BA, Zahoor M, Ullah R, Khan FA, Sultan MA. Modern diagnostic imaging technique applications and risk factors in the medical field: a review. Biomed Res Int. 2022;2022:5164970–5164970. doi: 10.1155/2022/5164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruiz Santiago, Láinez Ramos-Bossini, Wáng YXJ, Martínez Barbero, García Espinosa, Martínez Martínez. The value of magnetic resonance imaging and computed tomography in the study of spinal disorders. Quant Imaging Med Surg. 2022;12(7):3947–3986. doi: 10.21037/qims-2022-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xian JF, Chen M, Jin ZY. Magnetic resonance imaging in clinical medicine: current status and potential future developments in China. Chin Med J (Engl) 2015;128(5):569–570. doi: 10.4103/0366-6999.151637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garvey CJ, Hanlon R. Computed tomography in clinical practice. BMJ. 2002;324(7345):1077–1080. doi: 10.1136/bmj.324.7345.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crişan G, Moldovean-Cioroianu NS, Timaru DG, Andrieş G, Căinap C, Chiş V. Radiopharmaceuticals for PET and SPECT imaging: a literature review over the last decade. Int J Mol Sci. 2022;23(9):5023–5023. doi: 10.3390/ijms23095023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu FM, Yuan Z. PET/SPECT molecular imaging in clinical neuroscience: recent advances in the investigation of CNS diseases. Quant Imaging Med Surg. 2015;5(3):433–447. doi: 10.3978/j.issn.2223-4292.2015.03.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boese A, Wex C, Croner R, Liehr UB, Wendler JJ, Weigt J, Walles T, Vorwerk U, Lohmann CH, Friebe M, Illanes A. Endoscopic imaging technology today. Diagnostics (Basel) 2022;12(5):1262–1262. doi: 10.3390/diagnostics12051262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nam D, Barrack RL, Potter HG. What are the advantages and disadvantages of imaging modalities to diagnose wear-related corrosion problems. Clin Orthop Relat Res. 2014;472(12):3665–3673. doi: 10.1007/s11999-014-3579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mercuri M, Sheth T, Natarajan MK. Radiation exposure from medical imaging: a silent harm. CMAJ. 2011;183(4):413–414. doi: 10.1503/cmaj.101885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sim S, Wong NK. Nanotechnology and its use in imaging and drug delivery (Review) Biomed Rep. 2021;14(5):42–42. doi: 10.3892/br.2021.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palanikumar L, Kalmouni M, Houhou T, Abdullah O, Ali L, Pasricha R, Straubinger R, Thomas S, Afzal AJ, Barrera FN, Magzoub M. pH-responsive upconversion mesoporous silica nanospheres for combined multimodal diagnostic imaging and targeted photodynamic and photothermal cancer therapy. ACS Nano. 2023;17(19):18979–18999. doi: 10.1021/acsnano.3c04564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laranjeira MS, Ribeiro TP, Magalhães AI, Silva PC, Santos JAM, Monteiro FJ. Magnetic mesoporous silica nanoparticles as a theranostic approach for breast cancer: loading and release of the poorly soluble drug Exemestane. Int J Pharm. 2022;619:121711–121711. doi: 10.1016/j.ijpharm.2022.121711. [DOI] [PubMed] [Google Scholar]
- 70.Bock S, Choi YS, Kim M, Yun Y, Pham XH, Kim J, Seong B, Kim W, Jo A, Ham KM, Lee SG, Lee SH, Kang H, Choi HS, Jeong DH, Chang H, Kim DE, Jun BH. Highly sensitive near-infrared SERS nanoprobes for in vivo imaging using gold-assembled silica nanoparticles with controllable nanogaps. J Nanobiotechnology. 2022;20(1):130–130. doi: 10.1186/s12951-022-01327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mao AS, Mooney DJ. Regenerative medicine: current therapies and future directions. Proc Natl Acad Sci U S A. 2015;112(47):14452–14459. doi: 10.1073/pnas.1508520112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McKinley KL, Longaker MT, Naik S. Emerging frontiers in regenerative medicine. Science. 2023;380(6647):796–798. doi: 10.1126/science.add6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petrosyan A, Martins PN, Solez K, Uygun BE, Gorantla VS, Orlando G. Regenerative medicine applications: an overview of clinical trials. Front Bioeng Biotechnol. 2022;10:942750–942750. doi: 10.3389/fbioe.2022.942750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peña OA, Martin P. Cellular and molecular mechanisms of skin wound healing. Nat Rev Mol Cell Biol. 2024;25:1–18. doi: 10.1038/s41580-024-00715-1. [DOI] [PubMed] [Google Scholar]
- 75.Tottoli EM, Dorati R, Genta I, Chiesa E, Pisani S, Conti B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics. 2020;12(8):735–735. doi: 10.3390/pharmaceutics12080735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hooshmand S, Mollazadeh S, Akrami N, Ghanad M, El-Fiqi A, Baino F, Nazarnezhad S, Kargozar S. Mesoporous silica nanoparticles and mesoporous bioactive glasses for wound management: from skin regeneration to cancer therapy. Materials (Basel) 2021;14(12):3337–3337. doi: 10.3390/ma14123337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Masood A, Maheen S, Khan HU, Shafqat SS, Irshad M, Aslam I, Rasul A, Bashir S, Zafar MN. Pharmaco-technical evaluation of statistically formulated and optimized dual drug-loaded silica nanoparticles for improved antifungal efficacy and wound healing. ACS Omega. 2021;6(12):8210–8225. doi: 10.1021/acsomega.0c06242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maheen S, Younis H, Khan HU, Salman Shafqat, Ali S, Rehman AU, Ilyas S, Zafar MN, Shafqat SR, Kalam A, Al-Ghamdi AA. Enhanced antifungal and wound healing efficacy of statistically optimized, physicochemically evaluated Econazole-Triamcinolone loaded silica nanoparticles. Front Chem. 2022;10:836678–836678. doi: 10.3389/fchem.2022.836678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hamam F, Nasr A. Curcumin-loaded mesoporous silica particles as wound-healing agent: an in vivo study. Saudi J Med Med Sci. 2020;8(1):17–24. doi: 10.4103/sjmms.sjmms_2_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xue L, Deng T, Guo R, Peng L, Guo J, Tang F, Lin J, Jiang S, Lu H, Liu X, Deng L. A composite hydrogel containing mesoporous silica nanoparticles loaded with Artemisia argyi extract for improving chronic wound healing. Front Bioeng Biotechnol. 2022;10:825339–825339. doi: 10.3389/fbioe.2022.825339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu M, Guinart A, Granados A, Gimbert-Suriñach C, Fernández E, Pleixats R, Vallribera A. Coated cotton fabrics with antibacterial and anti-inflammatory silica nanoparticles for improving wound healing. ACS Appl Mater Interfaces. 2024;16(12):14595–14604. doi: 10.1021/acsami.4c00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lyons JG, Plantz MA, Hsu WK, Hsu EL, Minardi S. Nano-structured biomaterials for bone regeneration. Front Bioeng Biotechnol. 2020;8:922–922. doi: 10.3389/fbioe.2020.00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Q, Xiao L, Xiao Y. Porous nanomaterials targeting autophagy in bone regeneration. Pharmaceutics. 2021;13(10):1572–1572. doi: 10.3390/pharmaceutics13101572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu C, Xiao L, Cao Y, He Y, Lei C, Xiao Y, Sun W, Ahadian S, Zhou X, Khademhosseini A, Ye Q. Mesoporous silica rods with cone shaped pores modulate inflammation and deliver BMP-2 for bone regeneration. Nano Res. 2020;13(9):2323–2331. [Google Scholar]
- 85.Shen M, Wang L, Feng L, Gao Y, Li S, Wu Y, Xu C, Pei G. bFGF-loaded mesoporous silica nanoparticles promote bone regeneration through the Wnt/β-catenin signalling pathway. Int J Nanomedicine. 2022;17:2593–2608. doi: 10.2147/IJN.S366926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao H, Jia Y, Wang F, Chai Y, Zhang C, Xu J, Kang Q. Cobalt-doped mesoporous silica coated magnetic nanoparticles promoting accelerated bone healing in distraction osteogenesis. Int J Nanomedicine. 2023;18:2359–2370. doi: 10.2147/IJN.S393878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chadha U, Bhardwaj P, Agarwal R, Rawat P, Agarwal R, Gupta I, Panjwani M, Singh S, Ahuja C, Selvaraj SK, Banavoth M, Sonar P, Badoni B, Chakravorty A. Recent progress and growth in biosensors technology: a critical review. J Ind Eng Chem. 2022;109:21–51. [Google Scholar]
- 88.Mehrotra P. Biosensors and their applications - a review. J Oral Biol Craniofac Res. 2016;6(2):153–159. doi: 10.1016/j.jobcr.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andryukov BG, Lyapun IN, Matosova EV, Somova LM. Biosensor technologies in medicine: from detection of biochemical markers to research into molecular targets (Review) Sovrem Tekhnologii Med. 2021;12(6):70–83. doi: 10.17691/stm2020.12.6.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haleem A, Javaid M, Singh RP, Suman R, Rab S. Biosensors applications in medical field: a brief review. Sens Int. 2021;2:100100–100100. [Google Scholar]
- 91.Malik S, Singh J, Goyat R, Saharan Y, Chaudhry V, Umar A, Ibrahim AA, Akbar S, Ameen S, Baskoutas S. Nanomaterials-based biosensor and their applications: a review. Heliyon. 2023;9(9):e19929–e19929. doi: 10.1016/j.heliyon.2023.e19929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.en Karaman, Pamukçu A, Karakaplan MB, Kocaoglu O, Rosenholm JM. Recent advances in the use of mesoporous silica nanoparticles for the diagnosis of bacterial infections. Int J Nanomedicine. 2021;16:6575–6591. doi: 10.2147/IJN.S273062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chitra K, Annadurai G. Fluorescent silica nanoparticles in the detection and control of the growth of pathogen. J Nanotechnology. 2013;2013:509628–509628. [Google Scholar]
- 94.Jenie SNA, Kusumastuti Y, Krismastuti FSH, Untoro YM, Dewi RT, Udin LZ, Artanti N. Rapid fluorescence quenching detection of Escherichia coli using natural silica-based nanoparticles. Sensors (Basel) 2021;21(3):881–881. doi: 10.3390/s21030881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shams A, Rahimian Zarif. Designing an immunosensor for detection of Brucella abortus based on coloured silica nanoparticles. Artif Cells Nanomed Biotechnol. 2019;47(1):2562–2568. doi: 10.1080/21691401.2019.1626403. [DOI] [PubMed] [Google Scholar]
- 96.Udomsom S, Kanthasap K, Paengnakorn P, Jantrawut P, Kumphune S, Auephanwiriyakul S, Mankong U, Theera-Umpon N, Baipaywad P. Itaconic acid cross-linked biomolecule immobilization approach on amine-functionalized silica nanoparticles for highly sensitive enzyme-linked immunosorbent assay (ELISA) ACS Omega. 2024;9(12):13636–13643. doi: 10.1021/acsomega.3c07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Z, Mu Y, Peng C, Lavin MF, Shao H, Du Z. Understanding the mechanisms of silica nanoparticles for nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13(1):e1658–e1658. doi: 10.1002/wnan.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tng DJH, Low JGH. Current status of silica-based nanoparticles as therapeutics and its potential as therapies against viruses. Antiviral Res. 2023;210:105488–105488. doi: 10.1016/j.antiviral.2022.105488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gonçalves MC. Sol-gel silica nanoparticles in medicine: a natural choice. Design, synthesis and products. Molecules. 2018;23(8):2021–2021. doi: 10.3390/molecules23082021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xiao J, Weng J, Wen F, Ye J. Red blood cell membrane-coated silica nanoparticles codelivering DOX and ICG for effective lung cancer therapy. ACS Omega. 2020;5(51):32861–32867. doi: 10.1021/acsomega.0c01541. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101.Santino F, Stavole P, He T, Pieraccini S, Paolillo M, Prodi L, Rampazzo E, Gentilucci L. Preparation of non-toxic fluorescent peptide-coated silica/PEG nanoparticles from peptide-block copolymer conjugates. Micro. 2022;2(2):240–256. [Google Scholar]
- 102.MacCuaig WM, Samykutty A, Foote J, Luo W, Filatenkov A, Li M, Houchen C, Grizzle WE, McNally LR. Toxicity assessment of mesoporous silica nanoparticles upon intravenous injection in mice: implications for drug delivery. Pharmaceutics. 2022;14(5):969–969. doi: 10.3390/pharmaceutics14050969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang M, Li J, Dong S, Cai X, Simaiti A, Yang X, Zhu X, Luo J, Jiang LH, Du B, Yu P, Yang W. Silica nanoparticles induce lung inflammation in mice via ROS/PARP/TRPM2 signaling-mediated lysosome impairment and autophagy dysfunction. Part Fibre Toxicol. 2020;17(1):23–23. doi: 10.1186/s12989-020-00353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ogawa T, Okumura R, Nagano K, Minemura T, Izumi M, Motooka D, Nakamura S, Iida T, Maeda Y, Kumanogoh A, Tsutsumi Y, Takeda K. Oral intake of silica nanoparticles exacerbates intestinal inflammation. Biochem Biophys Res Commun. 2021;534:540–546. doi: 10.1016/j.bbrc.2020.11.047. [DOI] [PubMed] [Google Scholar]
- 105.Feng L, Ning R, Liu J, Liang S, Xu Q, Liu Y, Liu W, Duan J, Sun Z. Silica nanoparticles induce JNK-mediated inflammation and myocardial contractile dysfunction. J Hazard Mater. 2020;391:122206–122206. doi: 10.1016/j.jhazmat.2020.122206. [DOI] [PubMed] [Google Scholar]
- 106.Yin H, Fang L, Wang L, Xia Y, Tian J, Ma L, Zhang J, Li N, Li W, Yao S, Zhang L. Acute silica exposure triggers pulmonary inflammation through macrophage pyroptosis: an experimental simulation. Front Immunol. 2022;13:874459–874459. doi: 10.3389/fimmu.2022.874459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu Y, Wang Z, Wang R, Jin J, Zhu YZ. Short-term oral administration of mesoporous silica nanoparticles potentially induced colon inflammation in rats through alteration of gut microbiota. Int J Nanomedicine. 2021;16:881–893. doi: 10.2147/IJN.S295575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang YS, Cao MD, Wang A, Liu QM, Zhu DX, Zou Y, Ma LL, Luo M, Shao Y, Xu DD, Wei JF, Sun JL. Nano-silica particles synergistically IgE-mediated mast cell activation exacerbating allergic inflammation in mice. Front Immunol. 2022;13:911300–911300. doi: 10.3389/fimmu.2022.911300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salve R, Kumar P, Ngamcherdtrakul W, Gajbhiye V, Yantasee W. Stimuli-responsive mesoporous silica nanoparticles: a custom-tailored next generation approach in cargo delivery. Mater Sci Eng C Mater Biol Appl. 2021;124:112084–112084. doi: 10.1016/j.msec.2021.112084. [DOI] [PubMed] [Google Scholar]
- 110.Moodley T, Singh M. Current stimuli-responsive mesoporous silica nanoparticles for cancer therapy. Pharmaceutics. 2021;13(1):71–71. doi: 10.3390/pharmaceutics13010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.García-Fernández A, Vivo-Llorca G, Sancho M, García-Jareño AB, Ramírez-Jiménez L, Barber-Cano E, Murguía JR, Orzáez M, Sancenón F, Martínez-Máñez R. Nanodevices for the efficient codelivery of CRISPR-Cas9 editing machinery and an entrapped cargo: a proposal for dual anti-inflammatory therapy. Pharmaceutics. 2022;14(7):1495–1495. doi: 10.3390/pharmaceutics14071495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu X, Koivisto O, Liu C, Zhou J, Miihkinen M, Jacquemet G, Wang D, Rosenholm JM, Shu Y, Zhang H. Effective delivery of the CRISPR/Cas9 system enabled by functionalized mesoporous silica nanoparticles for GFP‐tagged paxillin knock‐in. Adv Ther. 2021;4(1):2000072–2000072. [Google Scholar]


