Abstract
There has been an alarming trend toward earlier puberty in girls, suggesting the influence of an environmental factor(s). As the reactivation of the reproductive axis during puberty is thought to be mediated by the hypothalamic neuropeptides kisspeptin and gonadotropin-releasing hormone (GnRH), we asked whether an environmental compound might activate the kisspeptin (KISS1R) or GnRH receptor (GnRHR). We used GnRHR or KISS1R-expressing HEK293 cells to screen the Tox21 10K compound library, a compendium of pharmaceuticals and environmental compounds, for GnRHR and KISS1R activation. Agonists were identified using Ca2+ flux and phosphorylated extracellularly regulated kinase (p-ERK) detection assays. Follow-up studies included measurement of genes known to be upregulated upon receptor activation using relevant murine or human cell lines and molecular docking simulation. Musk ambrette was identified as a KISS1R agonist, and treatment with musk ambrette led to increased expression of Gnrh1 in murine and human hypothalamic cells and expansion of GnRH neuronal area in developing zebrafish larvae. Molecular docking demonstrated that musk ambrette interacts with the His309, Gln122, and Gln123 residues of the KISS1R. A group of cholinergic agonists with structures similar to methacholine was identified as GnRHR agonists. When applied to murine gonadotrope cells, these agonists upregulated Fos, Jun, and/or Egr1. Molecular docking revealed a potential interaction between GnRHR and 5 agonists, with Asn305 constituting the most conservative GnRHR binding site. In summary, using a Tox21 10K compound library screen combined with cellular, molecular, and structural biology techniques, we have identified novel environmental agents that may activate the human KISS1R or GnRHR.
Keywords: GnRHR, KISS1R, puberty, environment, agonist, qHTS
Over the past decade, a worldwide secular trend of earlier breast development, or thelarche, in girls has emerged (1). This trend has important health implications, as earlier puberty is associated with an increased risk of psychosocial problems, obesity, diabetes, cardiovascular disease, and breast cancer (2, 3). While the timing of puberty is determined both by genetics and the environment (4), the rapid pace of the current trend precludes a genetic etiology and instead, points to environmental factors. A number of studies have attempted to identify potential environmental triggers by measuring one or more endocrine-disrupting chemicals of interest in peripubertal or pubertal girls in relation to pubertal timing, yet they have failed to demonstrate any compelling associations (5). These studies suffer from a number of methodological limitations: 1) the use of blood or urine samples, which often reflect only recent (on the order of hours/days) exposures (6), 2) inability to capture critical windows of developmental exposure (eg, fetal), and 3) importantly, these studies do not reflect exposures in the tissue that is most relevant to puberty—the hypothalamus.
Puberty is controlled by a network of gonadotropin-releasing hormone (GnRH) neurons. The cell bodies of GnRH neurons that control reproductive function are primarily found in the arcuate nucleus (ARC, or infundibular nucleus), a part of the medial basal hypothalamus. Pulsatile secretion of GnRH stimulates cells of the anterior pituitary called gonadotropes to secrete 2 gonadotropins, follicle-stimulating hormone and luteinizing hormone, which in turn, signal the ovaries and testes to synthesize estrogen and testosterone, respectively. The precise biological mechanisms underlying GnRH neuronal activation at the onset of puberty remain to be determined, but the neuropeptide kisspeptin, a potent stimulant of GnRH secretion (7, 8), is believed to play a critical role. Of note, kisspeptin-expressing neurons have receptors for glucocorticoids, leptin, and insulin, indicating that they are well-poised to modulate reproductive axis activity in response to environmental cues, such as stress and nutritional status (9). It is therefore plausible that kisspeptin and/or GnRH neurons may be the targets of environmental compounds responsible for earlier puberty in girls.
Both the GnRH receptor (GnRHR) and kisspeptin receptor (KISS1R) are members of the G protein–coupled receptor (GPCR) family (2, 10) (Fig. 1, created with BioRender.com.). On ligand binding, the GPCR undergoes a conformational change that activates the heterotrimeric GTP-binding protein (G protein), Gq, causing it to dissociate into α and β/γ subunits. The Gαq subunit binds to phosphoinositide-specific phospholipase C (PLC), which hydrolyzes phosphatidylinositol-4,5-biphosphate (PIP2) into the second messengers sn-1,2 diacylglycerol (DAG) and inositol-1,4,5-triphosphate (IP3). IP3 interacts with a calcium channel within the endoplasmic reticulum (ER), triggering an efflux of calcium ions (Ca2+) into the cytoplasm (11). DAG activates protein kinase C, which then stimulates the RAS-RAF-MEK-ERK signaling cascade that culminates in the phosphorylation of extracellularly regulated kinase (ERK). Phosphorylated ERK (p-ERK) translocates into the nucleus, where it modulates the expression of downstream genes specific to the given cell type. Thus, measurement of Ca2+ efflux and p-ERK have served as useful tools in demonstrating cellular activation following the administration of a chemical to most GqPCR-expressing cells (11).
Figure 1.
Pathway of gonadotropin-releasing hormone receptor (GnRHR) and/or kisspeptin receptor (KISS1R) activation.
To overcome the limitations inherent to observational studies in pediatric research participants, we used a quantitative high-throughput screening (qHTS) approach to identify environmental compounds that may activate the neuroreproductive axis. To this end, we used HEK293 cells genetically-engineered to express the human GnRHR or KISS1R along with Ca2+ flux and p-ERK detection assays to interrogate the Tox21 compound library, which comprises approximately 10 000 (10K) compounds, including a combination of licensed pharmaceuticals and environmental chemicals, of which 8312 are unique (12). Further contextualization using molecular docking studies and activation of downstream gene expression (transcriptional) in vitro and in vivo provided additional support for the agonist activity of the primary screen hits.
Materials and Methods
Materials
Kisspeptin-10 was purchased from TOCRIS (TOCRIS, catalog No. 2570). Goserelin (MedChemExpress, HY-13673A) and GnRH-I (MedChemExpress, HY-P0292) were purchased from MedChemExpress. All other chemicals were supplied by the National Center for Advancing Translational Sciences (NCATS) compound management group.
Cell Culture
The wild-type (WT) HEK293 cells, HEK293-WT, were purchased from the American Type Culture Collection (ATCC) and cultured with 90% Dulbecco’s modified Eagle’s medium (DMEM) (Thermo Fisher Scientific, catalog No. 11995-065) and supplemented with 10% fetal bovine serum (FBS, Hyclone, SH30071.03), as well as 100 U/mL penicillin and 100 μg/mL streptomycin (Thermo Fisher Scientific). Human GnRHR-expressing stably transfected HEK293 cells (Codex), HEK293-GnRHR cells, were cultured with 90% DMEM and supplemented with 10% FBS, 500 μg/mL Geneticin (G418, Thermo Fisher Scientific), as well as 100 U/mL penicillin and 100 μg/mL streptomycin (Thermo Fisher Scientific). Human KISS1R-expressing stably transfected HEK293 cells (Codex), HEK293-KISS1R cells, were cultured with 90% DMEM and supplemented with 10% FBS, 1 μg/mL puromycin (Thermo Fisher Scientific), as well as 100-U/mL penicillin and 100-μg/mL streptomycin. All cells were maintained at 37 °C in a humidified atmosphere with 5% CO2.
The adult mouse hypothalamic GnRH/GFP cell line, mHypoA-GnRH/GFP (CLU498), was purchased from the CELLution Biosystem Inc and cultured with 90% DMEM (Sigma, catalog No. D5796) and supplemented with 10% FBS, as well as 100 U/mL penicillin and 100 μg/mL streptomycin. Cells were maintained at 37 °C in a humidified atmosphere with 5% CO2. To generate spheroids for testing, 1000 cells were seeded in each well of the 96-well ultra-low attachment plates (Corning, catalog No. 4515). The culture medium is the same as the monolayer cell culture mentioned previously. After centrifuging at 1000 rpm for 5 minutes, plates were incubated at 37 °C in a humidified atmosphere with 5% CO2 for 6 days to allow spheroid formation, and the medium was changed every other day.
Human hypothalamic neurons were derived from induced pluripotent stem cells (iPSCs) at high efficiency in chemically defined conditions. Hypothalamus spheres were generated by optimizing the human hypothalamic neuron protocol. In brief, ventral diencephalon progenitor cells, derived from the LiPSC GR 1.1 line on day 7, were dissociated using StemPro Accutase (ThermoFisher, A1110501). A cell suspension of 2.5 million cells per milliliter was prepared in Arc-2 medium with Chroman-1, Emricasan, trans-Isrib and polyamines (CEPT) (13). For sphere formation, 3 mL of the cell suspension was added to each well of an AggreWell 800 6-well plate (StemCell Technologies, 34825), resulting in 5000 cells per microwell. The plates were incubated overnight. The next day, the spheres were carefully transferred using a 1-mL wide-bore pipette into 40 mL of Arc-2 medium and placed in T175 flasks prerinsed with an antiadherence solution (StemCell Technologies, 07010). One full AggreWell plate was transferred into each T175 flask. Spheres were maintained in Arc-2 medium with daily medium changes until day 11. On day 11, the medium was replaced with Arc-3 medium, and the medium was changed every other day. Spheres were maintained in Arc-3 medium until day 28. On day 28, 2 to 4 spheroids were dispensed into each well of a 96-well plate for subsequent drug treatment.
The mouse pituitary tumor cell line, LβT2, was kindly provided by Dr Pamela Mellon from the University of California at San Diego and cultured with 90% DMEM (Sigma, catalog No. D5796) and supplemented with 10% FBS, as well as 100 U/mL penicillin and 100 μg/mL streptomycin. Cells were maintained at 37 °C in a humidified atmosphere with 5% CO2. To generate spheroids for testing, 1000 cells were seeded in each well of 96-well ultra-low attachment plates (Corning, catalog No. 4515). The culture medium is the same as the monolayer cell culture mentioned previously. After centrifuge at 1000 rpm for 5 minutes, the plates were incubated at 37 °C in a humidified atmosphere with 5% CO2 for 4 days to allow spheroid formation, and the medium was changed every other day.
All cells underwent mycoplasma testing before being used for screening and follow-up studies. The MycoAlert Mycoplasma Detection Kit (Lonza, catalog No. LT07-318) was employed for the mycoplasma test, while the MycoAlert Assay Control Set (Lonza, catalog No. LT07-518) was employed for the positive and negative controls. Briefly, 100 μL of cell culture medium was transferred into each well of a 96-well plate. Next, 100 μL of MycoAlert reagent was added to each sample and incubated for 5 minutes at room temperature. PHERAstar (BMG Labtech) was used to quantify the luminescence intensity (reading A). Subsequently, the luminescence intensity was measured again (reading B) after 100 μL of MycoAlert substrate was added to each sample and incubated at room temperature for 10 minutes. The ratio was calculated by reading B/reading A.
Calcium Flux Assay in Quantitative High-Throughput Screening
Cells were suspended in culture media and dispensed at 1500 cells/3.5 μL/well using a Multidrop Combi (Thermo Fisher Scientific) dispenser into 1536-well black-wall/clear-bottom plates. After 18 hours of incubation at 37°/5% CO2, 3.5 μL/well of calcium dye (AAT Bioquest) was applied to the assay plate using a Flying Reagent Dispenser (Aurora Discovery). The assay plates were incubated at 37 °C/5% CO2 for 1 hour, followed by 15 minutes at room temperature, and then 23 nL of compounds dissolved in DMSO were transferred to the assay plate by Pintool inside the FDSS 7000EX kinetic plate reader system (Hamamatsu), resulting in a 217-fold dilution. There were 15 concentrations of each compound, and the final concentration ranged from 0.7 nM to 65.7 μM. For agonist mode, the fluorescence intensity was measured using an FDSS 7000EX kinetic plate reader equipped with an Ex/Em = 480/540 filter for 5 minutes at 1-second intervals. Kisspeptin-10 served as a positive control for HEK293-KISS1R cells, goserelin served as a positive control for HEK293-GnRHR cells, and DMSO served as a negative control.
Quantitative High-Throughput Screening Assay Data Analysis
Analysis of compound concentration-response data was performed as described previously (14, 15). First, raw plate readings for each titration point were normalized relative to the positive control compound (kisspeptin-10 and goserelin for HEK293-KISS1R and HEK293-GnRHR cells, respectively; 100%) and DMSO-only wells (0%) according to the following formula: % activity = [(Vcompound − VDMSO)/(Vpos − VDMSO)] × 100, where Vcompound represents compound well values. The median well values of the positive control and DMSO are represented by Vpos and VDMSO, respectively. An in-house pattern correction algorithm was applied to the data set using the DMSO-only compound plates at the beginning and end of the compound plate stack (16). To obtain each compound's half maximum effective value (EC50) and maximum response (efficacy) value, concentration-response curves of each compound were fitted to a 4-parameter Hill equation (17). Compounds were assigned a class designation between 1 and 4 (1.1, 1.2, 1.3, 1.4, 2.1, 2.2, 2.3, 2.4, and 3 for activators; 4 for inactive), depending on the type of concentration-response curve observed (15). Curve classes are heuristic measures of data confidence, classifying concentration-responses based on efficacy, the number of data points observed above background activity, and the quality of fit (18). Curve classes were further converted to curve ranks, which are integers between −9 and 9, where a positive number indicates activation, a negative number indicates inhibition, and 0 means inactive compounds, according to the criteria previously described (18, 19). The activity outcome of each test compound was determined by the average curve rank from the triplicate runs and data reproducibility, which was designated as 1 of the 4 categories: active match, inactive match, inconclusive, and mismatch, according to the previously described criterion (18, 19). Compounds with an activity outcome assignment of “active agonist” were considered active in this study. The screening data sets were deposited in the PubChem Bioassay Database: BioAssay AID 1920064 (HEK293-KISS1R); BioAssay AID 1920063 (HEK293-GnRHR); BioAssay AID 1920065 (HEK293 WT); BioAssay AID 1920067 (HEK293-GnRHR assay summary); BioAssay AID 1920068 (HEK293-KISS1R assay summary).
Chemical Structure-Activity Cluster Analysis
The Tox21 10K compound collection was grouped into 1014 clusters based on structural similarity (9242-bit fingerprints; Leadscope) using the self-organizing map (SOM) algorithm (20, 21). Fingerprints are digital representations of chemical structures. A fingerprint of a chemical is a bit vector composed of ones and zeros, with each bit representing a structural feature. A bit is set to 1 if the corresponding feature is present in the chemical and to 0 if the feature is absent. Each cluster was evaluated for its enrichment of active chemicals by comparing the fraction of actives in the cluster with the fraction of actives not in the cluster. A cluster is considered enriched with actives if the former fraction is larger than the latter. The statistical significance of enrichment was determined by the Fisher exact test (P < .01).
Homogeneous Time-Resolved Fluorescence-based Phosphorylated Extracellularly Regulated Kinase Detection Assay
The experiment was conducted in accordance with the instructions of the advanced p-ERK (Thr202/Tyr204) kit (Revvity). Briefly, cells were suspended in 1% FBS culture media and dispensed at 4000 cells/4 μL/well into 1536-well white-wall/solid-bottom plates using a Multidrop Combi dispenser. After overnight incubation at 37 °C/5% CO2, 23 nL of compounds dissolved in DMSO were transferred to the assay plate using Pintool (Wako Pure Chemical Industries Ltd), resulting in a 175-fold dilution. This was followed by 5 minutes of incubation at 37 °C for HEK293-KISS1R cells and 30 minutes of incubation for HEK293-GnRHR cells. Then, a 1.33 μL/well 4 × lysis buffer (containing blocking buffer) was added into each well by BioRAPTR FRD (Beckman Coulter), followed by incubating cells for 30 minutes at room temperature. After incubation, 1 μL/well of a premixed detection solution was added to each well, followed by 2-hour incubation at room temperature. PHERAstar (BMG Labtech) read the homogeneous time-resolved fluorescence (HTRF) signal, and the HTRF ratio was calculated as follows: ratio = signal 665 nm/signal 620 nm × 10 000.
Real-Time Reverse-Transcription Polymerase Chain Reaction on Cell Models
RNA was extracted using the SingleShot Cell Lysis Kit (Bio-Rad Laboratories) per the manufacturer's instructions. Briefly, a 96-well plate was loaded with 2000 CLU498 cells per well in complete culture medium or 6000 LβT2 cells per well in FBS-free culture medium. After each treatment, cells were lysed for 10 minutes at room temperature in 50 μL of lysis buffer containing DNase and proteinase, followed by 5 minutes at 37 °C and 5 minutes at 75 °C. Each sample underwent reverse-transcription polymerase chain reaction (RT-PCR) in triplicate using Reliance One-Step Multiplex Supermix (Bio-Rad Laboratories) and specific probe sets for each gene. The total volume of each reaction was 10 μL, and the final concentration of each gene's primer is 1×. On a QuantStudioTM 3 Real-Time PCR System, the real-time PCR reactions were performed according to the manufacturer's instructions (1 cycle of 50 °C for 10 minutes and 95 °C for 10 minutes for RT and enzyme activation, followed by 40 cycles of 95 °C for 15 seconds and 60 °C for 30 seconds for annealing and extension). Primer probe sets Gapdh (Mm99999915_g1), H3f3a (Mm01612808_g1), Gnrh1 (Mm01315604_m1), Jun (Mm07296811_s1), Egr1 (Mm00656724_m1), Fos (Mm00487425_m1), KISS1R (Hs00261399_m1), GNRH1 (Hs00171272_m1), and GAPDH (4310884E) were all purchased from ThermoFisher Scientific. Using the comparative threshold cycle (Ct) method, the target gene's expression level was normalized to that of the housekeeping gene Gapdh, or H3f3a.
Molecular Docking Simulations
The x-ray crystal structure of the human GnRHR was retrieved from the Protein Data Bank (PDB code 7BR3) (22). Before performing molecular docking simulations, the original ligand (elagolix) was separated from the receptor structures. The structure of the KISS1R was retrieved from the Protein Data Bank (PDB code 8ZJE) (23). Before performing molecular docking simulations, the original ligand (TAK-448) was separated from the receptor structures. On the PubChem website, 3-dimensional structures of compounds serving as ligands were downloaded. CB-Dock2 was used to simulate molecular docking (24, 25). The best ligand conformation binding to the receptor pocket was used for the next stage of the analysis. Docking models were visualized by Discovery Studio Visualizer.
Zebrafish Husbandry
Animal procedures were approved by the Institutional Animal Care and Use Committee at Northwestern University. WT AB and transgenic gnrh3:gfp (26) adult fish were maintained in standard conditions with a 14-hour light and 10-hour dark cycle. Embryos were obtained from natural mating of adult zebrafish and were reared at 28.5 °C in embryo medium (0.3 g/L NaCl, 75 mg/L CaSO4, 37.5 mg/L NaHCO3, and 0.003% methylene blue) until experimental procedures or tissue harvest.
Musk Ambrette Treatment of Embryos
Musk ambrette was obtained from Millipore Sigma (catalog No. S342998) and dissolved in 100% DMSO (Sigma). For zebrafish embryo treatments, musk ambrette was diluted further in embryo medium and the final concentration of DMSO was 0.1% across every condition. We outcrossed heterozygous gnrh3:gfp adults to WT AB and the resulting embryos were transferred to 12-well plates (15 embryos/well in 5 mL of embryo medium). At 24 hours post fertilization (hpf), treatment groups were incubated in increasing concentrations of musk ambrette (0.1 μg/mL, 0.3 μg/mL, 0.5 μg/mL, or 1 μg/mL in embryo medium) or 0.1% DMSO and had continuous treatment until 5 days post fertilization (dpf). During the treatment course, embryo medium was removed and replaced with fresh medium containing the respective concentrations of musk ambrette/DMSO at 2 dpf and again at 4 dpf. At the experimental end point (5 dpf), larvae were screened for fluorescent signal. Green fluorescent protein (GFP) positive larvae were used immediately for image acquisition of GnRH3-GFP neurons while the GFP negative larvae were kept at 28.5 °C in embryo media until total RNA harvest for gene expression studies.
In Vivo Imaging of Gonadotropin-Releasing Hormone Receptor 3:Green Fluorescent Protein Neurons
To visualize GnRH3 neuronal patterning, the GFP-positive larvae were subjected to automated live imaging, as described elsewhere (27). Briefly, larvae were anesthetized with tricaine and passed through the Vertebrate Automated Screening Technology (VAST; Union Biometrica; software version 1.2.6.7) BioImager connected to an AxioImager.M2 m fluorescent microscope (Zeiss). Dorsal fluorescent images were obtained using 65% minimum similarity for the pattern-recognition algorithm. To quantify GnRH3-GFP positive signal, images were converted from CZI format to individual TIFF files, opened in ImageJ (National Institutes of Health [NIH]), stacked, and the GFP-positive area (region of interest) was measured. GFP area was quantified with the investigator masked to the experimental condition and experiments were repeated at least twice using independent biological replicates. The raw arbitrary values from each treatment group within an experiment were first individually normalized to represent percentage vs DMSO control; biological replicates were then pooled together in a single graph for presentation.
Quantitative Real-Time Polymerase Chain Reaction Assays on Zebrafish
A total of 5 dpf GFP-negative larvae were decapitated and larval heads (25 heads per treatment group) were harvested in Trizol (Invitrogen) for total RNA extraction. The QuantiTect RT kit (Qiagen) was used to synthesize complementary DNA, which was used as a template for gene expression studies. To identify zebrafish orthologues for each relevant human gene of interest, we performed reciprocal BLAST of human protein against the translated D rerio genome and identified one-to-one or one-to-many orthologues in zebrafish (Supplementary Table S1) (28). We performed qRT-PCR using Power SYBR Green PCR Master Mix (Applied Biosystems) according to manufacturer's instructions, normalized the expression of genes of interest to eef1a1l1 and assessed relative expression using the 2−ΔΔCt method.
Statistical Analysis
All data were presented as mean ± SD with at least 3 independent experiments, unless otherwise stated, and plotted using GraphPad Prism software. The 2-tailed unpaired t test of the mean was used for single comparisons of statistical significance, and the analysis of variance test with Tukey multiple comparison was used for multiple comparisons of statistical significance between experimental groups. Differences were considered statistically significant at P less than .05.
Results
Primary Screen Identifies Chemicals That Activate Human Kisspeptin Receptor and/or Gonadotropin-Releasing Hormone Receptor
We used an HEK293 cell line genetically-engineered to overexpress GnRHR or KISS1R and a Ca2+ flux assay to screen the Tox21 10K compound collection for receptor agonists. Goserelin, a commercially available GnRHR agonist, and kisspeptin-10 were used as positive controls for each screening. Goserelin and kisseptin-10 induced Ca2+ flux in HEK293-GnRHR and HEK293-KISS1R cells in a concentration-dependent manner, with an EC50 of 4.26 ± 3.40 nM and 0.74 ± 0.44 nM for GnRHR and KISS1R, respectively (Supplementary Fig. S1A and S1B) (28). Both online screenings performed well: For the GnRHR screen, the signal-to-background ratio (S/B) and Z’ factor were 31.53 ± 3.70 and 0.74 ± 0.051, respectively, and for the KISS1R screen, the S/B and Z’ factor were 26.77 ± 19.62 and 0.73 ± 0.098, respectively (Supplementary Fig. S1C) (28). Evaluation of screening reproducibility revealed that the mismatch rates of both Tox21 10K screens were zero, indicating robust assay performance (Supplementary Fig. S1D) (28).
We identified 1014 clusters of compounds based on their structural similarity using the self-organized map (SOM) algorithm. A SOM is a type of artificial neural network that maps high-dimensional data to a lower-dimensional representation, such as clustering objects onto a 2-dimensional map, to facilitate visualization and interpretation (Fig. 2). Ninety-eight compound clusters were enriched for active GnRHR agonists, and 43 compound clusters were enriched for active KISS1R agonists. Many agonist compounds activate both receptors (see Fig. 2). Several compounds exhibited GnRHR specificity, such as the known GnRHR agonists fertirelin (see Fig. 2) and gonadorelin (Supplementary Fig. S2A) (28). A cluster of cholinergic receptor agonists (see Fig. 2, row 24, column 30), including acetylcholine, methacholine, carbachol, bethanechol, and 2-(acryloyloxy)ethyltrimethylammonium, were identified as novel GnRHR-specific agonists (see Fig. 2 and Supplementary Fig. S2B) (28).
Figure 2.
Primary screening for gonadotropin-releasing hormone receptor (GnRHR) and kisspeptin receptor (KISS1R) agonists. The heat map shows the active agonist enrichment data for GnRHR (left) and KISS1R (right) based on the chemical structure similarity clusters generated by the self-organizing map algorithm. In the heat maps, each hexagon represents a cluster of structurally similar compounds. The color gradient indicates the enrichment of active agonists in that specific cluster (negative logarithmic scale of the P value, –log [P value]). Clusters enriched with active agonists range from yellow to red, whereas clusters deficient in active agonists are colored in shades of blue or green. A light gray color indicates that the fraction of active inhibitors in that cluster is close to the library average. Empty clusters with no compounds in them are a darker shade of gray. Each cluster was evaluated for its enrichment of active agonists by comparing the fraction of actives in the cluster with the fraction of actives not in the cluster. Three representative compounds are shown in the center.
To select chemicals for further investigation, we chose those chemicals with a curve rank greater than 3.5 and efficacy greater than 40% in the target cells that also met at least one of the following criteria (1): a curve rank less than 0.5 in HEK293-WT cells; (2) efficacy less than 40% in the HEK293-WT cells; or (3) an EC50(WT)/EC50(target) ratio greater than 1.7 (Supplementary Fig. S3) (28). A total of 58 agonist candidates met these criteria. Forty-seven compounds exhibited agonist activity exclusively in HEK293-GnRHR cells, 3 compounds exhibited agonist activity exclusively in HEK293-KISS1R cells, and 8 compounds displayed agonist activity in both cell lines (Fig. 3A and Supplementary Table S2) (28).
Figure 3.
Secondary Ca2+ flux assay confirmation. A, The Venn diagram shows the secondary Ca2+ flux assay confirmation results. B, The heat map shows the half maximum effective value (EC50) distribution of all 45 compounds identified and confirmed from secondary assays based on the self-organizing map (SOM) algorithm. The left panel shows the data from the HEK293-GnRHR cell line, and the right panel shows the data from the HEK293-KISS1R cell line. Red indicates a compound with a low EC50 and high potency, and blue indicates a compound with a high EC50 and low potency. Dark blue indicates a compound was inactive in the tested cell line. Compounds can be separated into 3 groups based on their EC50 in each cell line through the SOM algorithm, as indicated by the bold black line. A representative compound for each group and its concentration-response curve are shown in the middle panel.
A Subset of Gonadotropin-Releasing Hormone Receptor and Kisspeptin Receptor Agonists Were Confirmed in Repeat Calcium Flux Assays
These 58 potential agonists were subjected to a second Ca2+ flux assay. Forty-three of 55 (78.18%) potential GnRHR agonists were confirmed in the second Ca2+ flux assay, while 9 of 11 (81.82%) potential KISS1R agonists were confirmed in the second Ca2+ flux assay (see Fig. 3A). In concordance with the primary screening data, 36 compounds were identified as GnRHR-specific agonists, 2 as KISS1R-specific agonists, and 7 as active at both receptors.
We then applied the SOM algorithm and used the EC50 values obtained from the secondary Ca2+ flux assays in GnRHR and KISS1R cell lines; 45 confirmed compounds were categorized into 3 distinct categories, including GnRHR-specific agonists (eg, methacholine), KISS1R-specific agonists (eg, musk ambrette), and agonists that exhibit activity in both cell lines (eg, mefloquine) (see Fig. 3B).
Several Potential Gonadotropin-Releasing Hormone Receptor and Kisspeptin Receptor Agonists Stimulate Extracellularly Regulated Kinase Phosphorylation
To confirm that these potential agonists indeed activate downstream signaling pathways of their respective receptors, we next used a homogeneous time-resolved fluorescence–based p-ERK detection assay. Thirty of 43 (69.77%) GnRHR agonist candidates, including 2 well-known GnRH agonists (gonadorelin and fertirelin), stimulated p-ERK activity in HEK293-GnRHR cells (Fig. 4A, Supplementary Fig. S4A) (28). Several novel GnRHR agonists, including 5 cholinergic receptor agonists, also displayed activity in the p-ERK detection assay in HEK293-GnRHR cells but not in HEK293-WT cells (Fig. 4B-4F).
Figure 4.
Phosphorylated extracellularly regulated kinase (p-ERK) assay confirmation of gonadotropin-releasing hormone receptor (GnRHR) agonists. A, The Venn diagram shows the p-ERK assay confirmation rate of GnRHR agonist candidates in the HEK293-GnRHR cells. B to F, Concentration-response curves demonstrate that cholinergic agonists, including B, methacholine; C, bethanechol; D, (2-(acryloyloxy)ethyl)trimethylammonium; E, acetylcholine; and F, carbachol, stimulated ERK phosphorylation in HEK293-GnRHR cells but not in wild-type (WT) cells. All values represent the mean ± SD (n = 3 replicates).
Six out of 9 (66.67%) potential KISS1R agonists stimulated p-ERK activity (Fig. 5A), including musk ambrette (Fig. 5B), mercuric iodide (Supplementary Fig. S5A) (28), and 4 long-chain detergent compounds that belong to the quaternary ammonium compounds (QAC) class (Supplementary Fig. S5B-S5E) (28). These 6 KISS1R agonists were further evaluated in HEK293-WT cells using the p-ERK assay. Musk ambrette was the only compound to demonstrate specific ERK phosphorylation-stimulating activity in HEK293-KISS1R cells (2.7-fold more potent in KISS1R cells vs WT cells at the highest testing concentration) (see Fig. 5B and Supplementary Fig. S5A-S5E) (28).
Figure 5.
Phosphorylated extracellularly regulated kinase (p-ERK) assay confirmation of a kisspeptin receptor (KISS1R) agonist. A, The Venn diagram shows the p-ERK assay confirmation rate of KISS1R agonist candidates in the HEK293-KISS1R cells. B, Dose-response curves demonstrate that musk ambrette treatment stimulated ERK phosphorylation in HEK293-KISS1R cells but not in wild-type (WT) cells. All values represent the mean ± SD (n = 3 replicates).
Musk Ambrette Triggers Gnrh1 (GNRH1) Expression in Hypothalamic Cells Expressing the Kisspeptin Receptor
We next focused our attention on musk ambrette, a candidate KISS1R-specific agonist. We used the mHypoA-GnRH/GFP cell line, a murine hypothalamic cell line that expresses Kiss1r (82% homologous to the human KISS1R) (29, 30). We measured the expression of Gnrh1, a gene previously shown to be actively transcribed on Kiss1r activation (29, 31), in the presence of musk ambrette. A time-course study revealed that Gnrh1 expression significantly increased in mHypoA-GnRH/GFP cells after 1 hour of exposure to musk ambrette, began to decrease after 2 hours of treatment, and then returned to baseline after 6 hours of treatment (Fig. 6A and Supplementary Fig. S6) (28). Notably, after 1 hour of treatment with musk ambrette, Gnrh1 expression increased in a concentration-dependent manner with an EC50 of 21.94 μM, which is very similar to its EC50 of 16.71 μM in the Ca2+ flux assay in HEK293-KISS1R cells (Supplementary Fig. S6B) (28). Furthermore, after 1 hour of treatment, musk ambrette induced Gnrh1 expression in mHypoA-GnRH/GFP spheroid cells more strongly than the basal level (see Fig. 6B). In addition, musk ambrette induced GNRH1 expression in human hypothalamic neurons derived from iPSCs after 4 hours of treatment, with levels returning to baseline 6 hours later (see Fig. 6C). Interestingly, when applied to iPSC-derived human hypothalamic spheroids/organoids, musk ambrette also stimulated GNRH1 expression (Supplementary Fig. S6C-S6D) (28). Molecular docking demonstrated that musk ambrette fits into the binding pocket of the KISS1R with a binding energy of −6.7 kcal/mol, as indicated by the hydrogen bonds between musk ambrette and the His309, Gln122, and Gln123 residues of the KISS1R (see Fig. 6D), akin to that reported for the endogenous ligand (kisspeptin-10) and a synthetic analogue (TAK-448) in recent cryoelectron microscopy studies (23).
Figure 6.
Musk ambrette is a candidate kisspeptin receptor (KISS1R) agonist. A, Gonadotropin-releasing hormone receptor (Gnrh1) expression levels (determined with quantitative reverse-transcription polymerase chain reaction [qRT-PCR]) in murine hypothalamic cells after various concentrations of musk ambrette treatment at 1, 2, 4, or 6 hours. B, Gnrh1 expression levels (determined with qRT-PCR) in murine hypothalamic mHypo-GnRH/GFP spheroids after various concentrations of musk ambrette at 1 hour. C, mHypo-GnRH/GFP spheroid morphology at 6 days pre treatment. There was no change in morphology after treatment (data not shown). Spheroids continued to appear as uniform, solid cell aggregates with a spherical shape and a diameter of approximately 200 to 250 μm. D, GNRH1 expression levels (determined with qRT-PCR) in induced pluripotent stem cells–derived human hypothalamic neurons after 25 μM musk ambrette treatment at 1, 2, 4, or 6 hours. E, Molecular docking predicts the potential binding sites between musk ambrette and KISS1R. All values represent the mean ± SD (n = 3 replicates). Statistical analysis was performed using a 2-way analysis of variance with Tukey multiple-comparison test, and the P value is shown in each graph.
Musk Ambrette Expands Gonadotropin-Releasing Hormone Receptor 3 Neuronal Area in Larval Zebrafish
Zebrafish (D rerio) are a tractable screening model for detecting toxicity and efficacy of small molecules due to the high conservation and physiological relevance of developmental characteristics and underlying pathways (27, 32). To complement our in vitro findings, we assessed the effect of musk ambrette in vivo by monitoring GnRH3 (a functionally relevant hypophysiotropic orthologue of human GNRH1; Supplementary Table S1 (28) and (26)) neuronal patterning as a proxy for early establishment of the zebrafish neuroreproductive axis. Musk ambrette treatment from 6 to 72 hpf has been shown previously in zebrafish to induce dose-dependent developmental anomalies at concentrations greater than or equal to 1 μg/mL (33). To avoid gross morphological abnormalities, we soaked embryos in compound starting from 24 hpf (Fig. 7A), a time point post-gastrulation that marks previously documented initiation of Gnrh3 transcript expression (26). Treatment of transgenic Gnrh3:gfp zebrafish (26) embryos with the lowest dose (0.1 μg/mL) did not induce any significant differences in the GnRH3 GFP-positive area compared to DMSO-treated control larvae when imaged at 5 dpf (Fig. 7B and 7C). Importantly, subeffective doses (0.3 μg/mL and 0.5 μg/mL) induced a modest but reproducible dose-dependent increase in the GnRH3 GFP-positive area (P vs DMSO: .0162 and .0003, respectively; see Fig. 7B and 7C). By contrast, the highest dose tested (1 μg/mL) resulted in significant expansion of the GnRH3 GFP-positive area compared to DMSO control (P vs DMSO, < .0001; see Fig. 7B and 7C). Notably, larvae treated with low-dose musk ambrette (0.1 μg/mL) were morphologically indistinguishable from DMSO controls. We observed no anatomical abnormalities in any other musk ambrette treatment group except for a dose-dependent developmental delay hallmarked by swim bladder formation defects at 5 dpf (Supplementary Fig. S7) (28), suggesting that the altered GnRH3-GFP area is likely specific to compound treatment.
Figure 7.
Musk ambrette treatment induces dose-dependent effects on GnRH3 neurons in developing zebrafish. A, Schematic of the experimental workflow. Embryos obtained from adult heterozygous gnrh3:gfp outcrossed with AB wild-type fish were treated with increasing doses of musk ambrette, starting at 1 day post fertilization (dpf) till 5 dpf (n = 15/well/treatment group). At 5 dpf, larvae were sorted for green fluorescent protein (GFP) signal and subjected to live GnRH3 fluorescence imaging or quantitative reverse-transcription polymerase chain reaction (qRT-PCR) based on the presence or absence of GFP signal, respectively. B, Representative dorsal fluorescent images of gnrh3:gfp larvae at 5 dpf. Anterior, left; posterior, right. Dotted lines indicate eyes and the most anterior region of the head. Scale bar shown in blue, 200 μm. C, Column scatter dot plots show quantification of the GnRH3 GFP-positive area in musk ambrette- or dimethyl sulfoxide (DMSO)-treated larvae. At least 2 independent biological replicates for each condition were normalized and pooled to represent percentage vs DMSO control in a single graph. Statistical differences were calculated using an unpaired t test (GraphPad); ns, not significant; error bars represent SD. N = number of larvae. D, Bar graph showing endogenous messenger RNA (mRNA) expression of genes of interest in 5 dpf zebrafish larval heads (human orthologues shown in Supplementary Table S1 (28); kiss2 is a zebrafish paralogue of kiss1 (34). Expression was normalized to eef1a1l1, and statistical differences were calculated using an unpaired t test (GraphPad); ns, not significant; error bars represent SD; n = 25 heads per batch, 3 technical replicates per condition, 3 biological replicates.
In addition to the GnRH neuronal area, we also assessed endogenous expression of Gnrh3 and other pathway-relevant genes (Kiss1, Kiss2, Kiss1rb, Jun, and Egr1) in larval heads of decapitated 5 dpf zebrafish (Fig. 7A; Supplementary Table S1) (28). We observed a modest increase in Gnrh3 and Kiss2 expression in musk ambrette–treated larvae compared to DMSO control (P, 1 μg/mL vs DMSO: .0156 and .0205, respectively; Fig. 7D). However, we did not detect any significant differences in Kiss1, Kiss1rb, Jun, and Egr1 expression in compound-treated larvae (see Fig. 7D). These data, especially Gnrh3 expression, are concordant with the GnRH3 neuronal expansion phenotype observed in musk ambrette–treated zebrafish larvae. Together, our in vivo data support findings from in vitro studies and highlight the potential for musk ambrette to activate the GnRHR and trigger earlier puberty.
Cholinergic Agonists Stimulate Gene Expression in the Pathway Downstream of the Gonadotropin-Releasing Hormone Receptor
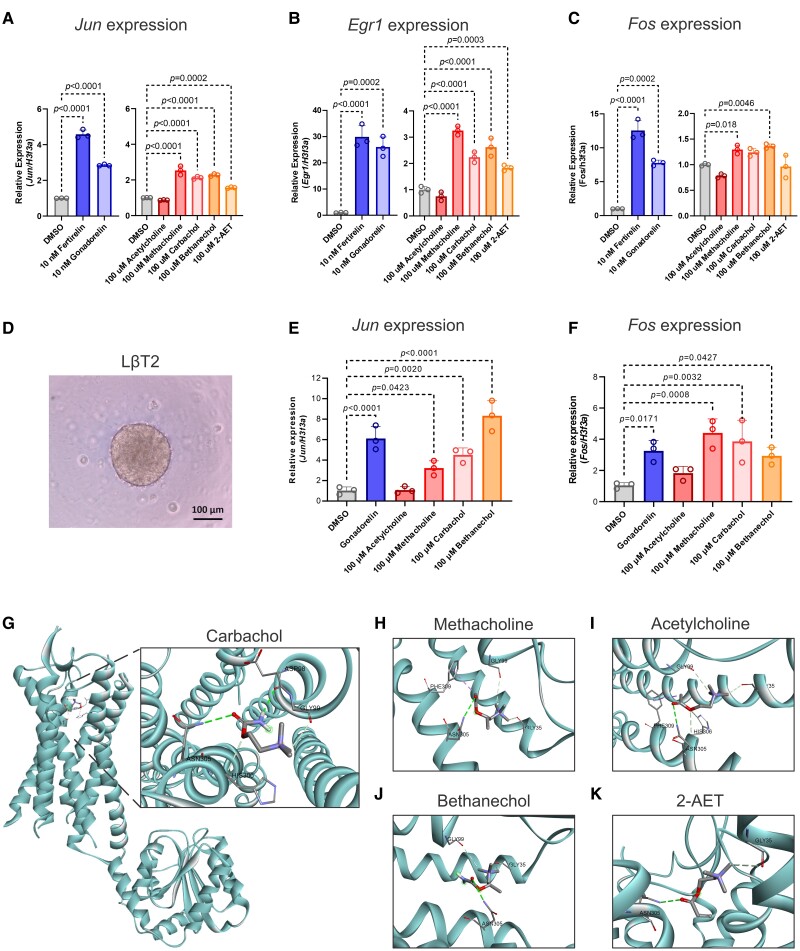
We also investigated the effect of 5 cholinergic receptor agonists, which demonstrated consistent agonist activity at the GnRHR. Here we used the LβT2 cell line, a murine gonadotrope cell line that expresses the Gnrhr (35), and measured changes in the expression of 3 genes that are downstream of the Gnrhr signaling pathway: Jun, Egr1, and Fos (36). With the exception of acetylcholine, the cholinergic receptor agonists significantly stimulated the expression of Jun and Egr1 after 2 hours of incubation but had little effect on the expression of Fos (Fig. 8A-8C and Supplementary Fig. S8A-S8E) (28). The same induction effect was also observed in the 3-dimensional LβT2 spheroids for Jun and Fos (Fig. 8D-8F). (Egr1 was not expressed in the spheroids in the presence of vehicle control nor gonadorelin.) Using molecular docking models, we found that all of these compounds could bind to the receptor pocket of GnRHR (22), and that Asn305, Gly99, and Gly35 are the most likely interaction residues (Fig. 8G-8I).
Figure 8.
Methacholine-like cholinergic agonists stimulated gene expression in the downstream pathway of the Gonadotropin-releasing hormone receptor (GnRHR). Expression levels (via quantitative reverse-transcription polymerase chain reaction [qRT-PCR]) of A, Jun; B, Egr1; or C, Fos in murine pituitary cells after compound treatment for 2 hours. D, Morphology of LβT2 3-dimensional spheroids at 4 days before treatment. There was no change in morphology after treatment (data not shown). Expression levels (via qRT-PCR) of E, Jun, or F, Fos in murine pituitary LβT2 spheroids after compound treatment for 2 hours. G, Molecular docking studies predict the potential binding between carbachol and GnRHR. Docking energy: −4.2 kcal/mol. H, Molecular docking studies predict the potential binding sites between methacholine and the GnRHR. Docking energy: −4.7 kcal/mol. I, Molecular docking studies predict the potential binding between acetylcholine and GnRHR. Docking energy: −4.3 kcal/mol. J, Molecular docking studies predict the potential binding between bethanechol and GnRHR. Docking energy: −4.6 kcal/mol. K, Molecular docking studies predict the potential binding between (2-(acryloyloxy)ethyl)trimethylammonium (2-AET) and GnRHR. Docking energy: −4.6 kcal/mol. All values represent the mean ± SD (n = 3 replicates). Statistical analysis was performed using a one-way analysis of variance with Tukey multiple-comparison test, and the P value is shown in each graph.
Discussion
There has been an alarming global trend toward earlier breast development in girls over the past decade, but an environmental trigger has yet to be convincingly identified. As puberty is controlled by the hypothalamus, we hypothesized that we could identify one or more causative environmental factors by screening the Tox21 10K compound library for GnRHR or KISS1R agonists. Indeed, by conducting a HTS and relying on several different readouts (eg, Ca2+ flux, p-ERK, gene transcription) as well as a transgenic Gnrh3:gfp zebrafish reporter line, we identified compounds that can bind to and activate one or both receptors.
HTS involves screening large compound libraries for biologically-pertinent activity using a diverse set of end points. Compared to traditional single-concentration HTS, titration-based qHTS is particularly useful in toxicological studies due to its ability to profile thousands of chemicals in a concentration-response format with lower false-positive and false-negative rates (37). In the present studies, we developed and used the Ca2+ flux assay to screen the Tox21 10K compound library for environmental chemicals that stimulate the GnRHR or KISS1R. We were careful to use HEK293-WT cells as a counter-screen to eliminate compounds that trigger Ca2+ flux independent of GnRHR or KISS1R activation, and this method demonstrated that some of the agonists were nonspecific. Nonspecific activation has been reported to occur because the overexpression of a given GPCR can influence signaling at other, unrelated GPCRs (38). We also performed confirmatory testing of potential agonists identified in the primary screen by performing a round of p-ERK detection assays, measuring changes in downstream gene expression, and conducting molecular docking simulations.
Notably, a large collection of long-chain detergent or surfactant component compounds, classified as QACs, was identified as active in the calcium flux assay in both receptor-overexpressed cell lines. However, the EC50 was greater than 10 μM, and these compounds were not identified as active in the p-ERK assay (because p-ERK activation also occurred in the treated HEK293-WT cells) (Supplementary Fig. S5B-S5E) (28). This pattern may reflect a disruption of ion channel homeostasis by QACs. In fact, QACs can disrupt the calcium pump in cellular membranes, such as in the ER, causing unregulated calcium release (39, 40). QACs have also been reported to block the human ether-a-go-go–related gene (hERG) channel, a member of a family of voltage-gated potassium channels, demonstrating their ability to modulate ion channel activity in the cell membrane (41). Alternatively, the screening results may indicate that QACs interact with the overexpressed receptor in a unique manner, which triggers calcium release from the ER but not downstream gene expression via p-ERK. According to the US Environmental Protection Agency's Chemistry Dashboard (42, 43), some QACs do alter GPCR binding to their ligand. Lastly, the screening results may represent a false-positive finding due to assay interference: Detergents such as QACs may increase the solubility of specific proteins in the assay system and the binding potential of antibodies to their target, resulting in a disproportionately strong signal in the fluorescence resonance energy transfer (FRET) assay, such as the HTRF assay (44).
Our study identified a group of cholinergic agonists—acetylcholine, methacholine, carbachol, bethanechol, and 2-(acryloyloxy)ethyl)trimethylammonium—with agonist activity at the GnRHR. It has been reported that acetylcholine can modulate GnRH secretion by activating its receptors on GnRH neurons (45-47). In this study, we showed an additional possibility that a group of cholinergic agonists can activate the GnRHR pathway by binding directly to GnRHR. Although the majority of acetylcholine analogues are prescription medications that may not be encountered frequently in the environment, our studies indicate a potential for adverse effects of this class of compounds in the event of overexposure. These data also suggest that other environmental compounds with a structure similar to this group of cholinergic agonists that are not included in the Tox21 10K compound library deserve further study.
We also identified musk ambrette as a novel KISS1R agonist that not only activated the receptor but also triggered expression of the downstream gene Gnrh1 in a murine hypothalamic cell line (as well as GNRH1 in human hypothalamic cells) or Gnrh3 in developing zebrafish larvae. In our study, musk ambrette exhibited very similar EC50 values in the Ca2+ flux assay in HEK293-KISS1R cells (16.71 μM) and Gnrh1 expression stimulation in murine hypothalamic cells (21.94 μM), whereas it exhibited a slightly higher EC50 in the p-ERK assay (55.86 μM). These data indicate that treatment with musk ambrette stimulates Gnrh1 expression quickly, akin to the rapid rise in luteinizing hormone levels in response to intravenous kisspeptin in humans (48-50). Importantly, continuous treatment of musk ambrette in developing zebrafish larvae from 1 to 5 dpf expanded the GnRH3-GFP area in the brain in a dose-dependent manner with a concomitant increase in endogenous Gnrh3 expression. Musk ambrette, a form of nitro-musk, is a common fragrance component of soap, detergent, creams/lotions, perfume, and even some beverages and foods (51). Because its consumption was associated with neurotoxicity in rats, the European Union discontinued the use of musk ambrette in personal care and cosmetic products in the 1990s (52, 53). Nonetheless, musk chemicals are still produced in some countries (eg, China and India) and found in noncosmetic compounds in the United States (53, 54). These compounds have also been detected in freshwater fish, river water, and wastewater, suggesting the possibility of oral exposure in children through food or water (55-58). Given the environmental persistence and continued use of nitro-musks, even at a reduced level, the potential for long-term exposure is a concern (53). Thus, it is conceivable that musk ambrette and/or related synthetic nitro-musks in the environment could be contributing to the trend of earlier puberty in girls via KISS1R activation. Although our in vitro and in vivo data do not offer direct evidence supporting an effect on puberty, they represent a first step toward linking musk ambrette to the activity of the neuroreproductive axis during development. This possibility deserves further study.
In this investigation, we used the Tox21 10K compound library to screen and identify potential agonists of the GnRH or KISS1Rs at 15 concentrations in 3 independent runs. This large library contains approximately 10 000 compounds including a combination of licensed pharmaceuticals and environmental compounds, allowing us to broadly investigate the potential of environmental compounds to activate the human GnRHR and/or KISS1R. We used primary screening and orthogonal assays to identify compounds that specifically activated the receptor using a receptor-overexpressing human cell line. This strategy yielded reliable hits for subsequent gene expression studies and docking model analyses. Some of the active compounds identified in our screening, however, may require further investigation. We used murine cell lines to measure downstream gene expression, and while the human and mouse receptors are highly homologous, there may be functional differences. For example, variations in gene expression, posttranslational modifications, and other regulatory mechanisms can result in differences in receptor function between species. Thus, confirmatory studies should include additional human cells, such as human iPSC-derived pituitary cells, as well as population exposure studies.
In conclusion, by using the Tox21 10K library and a qHTS method followed by confirmatory and secondary testing in relevant cell types (ie, mHypoA-GnRH/GFP, LβT2, and iPSC-derived hypothalamic neurons) and a zebrafish reporter line of GnRH neuronal development, we discovered novel GnRHR and KISS1R agonists that may, in part, contribute to the secular trend of early puberty in girls. Future epidemiological investigations of pubertal timing should pay particular attention to synthetic nitro-musks and to cholinergic agonists, as well as to other chemicals with similar structures.
Acknowledgments
We thank Abhinav Asthana and Glenn Gomba (NCATS) for their assistance with compound management. We also thank Jenna Miller (NCATS) for conducting Mycoplasma testing in our cells.
Abbreviations
- ARC
arcuate nucleus
- Ca2+
calcium ions
- DAG
sn-1,2 diacylglycerol
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethyl sulfoxide
- dpf
days post fertilization
- EC50
half maximum effective value
- ER
endoplasmic reticulum
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- GnRHR
gonadotropin-releasing hormone receptor
- GPCR
G protein–coupled receptor
- hpf
hours post fertilization
- HTRF
homogeneous time-resolved fluorescence
- IP3
inositol-1,4,5-triphosphate
- iPSCs
induced pluripotent stem cells
- KISS1R
kisspeptin receptor
- p-ERK
phosphorylated extracellularly regulated kinase
- QAC
quaternary ammonium compound
- qHTS
quantitative high-throughput screening
- qRT-PCR
quantitative reverse-transcription polymerase chain reaction
- S/B
signal-to-background ratio
- SOM
self-organized map
Contributor Information
Shu Yang, Division of Preclinical Innovation, National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD 20892, USA.
Li Zhang, Division of Preclinical Innovation, National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD 20892, USA.
Kamal Khan, Stanley Manne Children's Research Institute, Ann & Robert H. Lurie Children's Hospital of Chicago, Northwestern University, Chicago, IL 60611, USA.
Jameson Travers, Division of Preclinical Innovation, National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD 20892, USA.
Ruili Huang, Division of Preclinical Innovation, National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD 20892, USA.
Vukasin M Jovanovic, Division of Preclinical Innovation, National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD 20892, USA.
Rithvik Veeramachaneni, Division of Preclinical Innovation, National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD 20892, USA.
Srilatha Sakamuru, Division of Preclinical Innovation, National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD 20892, USA.
Carlos A Tristan, Division of Preclinical Innovation, National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD 20892, USA.
Erica E Davis, Stanley Manne Children's Research Institute, Ann & Robert H. Lurie Children's Hospital of Chicago, Northwestern University, Chicago, IL 60611, USA; Department of Pediatrics, Department of Cell and Developmental Biology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA.
Carleen Klumpp-Thomas, Division of Preclinical Innovation, National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD 20892, USA.
Kristine L Witt, Division of Translational Toxicology, National Institute of Environmental Health Sciences, Research Triangle Park, NC 27709, USA.
Anton Simeonov, Division of Preclinical Innovation, National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD 20892, USA.
Natalie D Shaw, Pediatric Neuroendocrinology Group, Clinical Research Branch, National Institute of Environmental Health Sciences, Research Triangle Park, NC 27709 USA.
Menghang Xia, Division of Preclinical Innovation, National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD 20892, USA.
Funding
This work was supported in part by the Interagency Agreement No. NTR 12003 between the National Institute of Environmental Health Sciences (NIEHS)/Division of Translational Toxicology (DTT) and the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), and by the Intramural Research Program of NIEHS/NIH (Z01-ES103315 to N.D.S.). N.D.S. is also supported as a Lasker Clinical Research Scholar (1SI2ES025429-01). The human iPSC lines LiPSC GR 1.1 and NCRM5 were obtained from the NIH Common Fund. E.E.D. is funded by US NIH R01-DE031452 and is the Ann Marie and Francis Klocke, M.D. Research Scholar. The views expressed in this paper are those of the authors and do not necessarily reflect the statements, opinions, views, conclusions, or policies of NIEHS and NCATS, NIH. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Disclosures
The authors have nothing to disclose.
Data Availability
Original data generated and analyzed during this study are included in this published article, the supplementary material listed in “References,” or are available from the corresponding authors on reasonable request. The qHTS screening data sets have been deposited into the PubChem database.
References
- 1. Herman-Giddens ME, Kaplowitz PB, Wasserman R. Navigating the recent articles on girls’ puberty in pediatrics: what do we know and where do we go from here? Pediatrics. 2004;113(4):911‐917. [DOI] [PubMed] [Google Scholar]
- 2. Rhie YJ. Kisspeptin/G protein-coupled receptor-54 system as an essential gatekeeper of pubertal development. Ann Pediatr Endocrinol Metab. 2013;18(2):55‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoo JH. Effects of early menarche on physical and psychosocial health problems in adolescent girls and adult women. Korean J Pediatr. 2016;59(9):355‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spaziani M, Tarantino C, Tahani N. et al. et al. Hypothalamo-Pituitary axis and puberty. Mol Cell Endocrinol. 2021;520:111094. [DOI] [PubMed] [Google Scholar]
- 5. Parent AS, Franssen D, Fudvoye J, Pinson A, Bourguignon JP. Current changes in pubertal timing: revised vision in relation with environmental factors including endocrine disruptors. Endocr Dev. 2016;29:174‐184. [DOI] [PubMed] [Google Scholar]
- 6. Fisher MM, Eugster EA. What is in our environment that effects puberty? Reprod Toxicol. 2014;44:7‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349‐11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Messager S, Chatzidaki EE, Ma D, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102(5):1761‐1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479‐3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu F, Usui I, Evans LG. et al. et al. Involvement of both G(q/11) and G(s) proteins in gonadotropin-releasing hormone receptor-mediated signaling in L beta T2 cells. J Biol Chem. 2002;277(35):32099‐32108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wahab F, Atika B, Shahab M, Behr R. Kisspeptin signalling in the physiology and pathophysiology of the urogenital system. Nat Rev Urol. 2016;13(1):21‐32. [DOI] [PubMed] [Google Scholar]
- 12. Richard AM, Huang R, Waidyanatha S, et al. The Tox21 10 K compound library: collaborative chemistry advancing toxicology. Chem Res Toxicol. 2021;34(2):189‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Y, Tristan CA, Chen L, et al. A versatile polypharmacology platform promotes cytoprotection and viability of human pluripotent and differentiated cells. Nat Methods. 2021;18(5):528‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang R. A quantitative high-throughput screening data analysis pipeline for activity profiling. Methods Mol Biol. 2016;1473:111‐122. [DOI] [PubMed] [Google Scholar]
- 15. Inglese J, Auld DS, Jadhav A. et al. et al. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci U S A. 2006;103(31):11473‐11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Y, Huang R. Correction of microplate data from high-throughput screening. Methods Mol Biol. 2016;1473:123‐134. [DOI] [PubMed] [Google Scholar]
- 17. Wang Y, Jadhav A, Southal N, Huang R, Nguyen DT. A grid algorithm for high throughput fitting of dose-response curve data. Curr Chem Genomics. 2010;4:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang R, Xia M, Cho MH, et al. Chemical genomics profiling of environmental chemical modulation of human nuclear receptors. Environ Health Perspect. 2011;119(8):1142‐1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang R, Sakamuru S, Martin MT, et al. Profiling of the Tox21 10 K compound library for agonists and antagonists of the estrogen receptor alpha signaling pathway. Sci Rep. 2014;4:5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Attene-Ramos MS, Miller N, Huang R, et al. The Tox21 robotic platform for the assessment of environmental chemicals--from vision to reality. Drug Discov Today. 2013;18(15–16):716‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kohonen T. Self-organizing neural projections. Neural Netw. 2006;19(6–7):723‐733. [DOI] [PubMed] [Google Scholar]
- 22. Yan W, Cheng L, Wang W, et al. Structure of the human gonadotropin-releasing hormone receptor GnRH1R reveals an unusual ligand binding mode. Nat Commun. 2020;11(1):5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shen S, Wang D, Liu H. et al. et al. Structural basis for hormone recognition and distinctive Gq protein coupling by the kisspeptin receptor. Cell Rep. 2024;43(7):114389. [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Yang X, Gan J, Chen S, Xiao ZX, Cao Y. CB-Dock2: improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022;50(W1):W159‐W164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang X, Liu Y, Gan J, Xiao ZX, Cao Y. FitDock: protein-ligand docking by template fitting. Brief Bioinform. 2022;23(3):bbac087. [DOI] [PubMed] [Google Scholar]
- 26. Abraham E, Palevitch O, Gothilf Y, Zohar Y. The zebrafish as a model system for forebrain GnRH neuronal development. Gen Comp Endocrinol. 2009;164(2–3):151‐160. [DOI] [PubMed] [Google Scholar]
- 27. Lippincott MF, Xu W, Smith AA, et al. The p190 RhoGAPs, ARHGAP35, and ARHGAP5 are implicated in GnRH neuronal development: evidence from patients with idiopathic hypogonadotropic hypogonadism, zebrafish, and in vitro GAP activity assay. Genet Med. 2022;24(12):2501‐2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang S. 2024, Supplementary files for “Identification of environmental compounds that may trigger early female puberty by activating human GnRHR and KISS1R”. Harvard Dataverse, V1. 2024. Doi: 10.7910/DVN/QYPXUH [DOI] [PMC free article] [PubMed]
- 29. Gojska NM, Friedman Z, Belsham DD. Direct regulation of gonadotrophin-releasing hormone (GnRH) transcription by RF-amide-related peptide-3 and kisspeptin in a novel GnRH-secreting cell line, mHypoA-GnRH/GFP. J Neuroendocrinol. 2014;26(12):888‐897. [DOI] [PubMed] [Google Scholar]
- 30. Ohtaki T, Shintani Y, Honda S, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411(6837):613‐617. [DOI] [PubMed] [Google Scholar]
- 31. Novaira HJ, Ng Y, Wolfe A, Radovick S. Kisspeptin increases GnRH mRNA expression and secretion in GnRH secreting neuronal cell lines. Mol Cell Endocrinol. 2009;311(1–2):126‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patton EE, Zon LI, Langenau DM. Zebrafish disease models in drug discovery: from preclinical modelling to clinical trials. Nat Rev Drug Discov. 2021;20(8):611‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qu L, Zhao C, Wang C. et al. et al. A novel zebrafish (danio rerio) assay for assessing musk ambrette-induced toxicity. Bull Environ Contam Toxicol. 2018;101(1):80‐85. [DOI] [PubMed] [Google Scholar]
- 34. Zhao Y, Lin MC, Mock A, Yang M, Wayne NL. Kisspeptins modulate the biology of multiple populations of gonadotropin-releasing hormone neurons during embryogenesis and adulthood in zebrafish (Danio rerio). PLoS One. 2014;9(8):e104330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomas P, Mellon PL, Turgeon J, Waring DW. The L beta T2 clonal gonadotrope: a model for single cell studies of endocrine cell secretion. Endocrinology. 1996;137(7):2979‐2989. [DOI] [PubMed] [Google Scholar]
- 36. Ruf-Zamojski F, Fribourg M, Ge Y, et al. Regulatory architecture of the LbetaT2 gonadotrope cell underlying the response to gonadotropin-releasing hormone. Front Endocrinol (Lausanne). 2018;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Attene-Ramos MS, Austin CP, Xia M. High throughput screening. In: Wexler P, ed. Encyclopedia of Toxicology. 3rd ed. Academic Press; 2014:916‐917. [Google Scholar]
- 38. Tubio MR, Fernandez N, Fitzsimons CP. et al. et al. Expression of a G protein-coupled receptor (GPCR) leads to attenuation of signaling by other GPCRs: experimental evidence for a spontaneous GPCR constitutive inactive form. J Biol Chem. 2010;285(20):14990‐14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Herron J, Reese RC, Tallman KA, Narayanaswamy R, Porter NA, Xu L. Identification of environmental quaternary ammonium compounds as direct inhibitors of cholesterol biosynthesis. Toxicol Sci. 2016;151(2):261‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Inacio AS, Costa GN, Domingues NS. et al. et al. Mitochondrial dysfunction is the focus of quaternary ammonium surfactant toxicity to mammalian epithelial cells. Antimicrob Agents Chemother. 2013;57(6):2631‐2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xia M, Shahane SA, Huang R, et al. Identification of quaternary ammonium compounds as potent inhibitors of hERG potassium channels. Toxicol Appl Pharmacol. 2011;252(3):250‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Williams AJ, Grulke CM, Edwards J, et al. The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. J Cheminform. 2017;9(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arnold WA, Blum A, Branyan J, et al. Quaternary ammonium compounds: a chemical class of emerging concern. Environ Sci Technol. 2023;57(20):7645‐7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cho YK, Shusta EV. Antibody library screens using detergent-solubilized mammalian cell lysates as antigen sources. Protein Eng Des Sel. 2010;23(7):567‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shostak DM, Constantin S, Flannery J, Wray S. Acetylcholine regulation of GnRH neuronal activity: a circuit in the medial septum. Front Endocrinol (Lausanne). 2023;14:1147554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abreu AP, Kaiser UB. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016;4(3):254‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Spergel DJ. Modulation of gonadotropin-releasing hormone neuron activity and secretion in mice by non-peptide neurotransmitters, gasotransmitters, and gliotransmitters. Front Endocrinol (Lausanne). 2019;10:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jayasena CN, Nijher GM, Comninos AN, et al. The effects of kisspeptin-10 on reproductive hormone release show sexual dimorphism in humans. J Clin Endocrinol Metab. 2011;96(12):E1963‐E1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. George JT, Veldhuis JD, Roseweir AK. et al. et al. Kisspeptin-10 is a potent stimulator of LH and increases pulse frequency in men. J Clin Endocrinol Metab. 2011;96(8):E1228‐E1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chan YM, Butler JP, Pinnell NE. et al. et al. Kisspeptin resets the hypothalamic GnRH clock in men. J Clin Endocrinol Metab. 2011;96(6):E908‐E915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Musk ambrette and musk xylene. IARC Monogr Eval Carcinog Risks Hum. 1996;65:477‐495. [PMC free article] [PubMed] [Google Scholar]
- 52. Spencer PS, Bischoff-Fenton MC, Moreno OM, Opdyke DL, Ford RA. Neurotoxic properties of musk ambrette. Toxicol Appl Pharmacol. 1984;75(3):571‐575. [DOI] [PubMed] [Google Scholar]
- 53. Taylor KM, Weisskopf M, Shine J. Human exposure to nitro musks and the evaluation of their potential toxicity: an overview. Environ Health. 2014;13(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chisvert A, López-Nogueroles M, Miralles P, Salvador A. Chapter 10—perfumes in cosmetics: regulatory aspects and analytical methods. In: Salvador A, Chisvert A, eds. Analysis of Cosmetic Products. 2nd ed. Elsevier; 2018:225‐248. [Google Scholar]
- 55. Zhang X, Xu Q, Man S. et al. et al. Tissue concentrations, bioaccumulation, and biomagnification of synthetic musks in freshwater fish from Taihu Lake, China. Environ Sci Pollut Res Int. 2013;20(1):311‐322. [DOI] [PubMed] [Google Scholar]
- 56. Rimkus GG, Wolf M. Nitro musk fragrances in biota from freshwater and marine environment. Chemosphere. 1995;30(4):641‐651. [Google Scholar]
- 57. Peck AM, Hornbuckle KC. Synthetic musk fragrances in lake Michigan. Environ Sci Technol. 2004;38(2):367‐372. [DOI] [PubMed] [Google Scholar]
- 58. Hajkova K, Pulkrabova J, Hajslova J, Randak T, Zlabek V. Chub (Leuciscus cephalus) as a bioindicator of contamination of the Vltava River by synthetic musk fragrances. Arch Environ Contam Toxicol. 2007;53(3):390‐396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Yang S. 2024, Supplementary files for “Identification of environmental compounds that may trigger early female puberty by activating human GnRHR and KISS1R”. Harvard Dataverse, V1. 2024. Doi: 10.7910/DVN/QYPXUH [DOI] [PMC free article] [PubMed]
Data Availability Statement
Original data generated and analyzed during this study are included in this published article, the supplementary material listed in “References,” or are available from the corresponding authors on reasonable request. The qHTS screening data sets have been deposited into the PubChem database.