Abstract
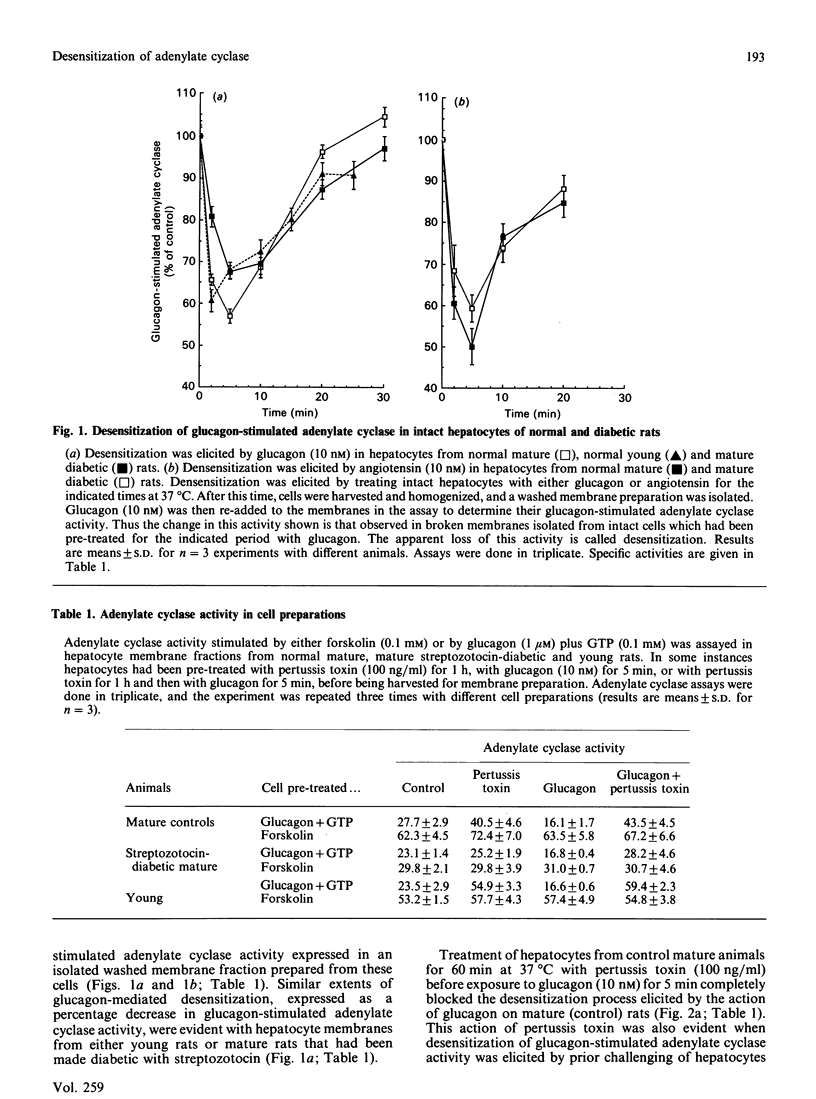
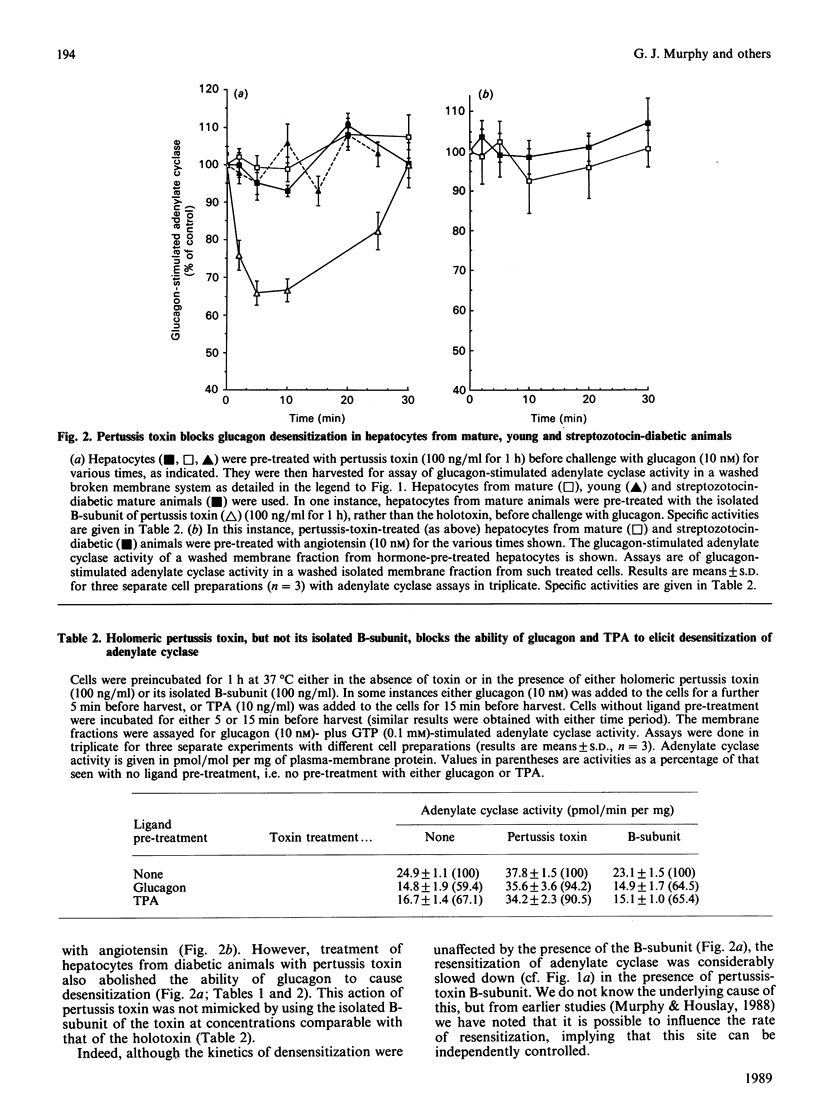
Brief exposure of hepatocytes to glucagon, angiotensin or the protein kinase C activator TPA (12-O-tetradecanoylphorbol 13-acetate) caused the inactivation of the inhibitory guanine nucleotide regulatory protein Gi. Glucagon-mediated desensitization of glucagon-stimulated adenylate cyclase activity was seen in hepatocytes from both normal rats and those made diabetic with streptozotocin, where Gi is not functionally expressed. Normal glucagon desensitization was seen in hepatocytes from young animals, 6 weeks of age, which had amounts of Gi in their hepatocyte membranes which were some 45% of that seen in mature animals (3.4 pmol/mg of plasma-membrane protein). Streptozotocin-induced diabetes in young animals abolished the appearance of functional Gi in hepatocyte plasma membranes. Pertussis-toxin treatment of hepatocytes from both normal mature animals and those made diabetic, with streptozotocin, blocked the ability of glucagon or angiotensin or TPA to elicit desensitization of adenylate cyclase. The isolated B (binding)-subunit of pertussis toxin was ineffective in blocking desensitization. Neither induction of diabetes nor treatment of hepatocytes with pertussis toxin inhibited the ability of glucagon and angiotensin to stimulate the production of inositol phosphates in intact hepatocytes. Thus (i) Gi does not appear to play a role in the molecular mechanism of glucagon desensitization in hepatocytes, (ii) the G-protein concerned with receptor-stimulated inositol phospholipid metabolism in hepatocytes appears not to be a substrate for the action of pertussis toxin, (iii) in intact hepatocytes, treatment with glucagon and/or angiotensin can elicit the inactivation of the inhibitory G-protein Gi, and (iv) pertussis toxin blocks desensitization by a process which does not involve Gi.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banga H. S., Walker R. K., Winberry L. K., Rittenhouse S. E. Pertussis toxin can activate human platelets. Comparative effects of holotoxin and its ADP-ribosylating S1 subunit. J Biol Chem. 1987 Nov 5;262(31):14871–14874. [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D. L., Manclark C. R. Adenine nucleotides promote dissociation of pertussis toxin subunits. J Biol Chem. 1986 Mar 25;261(9):4324–4327. [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Gawler D., Milligan G., Houslay M. D. Treatment of streptozotocin-diabetic rats with metformin restores the ability of insulin to inhibit adenylate cyclase activity and demonstrates that insulin does not exert this action through the inhibitory guanine nucleotide regulatory protein Gi. Biochem J. 1988 Jan 15;249(2):537–542. doi: 10.1042/bj2490537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawler D., Milligan G., Spiegel A. M., Unson C. G., Houslay M. D. Abolition of the expression of inhibitory guanine nucleotide regulatory protein Gi activity in diabetes. Nature. 1987 May 21;327(6119):229–232. doi: 10.1038/327229a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Heyworth C. M., Hanski E., Houslay M. D. Islet-activating protein blocks glucagon desensitization in intact hepatocytes. Biochem J. 1984 Aug 15;222(1):189–194. doi: 10.1042/bj2220189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth C. M., Houslay M. D. Challenge of hepatocytes by glucagon triggers a rapid modulation of adenylate cyclase activity in isolated membranes. Biochem J. 1983 Jul 15;214(1):93–98. doi: 10.1042/bj2140093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth C. M., Wallace A. V., Houslay M. D. Insulin and glucagon regulate the activation of two distinct membrane-bound cyclic AMP phosphodiesterases in hepatocytes. Biochem J. 1983 Jul 15;214(1):99–110. doi: 10.1042/bj2140099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth C. M., Whetton A. D., Kinsella A. R., Houslay M. D. The phorbol ester, TPA inhibits glucagon-stimulated adenylate cyclase activity. FEBS Lett. 1984 May 7;170(1):38–42. doi: 10.1016/0014-5793(84)81364-2. [DOI] [PubMed] [Google Scholar]
- Heyworth C. M., Whetton A. D., Wong S., Martin B. R., Houslay M. D. Insulin inhibits the cholera-toxin-catalysed ribosylation of a Mr-25000 protein in rat liver plasma membranes. Biochem J. 1985 Jun 15;228(3):593–603. doi: 10.1042/bj2280593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth C. M., Wilson S. P., Gawler D. J., Houslay M. D. The phorbol ester TPA prevents the expression of both glucagon desensitisation and the glucagon-mediated block of insulin stimulation of the peripheral plasma membrane cyclic AMP phosphodiesterase in rat hepatocytes. FEBS Lett. 1985 Aug 5;187(2):196–200. doi: 10.1016/0014-5793(85)81241-2. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. D., Hanoune J., Birnbaumer L. Guanine nucleotide inhibition of cyc- S49 mouse lymphoma cell membrane adenylyl cyclase. J Biol Chem. 1982 Dec 25;257(24):14723–14725. [PubMed] [Google Scholar]
- Houslay M. D., Elliott K. R. Cholera toxin mediated activation of adenylate cyclase in intact rat hepatocytes. FEBS Lett. 1979 Aug 15;104(2):359–363. doi: 10.1016/0014-5793(79)80852-2. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Gawler D. J., Milligan G., Wilson A. Multiple defects occur in the guanine nucleotide regulatory protein system in liver plasma membranes of obese (fa/fa) but not lean (Fa/Fa) Zucker rats: loss of functional Gi and abnormal Gs function. Cell Signal. 1989;1(1):9–22. doi: 10.1016/0898-6568(89)90016-8. [DOI] [PubMed] [Google Scholar]
- Houslay M. D. Insulin, glucagon and the receptor-mediated control of cyclic AMP concentrations in liver. Twenty-second Colworth medal lecture. Biochem Soc Trans. 1986 Apr;14(2):183–193. doi: 10.1042/bst0140183. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Metcalfe J. C., Warren G. B., Hesketh T. R., Smith G. A. The glucagon receptor of rat liver plasma membrane can couple to adenylate cyclase without activating it. Biochim Biophys Acta. 1976 Jun 17;436(2):489–494. doi: 10.1016/0005-2736(76)90210-8. [DOI] [PubMed] [Google Scholar]
- Itoh H., Okajima F., Ui M. Conversion of adrenergic mechanism from an alpha- to a beta-type during primary culture of rat hepatocytes. Accompanying decreases in the function of the inhibitory guanine nucleotide regulatory component of adenylate cyclase identified as the substrate of islet-activating protein. J Biol Chem. 1984 Dec 25;259(24):15464–15473. [PubMed] [Google Scholar]
- Jakobs K. H., Bauer S., Watanabe Y. Modulation of adenylate cyclase of human platelets by phorbol ester. Impairment of the hormone-sensitive inhibitory pathway. Eur J Biochem. 1985 Sep 2;151(2):425–430. doi: 10.1111/j.1432-1033.1985.tb09119.x. [DOI] [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Marchmont R. J., Ayad S. R., Houslay M. D. Purification and properties of the insulin-stimulated cyclic AMP phosphodiesterase from rat liver plasma membranes. Biochem J. 1981 Jun 1;195(3):645–652. doi: 10.1042/bj1950645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G. J., Houslay M. D. Resensitization of hepatocyte glucagon-stimulated adenylate cyclase can be inhibited when cyclic AMP phosphodiesterase inhibitors are used to elevate intracellular cyclic AMP concentrations to supraphysiological values. Biochem J. 1988 Jan 15;249(2):543–547. doi: 10.1042/bj2490543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G. J., Hruby V. J., Trivedi D., Wakelam M. J., Houslay M. D. The rapid desensitization of glucagon-stimulated adenylate cyclase is a cyclic AMP-independent process that can be mimicked by hormones which stimulate inositol phospholipid metabolism. Biochem J. 1987 Apr 1;243(1):39–46. doi: 10.1042/bj2430039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pyne N. J., Murphy G. J., Milligan G., Houslay M. D. Treatment of intact hepatocytes with either the phorbol ester TPA or glucagon elicits the phosphorylation and functional inactivation of the inhibitory guanine nucleotide regulatory protein Gi. FEBS Lett. 1989 Jan 16;243(1):77–82. doi: 10.1016/0014-5793(89)81221-9. [DOI] [PubMed] [Google Scholar]
- Rich K. A., Codina J., Floyd G., Sekura R., Hildebrandt J. D., Iyengar R. Glucagon-induced heterologous desensitization of the MDCK cell adenylyl cyclase. Increases in the apparent levels of the inhibitory regulator (Ni). J Biol Chem. 1984 Jun 25;259(12):7893–7901. [PubMed] [Google Scholar]
- Sauerheber R. D., Kuhn C. E., Hyslop P. A. Membrane structural/functional properties of adipocytes from normal and streptozotocin-diabetic rats. Diabetes. 1984 Mar;33(3):258–265. doi: 10.2337/diab.33.3.258. [DOI] [PubMed] [Google Scholar]
- Smith S. A., Elliott K. R., Pogson C. I. Differential effects of tryptophan on glucose synthesis in rats and guinea pigs. Biochem J. 1978 Dec 15;176(3):817–825. doi: 10.1042/bj1760817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Strnad C. F., Carchman R. A. Human T lymphocyte mitogenesis in response to the B oligomer of pertussis toxin is associated with an early elevation in cytosolic calcium concentrations. FEBS Lett. 1987 Dec 10;225(1-2):16–20. doi: 10.1016/0014-5793(87)81123-7. [DOI] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Yajima M., Ase K., Ui M. A role of the B-oligomer moiety of islet-activating protein, pertussis toxin, in development of the biological effects on intact cells. J Biol Chem. 1983 Jun 10;258(11):6756–6761. [PubMed] [Google Scholar]
- Vistica B. P., McAllister C. G., Sekura R. D., Ihle J. N., Gery I. Dual effects of pertussis toxin on lymphoid cells in culture. Cell Immunol. 1986 Aug;101(1):232–241. doi: 10.1016/0008-8749(86)90200-5. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Murphy G. J., Hruby V. J., Houslay M. D. Activation of two signal-transduction systems in hepatocytes by glucagon. Nature. 1986 Sep 4;323(6083):68–71. doi: 10.1038/323068a0. [DOI] [PubMed] [Google Scholar]
- Whetton A. D., Needham L., Dodd N. J., Heyworth C. M., Houslay M. D. Forskolin and ethanol both perturb the structure of liver plasma membranes and activate adenylate cyclase activity. Biochem Pharmacol. 1983 May 15;32(10):1601–1608. doi: 10.1016/0006-2952(83)90334-9. [DOI] [PubMed] [Google Scholar]
- Wilson P. D., Dixon B. S., Dillingham M. A., Garcia-Sainz J. A., Anderson R. J. Pertussis toxin prevents homologous desensitization of adenylate cyclase in cultured renal epithelial cells. J Biol Chem. 1986 Feb 5;261(4):1503–1506. [PubMed] [Google Scholar]


