Graphical Abstract
Keywords: Grass carp, Postmortem, Muscle degradation, Flavor evolution, Structural disruption, Correlation analysis
Highlights
-
•
Analyzed the muscle quality changes during postmortem chilled and ambient storage.
-
•
The degradation of muscular lipid, protein and ATP promoted its integrity disruption.
-
•
Freshness could be effectively characterized by VOCs profile and electronic sensors.
-
•
The correlation between muscle degradation and flavor evolution was established.
-
•
The mechanisms underlying the changes in postmortem flesh quality were elucidated.
Abstract.
This study investigated the dynamic changes in muscle degradation and flavor evolution of grass carp during postmortem refrigerated and ambient storage, exploring their interconnections. The results revealed gradual degradation in muscular chemical compositions, including lipid, protein, and ATP, alongside varying deterioration in associated biochemical indices during postmortem storage, leading to disrupted muscle integrity. SPME-GC–MS analysis unveiled that carbonyls and alcohols predominantly constituted the volatile compounds in postmortem muscle. E-nose analysis displayed intense responses to nitrogen oxides, broad-alcohols, aldehydes, and ketones, but weak responses to aroma components. E-tongue analysis manifested heightened bitterness and astringency, alongside reduced umami. Correlation analyses and mechanistic explorations revealed a strong association between muscle degradation and flavor evolution, jointly contributing to declining flesh quality. Overall, this quality analysis aided in comprehending the postmortem muscle changes and provided supports for improving the preservation techniques and quality control of freshwater fish.
1. Introduction
China is the world's largest producer and consumer of freshwater fish, with an annual output of nearly 30 million tons (Yi, An, Liu, Hu, & Xiong, 2023). Freshwater fish are renowned for their cost-effectiveness and high nutritional value, especially for their rich protein and polyunsaturated fatty acids (PUFAs) content. Among these species, grass carp (Ctenopharyngodon idellus) stands out for its highest cultivated yield in China (Tie et al., 2021). Traditionally, these fish are sold whole to consumers. However, with the accelerating development of cryogenic technology, changes in retail and agricultural practices, and evolving consumer preferences, there has been a growing demand for processing these fish into smaller, more convenient products like fillets and steaks (Liu, Liang, Xia, Regenstein, & Zhou, 2013). Inevitably, the quality of fish meat degrades over time, which may potentially affect consumer acceptance. Consequently, research into the dynamic quality changes in fish flesh during postmortem storage is essential for accurately assessing their economic value.
Raw fish, in comparison to other meat products, is more susceptible to oxidation and deterioration during postmortem storage and processing (Chu, Mei, & Xie, 2023a). This susceptibility arises from its high nutrient and moisture content, as well as the presence of numerous endogenous enzymes with high activity (Sheng et al., 2024). After slaughter, physical, chemical, and microbial changes occur within the fish muscle, causing it to soften. Macromolecules such as protein, lipid, and nucleotide are progressively degraded into low molecular compounds like free amino acids (FAAs), free fatty acids (FFAs), and 5′-nucleotide compounds. During these degradation processes, flavor is produced and evolves. Generally, flavor formation is quite complex, focusing mainly on odor and taste. Odor primarily consists of volatile compounds (VOCs) including aldehydes, ketones, alcohols, esters, hydrocarbons, and acids, while taste is composed of non-VOCs such as FAAs, nucleotides, and organic acids (Liu, You, Zhu, & Shi, 2024). Proteins in the proximate compositions of fish serve as precursors for taste FAAs. Additionally, some enzymes participate in the formation of nucleotides like adenosine monophosphate (AMP) and inosine monophosphate (IMP), which along with FAAs can contribute to umami (Chu, Ding, Wang, Xie, & Ding, 2023). Lipids in the proximate compositions of fish are precursors for fatty acids (FAs), which in turn are precursors for VOCs (Wang, Xie, Zhang, Xu, & Yang, 2022). Furthermore, the deterioration of flesh quality in fish is linked to disruptions in muscle integrity (Tie et al., 2022). Research into the microstructural features and evolution of postmortem fish muscle is crucial for elucidating the quality changes involved. However, to our knowledge, the specific correlation between component decomposition, microstructure transformation, and flavor development of fish muscle during postmortem aging and storage remains unclear. Particularly, up to now, the exact mechanisms underlying quality changes in postmortem fish flesh have not been fully elucidated.
Recently, increasing studies have been conducted to investigate the impact of postmortem time on the quality of terrestrial animal flesh (Dou et al., 2022; Zhang et al., 2013; Zou et al., 2022). Dou et al. (2022) assessed the effect of postmortem aging on the oxidative stability, meat quality, and flavor of lamb muscle. Zhang et al. (2013) explored the correlation between the postmortem apoptosis process and meat quality traits such as color, water holding capacity, and tenderness in duck skeletal muscle. In aquatic product studies, Tie et al. (2022) investigated the effect of postmortem time on grass carp muscle integrity via alterations in tight junction protein gene expressions and antioxidant capacity. Given the considerable cultivated yield and economic value of grass carp, it is crucial to dynamically evaluate its postmortem quality. The present study, therefore, aimed to elicit the dynamic shifts in postmortem quality of grass carp from the perspectives of muscle degradation and flavor evolution, and to explore the interconnections therein. The insights gained are expected to provide theoretical guidance for understanding flesh quality deterioration and for improving preservation processes and quality control in fish products.
2. Materials and methods
2.1. Samples collection
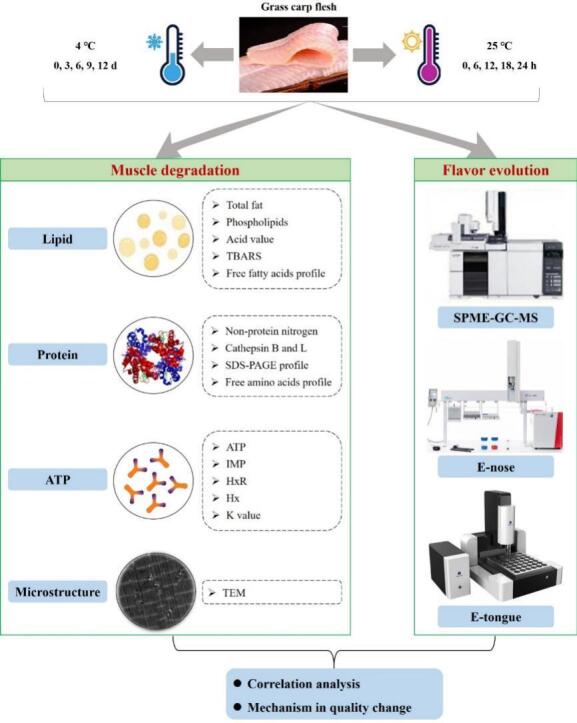
Alive grass carp (Ctenopharyngodon idellus) was procured from a local market (Changsha, Hunan province, China). The fishes, 2.50 ± 0.20 kg mean weight, were rested in oxygen-dissolved water tanks for 2 h. In the slaughtered lab, trained personnel administered a wooden hammer to stun the live grass carp into unconsciousness immediately, following the guidelines set forth by the World Organization for Animal Health (2015). Afterward, the head, guts, skin, and bones were quickly removed, and muscle tissue was collected and rinsed with cold sterile water (4 °C). The fish muscle was manually dissected into approximately 30 g fillets (2.0 cm × 3.0 cm × 2.5 cm) from the dorsal region of each individual. Subsequently, the fillets were packed in sterilized polyethylene bags and vacuum-sealed using a vacuum sealer (MJ-CF07X7–101, Midea, China). The sealed fillets were then individually stored in 4 °C refrigerator (BCD-180LLC2E0C9, Haier, China) and 25 °C incubator (LC-SPX-250B, lichen, China). Fish samples were taken on the day of processing (0 d/h), as well as at intervals of 3, 6, 9, 12 days post-refrigeration and 6, 12, 18, 24 h under ambient temperature (AT) conditions for subsequent analysis.
2.2. Determination of lipid related properties
2.2.1. Total fat, acid value (AV), and thiobarbituric acid reactive substances (TBARS)
In the analysis of fish muscle collected at various postmortem times, both total fat content and AV were evaluated in accordance with the Chinese National Food Standard (GB 9695.7–2008 and GB 5009. 37–2003, respectively). TBARS was assessed according to the literature (Yarnpakdee, Benjakul, Nalinanon, & Kristinsson, 2012), utilizing 0–2 ppm of malonaldehyde bis (dimethyl acetal) as the standard. The absorbance of the chromophore was measured at 532 nm, and the results were expressed as mg malonaldehyde (MDA)/kg muscle.
2.2.2. Phospholipid content
Phospholipids were extracted from fish muscle according to Yarnpakdee et al. (2012) with minor modifications. Briefly, 30 g of minced muscle samples were individually extracted with 300 mL of chloroform/methanol (2:1, v/v, containing 0.2 mg/mL butyl hydroxyl toluene) at 20 °C under ultrasonic treatment. Following extraction and filtration, the organic solvent was evaporated at 45 °C. Then, the resulting concentrated lipid extract was mixed with ten times its volume of cold acetone. The acetone-soluble supernatant was filtered off, while the insoluble phospholipid precipitate was repeatedly washed with cold acetone until the acetone layer became colorless. Afterwards, the phospholipid content was spectrophotometrically detected according to the AOCS standard method (AOCS, 2009).
2.2.3. FFAs composition
In FFAs determination, lipid was extracted following the method outlined by Wu, Forghani, Abdollahi, and Undeland (2022). About 10 g of each specimen was individually extracted with the solvent of an isooctane:methanol:HCl (1 N) mixture (60:20:1, v/v/v) using an ultrasonic treatment at 20 °C. Following extraction and centrifugation, the organic phase present in the under layer was collected and evaporated at 25 °C to yield the FFAs extraction solution. The FFAs extracts were then treated with methyl esters and analyzed via gas chromatography–mass spectrometry (GCMS-QP2010 Ultra, Shimadzu), as previously described (Chu, Mei, & Xie, 2023a). All detected concentrations of FFAs were quantified using external standard methods.
2.3. Determination of protein related properties
2.3.1. Non-protein nitrogen (NPN) content
The NPN (%) was determined according to Xiao et al. (2024) with slight modifications. A portion of ground muscle (2 g) was homogenized in 10 mL of 5% trichloroacetic acid (TCA). The homogenate was shocked for 1 h then centrifuged at 10,000 g for 10 min at 4 °C. The supernatant was collected, and the precipitate was re-extracted. Subsequently, the supernatants obtained twice were merged, filtered, and adjusted to 50 mL with 5% TCA. The NPN content was detected by the micro Kjeldahl method.
2.3.2. Cathepsin (B and L) activities assay
The crude cathepsin solution was extracted according to Li et al. (2022) with few modifications. In brief, ground fish samples weighing 2 g were mixed with 20 mL PBS buffer solution (0.1 M, pH 7.2). The mixture was homogenized and stirred in an ice water bath for 30 min. Subsequently, the solution was centrifuged at 10,000 g for 20 min at 4 °C, and the supernatant was collected as the crude enzyme extract. The enzymatic activities of both cathepsin B and L were then detected using commercial enzyme-linked immunosorbent assay kits (Shanghai Enzyme-linked Biotechnology, Shanghai, China).
2.3.3. Protein electrophoresis
Changes in protein patterns of myofibrillar and sarcoplasmic proteins from fish muscle during refrigeration at day 0, 6 and 12 were analyzed using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). These two protein fractions were prepared according to Zhao, Benjakul, and Eun (2019), and their concentrations were measured using the Lowry method. Each protein fraction was mixed with 2 × Laemmli loading buffer containing 2 mM β-mercaptoethanol, and then boiled in a water bath for 5 min. The electrophoretic analysis was performed using a 4% stacking gel and a 12% separating gel at a constant current of 23 mA. After electrophoresis, the protein bands were stained with 0.5 g/L Coomassie Brilliant Blue R-250 for 2–3 h, and then decolorized with a solution of 25% ethanol and 10% acetic acid.
2.3.4. FAAs composition
FAAs were extracted from fish samples following the procedure described by Li et al. (2022) with slight modifications. The minced fish sample was homogenized with 0.01 N HCl and left to stand for 30 min. The extract was then adjusted to 10 mL, added with an equal volume of 8% (w/v) sulfosalicylic acid solution, and kept at 4 °C overnight. Afterwards, the samples were centrifuged at 13,000 g for 10 min at 4 °C, and the supernatants were filtered through 0.22 μm microporous filters. FAAs were measured using an automatic amino acid analyzer (L-8900, Hitachi, Japan). The identification and quantification of FAAs were performed on the basis of the retention time and peak area of standard amino acids.
2.4. Determination of adenosine triphosphate (ATP)-related compounds
ATP derivative compounds, including ATP, inosine monophosphate (IMP), hypoxanthine riboside (HxR), and hypoxanthine (Hx), were analyzed using an HPLC system (Waters 2695, Milford, USA) equipped with an XDB C18 column (250 × 4.6 mm, 5 μm). The sample pretreatment and HPLC operation were carried out as previously described (Pongsetkul, Yongsawatdigul, Boonanuntanasarn, & Benjakul, 2022). A 2 g sample was combined with 20 mL of perchloric acid (10%, v/v), homogenized, and centrifuged at 8000 g and 4 °C for 10 min to obtain the supernatant. The pellet underwent re-extraction using the same procedure. All supernatants were amalgamated and diluted to 50 mL with 10% perchloric acid. This mixture was neutralized with NaOH and centrifuged again as described. The resulting supernatants were filtered through a 0.22 μm Millipore filter for HPLC analysis under the following conditions: mobile phase consisting of 20 mM KH2PO4–20 mM K2HPO4 (1:1, v/v), flow rate set at 1.0 mL/min, column temperature ranging from 35 to 85 °C, and detection wavelength set at 254 nm. Identification of ATP-related compounds was performed by comparing their retention times with those of commercial standards. The measurement of the K value was conducted following the modified procedure proposed by Liu, Mei, and Xie (2021).
2.5. Transmission electron microscope (TEM) analysis for structural integrity
Ultrastructural analysis was performed using a TEM according to the protocol described by Gherbawy, Thabet, and Sultan (2023). Fish muscle was sliced into tiny slices (5 mm × 3 mm × 3 mm) and fixed in 2.5% glutaraldehyde in PBS buffer (0.1 M, pH 7.4) for 2 h at 4 °C. The slices were postfixed in 2% osmium tetroxide buffered with PBS for 1 h at 4 °C. The slices were dehydrated in an ascending series of acetone and then soaked and embedded in Epon. Ultrathin sections were prepared utilizing a Leica Ultra Cut microtome (UCT), achieving an approximate thickness of 75–90 μm. Subsequently, these thin sections underwent staining processes with uranyl acetate and lead nitrate. A JEM-1400 TEM (JEOL Optical, Tokyo, Japan) was adopted for scanning observation and electron micrograph.
2.6. Determination of flavor evolution
2.6.1. VOCs analysis
The VOCs analysis was performed by solid-phase microextraction gas chromatography–mass spectrometry (SPME-GC–MS) following the previous method (Liu, Shen, Xiao, Jiang, & Shi, 2022). The minced muscle (2.5 g) was homogenized with 2.5 mL of 0.18 g/mL NaCl and transferred into a glass vial. Samples were then heated to 45 °C for 60 min before the VOCs were adsorbed onto an SPME fiber at 45 °C for another 30 min. The SPME fiber adopted was 65 μm of divinylbenzene/ polydimethylsiloxane (Supelco, Inc., Bellefonte, PA, USA). The VOCs were then desorbed in a thermal desorption unit and injected into a GC–MS (QP2010 Ultra, Shimadzu). The extract's compounds were segregated on a 30 m × 0.25 mm × 0.25 μm fused silica DB-5 ms capillary column (Agilent). The GC oven temperature program commenced at 45 °C for 3 min, escalated to 250 °C at a rate of 10 °C/min, and then held for 8 min. Helium was served as the carrier gas at a flow rate of 0.95 mL/min in non-split mode. The quadrupole mass spectrometer was operated in electron impact mode, with the anion source temperature maintained at 200 °C and the electron energy set at 70 eV. The spectral acquisition spanned from m/z 10 to 350 amu. Volatile compounds were identified utilizing NIST 11 database search, and their quantities were determined based on the content of internal standards (IS).
2.6.2. Electronic nose (E-nose) analysis
The PEN3 portable E-nose (Airsense, Schwerin, Germany) was employed to assess the overall odor characteristics conforming to the method of Limbo, Sinelli, Torri, and Riva (2009) with certain modifications. The sensor array incorporates 10 metal oxide sensors, each with sensitivities and detection limits detailed in Table S1. For the analysis, 10 g of minced fish muscle was encapsulated in a specialized headspace bottle and allowed to equilibrate at ambient temperature for 30 min. The E-nose parameters for sensors were configured as follows: chamber and injection flow rate of 300 mL/min, sample analysis time of 90 s, sample preparation time of 5 s, automatic zero adjustment time of 10 s, and sensor self-cleaning time of 100 s.
2.6.3. Electronic tongue (E-tongue) analysis
The taste attributes of fish muscle were evaluated using an electronic tongue system, following the methodology outlined by Li et al. (2023) with slight modifications. The minced muscle was homogenized with ultrapure water at a ratio of 1:5. The resulting homogenate underwent centrifugation at 3000 g and 4 °C for 10 min to yield the supernatant. Following filtration, the resultant filtrate was promptly analyzed using a TS-5000Z taste sensor system (Insent, Japan). This system was configured with an array of five different taste sensors capable of evaluating both initial and after taste (Table S2). The relative potentials acquired from AAE (umami), CT0 (saltiness), CA0 (sourness), C00 (bitterness), and AE1 (astringency) sensor probes were employed to assess selective initial tastes. The CPA (change in membrane potential due to adsorption) values derived from AAE (umami richness), C00 (bitterness), and AE1 (astringency) sensor probes were utilized to ascertain selective aftertastes. The variances in the intensity of each taste attribute were estimated according to Weber's law from the average of three parallel measurements. The results were represented as comparison values for each sensor.
2.7. Statistical analysis
All assays were performed in triplicate. Statistical analysis was conducted using SPSS 26.0 software (Chicago, IL, USA). Data were presented as means ± standard deviation (SD) and subjected to an analysis of variance (ANOVA). The least significant difference (LSD) procedure was applied to the comparison of means, with a significance level of p < 0.05. Heatmap construction and correlation analysis were performed via MetaboAnalyst 5.0.
3. Results and discussion
3.1. TEM observation
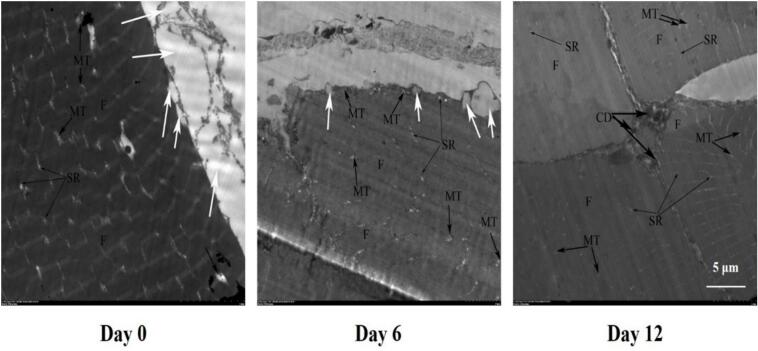
According to Fig. 1, at day 0, the muscle structure was clear with easily distinguishable sarcomeres and transverse striaes. Mild edema was observed in both the sarcoplasmic reticulum and mitochondria. Accumulated lipid droplets were found beneath the myofibrillar membrane with signs of efflux, while no lipid droplets were present between myofibrils. By the 6th day, the muscle structure turned blurred and disrupted, with numerous myofibers breaking down and dissolving. The presence of sarcomeres and transverse striaes had vanished, myofibrils showed loss of contour and watery changes, and partial swelling was observed in the sarcoplasmic reticulum and mitochondria. A decrease in the number of lipid droplets beneath the myofibrillar membrane, corresponding to the forthcoming demonstration of reduced total fat content, suggested ongoing lipid degradation. On the 12th day, it seemed that the enzymatic autolysis of myofibrils had become more severe. Both the sarcoplasmic reticulum and mitochondria were significantly swollen and vacuolated, accompanied by watery degeneration and an abundance of cell fragments between myofibers. The sarcolemma was destroyed, leading to the complete disappearance of submembrane lipid droplets. Collectively, these microstructural transformations implied that muscle integrity progressively disrupted with increasing postmortem refrigeration time.
Fig. 1.
The disruption of structural integrity observed by TEM photographs in grass carp muscle during postmortem refrigeration storage (scale bar = 5 μm). White arrow, lipid drops; SR, sarcoplasmic reticulum; MT, mitochondria; F, myofibrils; CD, cell debris.
3.2. Lipid degradation analysis
3.2.1. Total fat and phospholipids contents
From Fig. 2 A and 2B, the total fat content of fish muscle exhibited overall downward trends as postmortem time increased, regardless of whether the storage conditions were refrigerated or at AT. Following storage at 4 °C for 12 d and at 25 °C for 24 h, the total fat levels decreased by 38.25% and 29.70%, respectively. Phospholipids are crucial constituents of fish. Due to the abundant unsaturated fatty acids (UFAs) in the molecules, phospholipids are sensitive to oxidation during postmortem storage, leading to reduced nutritional value and increased generation of toxic and harmful components in the flesh. Herein, significant reductions in phospholipid levels were observed during the initial 9 days of refrigeration and the first 18 h of AT storage, before eventually stabilizing until the end of storage. Tracing it to its cause, there were sufficient substrates such as water and oxygen in the flesh matrix during the early postmortem phase, which facilitated rapid oxidation of phospholipids (Yarnpakdee et al., 2012). However, as the aging stage commenced, both water and oxygen were depleted, making it less conducive to oxidation. As such, lipids in postmortem grass carp muscle underwent lipolysis and oxidation during storage.
Fig. 2.
Changes in the total fat and phospholipids (A, B), AV and TBARS (C, D), and proportion of fatty acids category (E) of grass carp muscle; and Heatmap visualization of individual FFA (F) in grass carp muscle during postmortem refrigerated and ambient storage. Different letters indicate significant differences (p < 0.05). AV, acid value; TBARS, thiobarbituric acid reactive substance; FFA, free fatty acid; PUFA, polyunsaturated fatty acid; MUFA, monounsaturated fatty acid; SFA, saturated fatty acid.
3.2.2. AV and TBARS
AV serves as a significant indicator of lipolysis degree, while TBARS signifies secondary lipid oxidation products that arise from hydroperoxide decomposition. As depicted in Fig. 2C and 2D, AV demonstrated upward trends throughout postmortem storage. At the end of storage, the AV values for samples were 3.09 (4 °C) and 5.07 (25 °C) times greater than those of fresh flesh, respectively. Regarding TBARS, minor alterations were noted within the initial six days for the postmortem refrigeration group, followed by distinct increments, with values reaching 0.62 mg MDA/kg on day 12. By contrast, the TBARS values surged significantly from 0.15 to 0.54 mg MDA/kg within 24 h of postmortem AT storage. Accordingly, the results suggested AV and TBARS values in postmortem muscle exhibited more pronounced variations with increasing storage temperature, indicative of heightened levels of lipid hydrolysis and oxidation. Notably, the rates of lipid oxidation in postmortem muscle diminished after 9 days of refrigeration and 12 h of AT storage. It can be speculated that these two time points are critical turning points for the freshness changes in grass carp fillets.
3.2.3. FFAs profile
The results of total fat, phospholipids, AV and TBARS analyses fully indicated that the lipids in postmortem muscle were prone to lipolysis and oxidation during refrigerated and ambient storage, which had great influence on the quality of grass carp products. Importantly, as the lipolysis products, FFAs are more sensitive to oxidation and further promote protein denaturation and VOCs formation, thereby accelerating the spoilage process of postmortem fish flesh (Wu et al., 2022). As delineated in Table S3 and Fig. 2F, a total of 24 FFAs were detected. Specifically, saturated fatty acids (SFAs) predominantly comprised C16:0 and C18:0, monounsaturated fatty acids (MUFAs) chiefly included C18:1 and C16:1, and PUFAs primarily contained C18:2 and C20:4. As the duration postmortem elapsed, the relative contents of PUFAs decreased while SFAs increased gradually, while the relative contents of MUFAs changed insignificantly (Fig. 2E). Concerning PUFAs, their heightened unsaturation rendered them more susceptible to oxidation, resulting in higher oxidation rates than generation rates (Chu, Mei, & Xie, 2023a); conversely, for MUFAs, given that lipolysis was ongoing and MUFAs were continuously released, their relative levels remained roughly balanced. Consequently, the relative proportions of SFAs escalated. Notably, among PUFAs, the relative contents of n-3 PUFAs were obviously lower than those of n-6 PUFAs. Regardless of storage conditions, the reduction in n-3 PUFAs occurred earlier, while the decline in n-6 PUFAs intensified during the later stages of postmortem storage (Fig. 2E).
In comparison with fresh fish, the relative contents of total PUFAs decreased by 43.86% (4 °C) and 52.87% (25 °C) at the end of storage, respectively. Accordingly, lower temperature was more conducive to retarding PUFAs oxidation in postmortem flesh. Among PUFAs, linoleic acid (C18:2) and arachidonic acid (C20:4) were the most prominent UFAs that comprised 78.33% of the total PUFA level in fresh fish. These two acids were mainly responsible for the changes in total PUFAs during both refrigerated and ambient postmortem storage. Likewise, palmitic acid (C16:0) was the first major component among SFAs, accounting for 74.86% (4 °C) and 75.04% (25 °C) of the total SFA contents at the end of storage, and its variation amplitudes largely influenced the changes in total SFAs. Given these findings, linoleic, arachidonic, and palmitic acids were confirmed as characteristic FFAs in postmortem grass carp muscle, meaning their potential as valuable indicators for evaluating postmortem flesh quality.
3.3. Protein degradation analysis
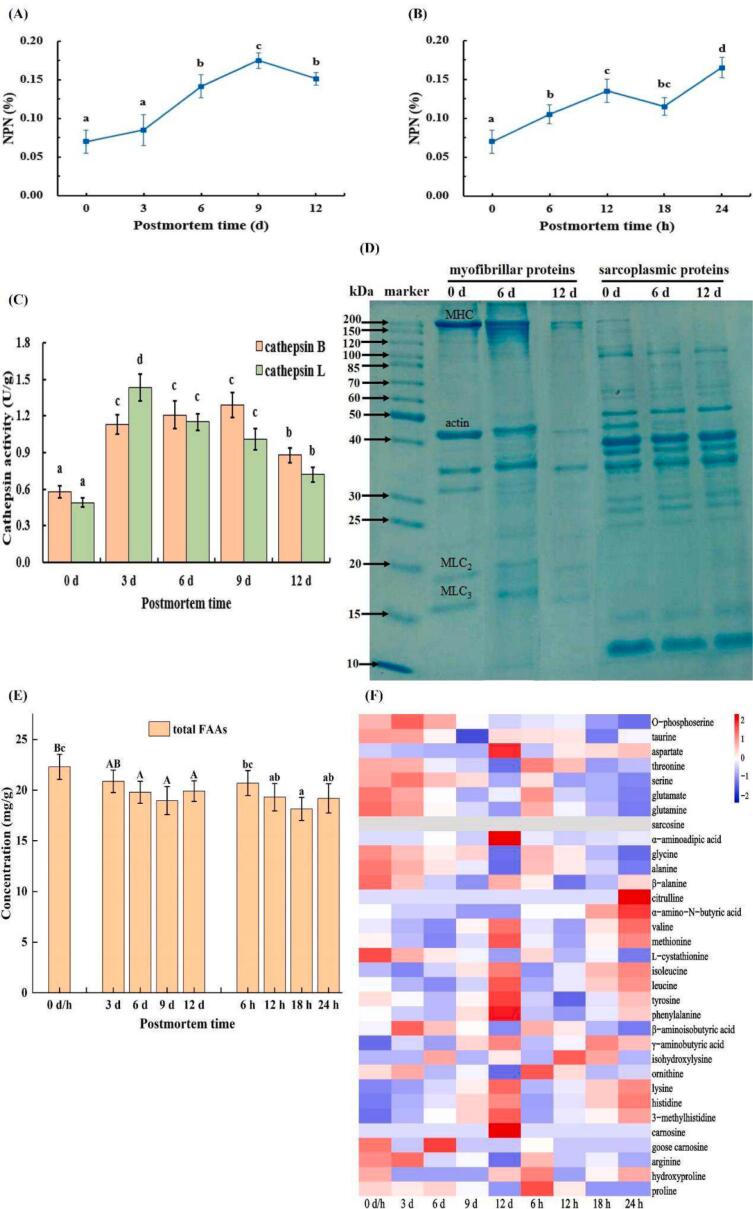
3.3.1. NPN content
NPN content reflects the protein degradation degree in the food matrix (Xiao et al., 2024). As depicted in Fig. 3 A and 3B, upon postmortem refrigeration, the NPN content increased to its peak (0.175%) on day 9 and decreased thereafter. This phenomenon may be attributed to the continuous degradation of muscular protein over the initial 9 days postmortem due to the action of microorganisms and endogenous proteases (Xiao et al., 2024), leading to a rapid accumulation of NPN. Afterwards, during the later stages of postmortem, muscle spoilage intensified, resulting in the formation of certain volatile nitrogen-containing compounds. Additionally, after undergoing prolonged consumption, the basal contents of protein gradually decreased, and its degradation rates slowed down as well. These factors collectively led to the reduction in NPN content. In the case of AT storage, the NPN content presented an overall upward trend with slight fluctuation, indicating ongoing protein degradation at 25 °C within 24 h postmortem.
Fig. 3.
Changes in the NPN content (A, B), cathepsin B and L activities (C), SDS-PAGE profile (D), and total FAAs content (E) of grass carp muscle; and Heatmap visualization of each FAA (F) in grass carp muscle during postmortem storage. Different letters indicate significant differences (p < 0.05). NPN, non-protein nitrogen; MHC, myosin heavy chain; MLC, myosin light chain; FAA, free amino acid.
3.3.2. Activities of endogenous cathepsin B and L
Cathepsin B and L are regarded as the main endogenous proteases in preserved fish or meat (Chéret, Delbarre-Ladrat, Lamballerie-Anton, & Verrez-Bagnis, 2007). Within a 12-day postmortem refrigeration period, the activities of cathepsin B and L firstly increased, especially in the initial 3 days (p < 0.05), and then gradually decreased (Fig. 3C). In contrast, Bahuaud et al. (2008) reported that these two enzymatic activities in Atlantic salmon initially rose and subsequently reduced within 48 h postmortem, suggesting that the properties of cathepsin were largely affected by fish species with varying growth environments. Zhao, Li, Wang, and Lv (2012) suggested that the heightened cathepsin activity in early postmortem muscle could be attributed to the sustained expression of genes involved in cathepsin synthesis and cell apoptosis. Whereas, the subsequent decline in activity might be due to the fact that in the late postmortem period, the pH of fish meat increased with the accumulation of amines, deviating from the optimal pH range for cathepsin (Bahuaud et al., 2008). Revealingly, cathepsin demonstrates strong activity at pH 3.0–6.5 but exhibits structural instability at pH levels exceeding 7 (Chéret et al., 2007).
3.3.3. SDS-PAGE profile
Myofibrillar and sarcoplasmic proteins are pivotal structural components of fish muscle, comprising approximately 55% and 35% of total protein, respectively (Zhao et al., 2019). According to the SDS-PAGE electrophoretogram (Fig. 3D), the protein bands in lanes corresponding to the 6 and 12 d samples exhibited a gradual attenuation across varying degrees. Intriguingly, unlike myofibrillar proteins, sarcoplasmic protein fractions manifested several novel protein bands within the 60–100 kDa range. Similar results were also reported by Yang, Xia, Zhang, Xu, and Jiang (2016) in silver carp. These emergent bands may be degradation fragments of high molecular weight proteins (such as sarcoplasmic protein), or aggregates of peptides and smaller molecular weight protein through disulfide and covalent bonds (Zhao et al., 2019). Particularly, the intensities of bands corresponding to myosin heavy chain (MHC, 210 kDa), myosin light chains (MLC, 15 and 17 kDa), and actin (42 kDa) markedly decreased in lanes 6 d, and completely disappeared or severely weakened in lanes 12 d. Myosin and actin are the main proteins that make up muscle, and their degradation can ultimately lead to the rupture of most myofibers, destruction of intramuscular membranes, and disruption of muscle structural integrity, as observed in the above-mentioned TEM detection.
3.3.4. FAAs profile
A total of 33 FAAs were detected and represented by glycine, alanine, lysine, histidine, and taurine (Table S4 and Fig. 3F). And, according to Fig. 3E, the total FAAs contents exhibited gradual downward trends in the previous storage stage (0–9 d and 0–18 h). This reduction could potentially be attributed to the conversion of FAAs into biogenic amines (BAs) via decarboxylase activity, as suggested by Li et al. (2022), or to their utilization as energy sources for bacterial reproduction (Guo, Chen, Ma, Yu, & Zhang, 2023). Afterwards, increasing the postmortem storage time caused rising shifts in total FAAs levels. This may be due to the fact that autolysase in muscle can degrade proteins into peptides and amino acids, and small peptides can release amino acids under the action of aminopeptidases (Zhao et al., 2024). Similar trends were obtained by Chu, Ding, Wang, and Xie (2023), who revealed that the total FAAs of groupers decreased to the lowest content at 12 d postmortem during refrigerated storage and increased gradually thereafter.
Among the five representative FAAs, taurine showed overall downward trends during storage without prominent variations, while the other four FAAs demonstrated heightened sensitivity to storage duration, with pronounced changes. This phenomenon posited that glycine, alanine, lysine, and histidine played pivotal roles in shaping the tastes of postmortem grass carp flesh. Notably, unlike postmortem refrigeration, most FAAs underwent more substantial variations in content during postmortem AT storage, indicating enhanced FAA transformation and microbial activity at elevated temperatures.
Hydrophobic amino acids, including histidine, valine, isoleucine, leucine, phenylalanine, lysine, and methionine, are known to contribute to a bitter taste (Pongsetkul et al., 2022). Our findings demonstrated that the levels of these bitter amino acids generally escalated during storage processes. In contrast, the levels of glycine, alanine, threonine, serine, glutamate, and glutamine showed constant decrements during storage. Of these, glycine, alanine, and glutamate are associated with umami, whereas threonine, serine, and glutamine provide sweetness (Ismail, Hwang, & Joo, 2020). Our results were in line with those reported by Chu, Ding, Wang, and Xie (2023) wherein the contents of bitter amino acids in grouper were positively correlated with cooling time, while the contents of umami and sweet amino acids were negatively correlated with chilling period. Furthermore, it appears that other amino acids may also be used as indicators of flesh quality changes. 3-Methylhistidine, for instance, was located mainly in myofibrillar protein and could be employed to monitor meat protein changes (Zhao et al., 2019). Herein, the increases in 3-methylhistidine content was positively correlated with the degradation of aforementioned myofibrillar protein during postmortem storage.
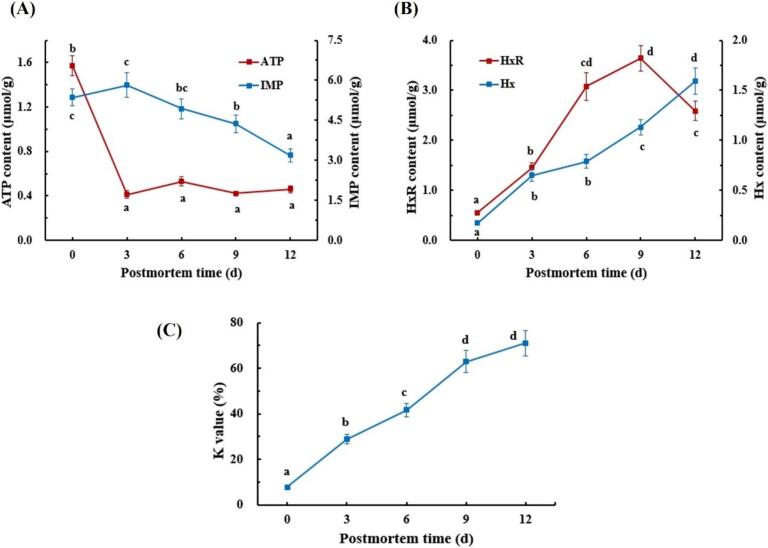
3.4. ATP derivative compounds analysis
ATP and its derivatives are the predominant constituents of nucleotides in aquatic muscle (Hong, Regenstein, & Luo, 2017). As depicted in Fig. 4 A, the initial ATP content in fish samples was 1.57 μmol/g. With extended postmortem time, the ATP levels decreased prominently (p < 0.05) during the first 3 days of storage, followed by a gradual stabilization until the end. The swift degradation of ATP in postmortem flesh may be caused by the activation of ATP enzymes (Bi et al., 2023). Similar results were obtained by Xu et al. (2020), who revealed that the ATP contents of flounder fillets diminished considerably within the initial 4 days of postmortem refrigeration.
Fig. 4.
Changes in ATP-related compounds (ATP, IMP, HxR, Hx) and K values of grass carp muscle during postmortem refrigerated storage. Means with different lowercase letters in the same index differ significantly (p < 0.05). ATP, adenosine triphosphate; IMP, inosine monophosphate; HxR, hypoxanthine riboside; Hx, hypoxanthine.
Reportedly, inosine 5′-monophosphate (IMP) is linked to the umami and sweetness characteristics of fish (Xu et al., 2020). The IMP content in grass carp muscle showed a slight rise within the initial 3 days of storage, followed by a remarkable reduction during subsequent storage periods (p < 0.05). This phenomenon may be due to the rapid decomposition of ATP and AMP into IMP during the early postmortem phase, which is facilitated by endogenous enzymes. Afterwards, IMP underwent degradation into inosine (HxR) and hypoxanthine (Hx), a process mediated by phosphomonoesterase and nucleoside hydrolase (Hong et al., 2017).
As shown in Fig. 4B, the HxR content enhanced distinctly (p < 0.05) to the highest level on day 9 and subsequently declined with increasing postmortem time, which was consistent with previous studies on tilapia (Cheng, Mei, & Xie, 2023). The increment may be due to the consumption of IMP by 5′-nucleotidase, while the decrement could result from the hydrolysis of HxR into Hx and microbial reproduction (Xu et al., 2020). Furthermore, Hx is intimately associated with the bitter off-taste that contributes to fish spoilage (Cheng et al., 2023). An increase in Hx content was noted in postmortem flesh throughout the storage period, signifying a decline in quality.
The K value is commonly employed to assess the freshness of fish. A K value <20% signifies first-grade freshness suitable for consumption as raw food. A K value ranging from 20% to 40% indicates the second freshness grade, while a value exceeding 60% suggests the onset of spoilage (Hong et al., 2017). Herein, the K value of fresh grass carp was 7.72%, progressively increasing with extended refrigerated postmortem time (Fig. 4C). By the sixth day, the K value had escalated to 41.71%, indicating that the flesh had not yet begun to decay. By day 9, the muscular K value peaked at 62.97%, accompanied by stickiness and discoloration, signaling the fish was in the initial stages of decay and unsuitable for consumption.
3.5. Flavor evolution
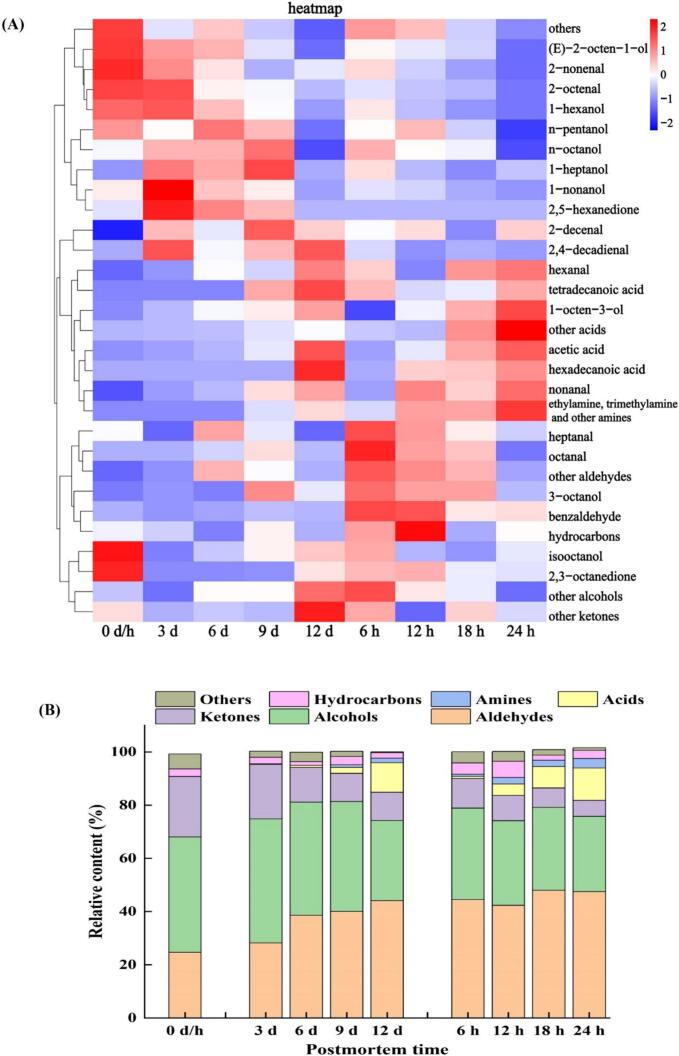
3.5.1. VOCs profile
Through SPME-GC–MS detection, the VOCs that had great impacts on the flavor characteristics of grass carp muscle were identified, as detailed in Table S5 and Fig. 5 A.
Fig. 5.
Heatmap visualization of volatile compounds (A), and proportion of volatile compounds category (B) in grass carp muscle during postmortem refrigerated and ambient storage.
3.5.1.1. Carbonyl compounds
In this study, aldehydes and alcohols comprised a significant portion of the VOCs found in postmortem grass carp muscle (Fig. 5B). Reportedly, they could significantly affect the overall flavor of fish due to their low odor thresholds (Chu, Mei, & Xie, 2023b). The study confirmed that regardless of storage at either 4 °C or 25 °C, hexanal and nonanal were the most abundant aldehydes, followed by octanal and heptanal, and then alkenyl aldehydes. It is similar to what Liu et al. (2021) reported for aldehyde compounds distribution in turbot during refrigerated postmortem periods. Moreover, the relative content of hexanal and nonanal changed significantly with the extension of storage time. These observations suggested that hexanal and nonanal could serve as key characteristic VOCs for assessing the flavor quality of postmortem grass carp muscle. Notably, Notably, aldehyde production was higher in AT storage than in refrigeration (Fig. 5B). Specifically, the relative content of hexanal with sour rotting odor increased continuously from an initial 8.34% to 21.70% (4 °C) and 22.10% (25 °C), while the relative content of nonanal with an unpleasant smell increased from 6.90% initially to 18.27% (4 °C) and 20.63% (25 °C). Consequently, FAs were more prone to oxidation at elevated postmortem storage temperatures, leading to intensified oxidative cracking of oleic, linoleic, and arachidonic acids, thereby increasing the production of hexanal and nonanal (Zhang et al., 2019).
In addition, ketones in postmortem fish are mainly produced through UFA autoxidation, protein/amino acid decomposition, and microorganism action (Chu, Mei, & Xie, 2023b), which may lead to the enhancement of fishy smell. Here, two specific ketones, namely, 2, 3-octanedione and 2, 5-hexanedione, were primarily identified in postmortem grass carp muscle. Interestingly, these two ketones have been found to significantly influence flavor development, as observed in studies on postmortem grouper (Chu, Mei, & Xie, 2023b) and silver carp (Liu et al., 2022).
3.5.1.2. Alcohol compounds
Unlike aldehydes, the total contents of alcohols in postmortem muscle stored at 25 °C were lower than those stored at 4 °C (Fig. 5B). Regardless of storage temperature, the volatile saturated alcohols in muscle predominantly comprised 1-heptanol, 1-nonanol, n-pentanol, n-octanol, isooctanol, 3-octanol, and 1-hexanol. The primary volatile unsaturated alcohols were identified as 1-octen-3-ol and (E)-2-octen-1-ol. Among them, 1-octen-3-ol and 1-hexanol displayed relatively higher contents and prominent variations, followed by (E)-2-octen-1-ol and n-octanol. Specifically, 1-octen-3-ol, characterized by earthy and mushroom-like flavors, gradually increased from an initial 12.43% to 21.28% (4 °C) and 25.01% (25 °C), which may be related to the accelerated degradation of linoleate hydroperoxide (Matsui, Sasahara, Akakabe, & Kajiwara, 2003). Conversely, 1-hexanol, known for its sweet alcohol and pleasant odor, showed downward trends throughout the storage processes, decreasing from an initial 17.97% to 3.55% (4 °C) and 1.37% (25 °C). It can be seen that both 1-octen-3-ol and 1-hexanol in flesh exhibited heightened sensitivity to elevated storage temperatures. Importantly, due to their low odor thresholds and great influence on flavor, 1-octen-3-ol and 1-hexanol were recognized as vital characteristic VOCs.
3.5.1.3. Other VOCs
Increasing the postmortem storage time caused accumulation of volatile acids (such as acetic, tetradecanoic, and hexadecanoic acids), amines (such as trimethylamine and ethylamine) and other substances (Fig. 5B), which may be caused by metabolic reactions (Zhao, Hu, & Chen, 2022). Similar phenomena have been observed in grouper (Chu, Ding, Wang, & Xie, 2023) and turbot (Liu et al., 2021), where postmortem aging hastened the decomposition of proteins or amino acids into volatile low molecular weight putrid substances such as amines, ammonia, acids, peroxides, and indole, resulting in flavor deterioration. Moreover, the hydrolysis of fatty acid alkoxy radicals yields various hydrocarbons, among which volatile alkenes such as 8-methylundeecene, 3,5,5-trimethyl-2-hexene, and 3,5,5-trimethylhexene were reported to transform into ketones or aldehydes under certain conditions (Pongsetkul et al., 2022), thus they have the potential to produce fishy odor. In addition, toluene, naphthalene, 1-methylnaphthalene, and other substances could also cause unpleasant flavors in postmortem flesh and may originate from the surrounding environment (Liu et al., 2021).
3.5.2. E-nose analysis
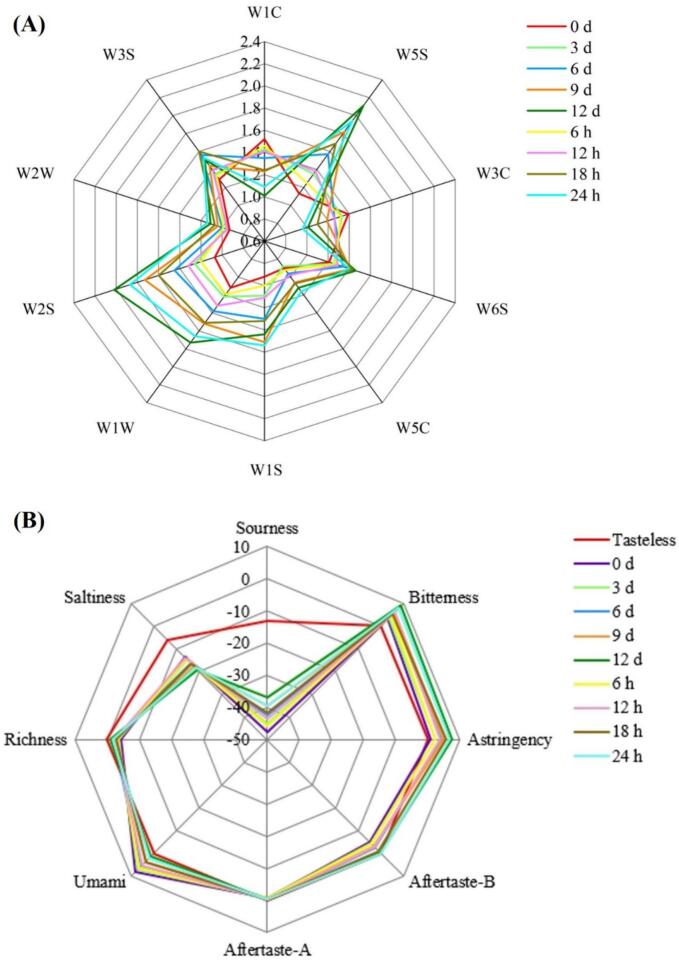
Revealingly, aromatic substances impart pleasant smells and contribute to the fresh meat-like properties of fish. Conversely, nitrogen oxides, which result from the degradation of proteins, FAAs, and nucleotides, are associated with undesirable odors and often manifest in fish samples as their quality deteriorates. According to Fig. 6 A, nitrogen oxides (W5S) and broad-alcohols, aldehydes, and ketones (W2S) were the predominant odorous substances in flesh. Their response values surged most significantly during postmortem refrigerated storage, succeeded by inorganic sulfides (W1W). In contrast, the response values for aroma component (W1C) and ammonium hydroxide and aroma component (W3C) were weak and decreased gradually over storage time. Upon AT storage, all odor signals changed gently within 0–12 h postmortem. But then the sensors for W5S, W2S, W1W, and methyl (W1S) showed strong responses, indicating that the muscle stored at 25 °C for 18–24 h postmortem may produce more sulfur-containing compounds, methyl, nitrogen oxides, and broad-alcohols, aldehydes and ketones. These observations indicated that the odor of postmortem fish flesh turned unacceptable, which aligned with Limbo et al. (2009) who investigated the shelf life and freshness decay of European sea bass.
Fig. 6.
Radar diagrams of E-nose (A) and E-tongue (B) response signal value for grass carp muscle during postmortem refrigerated and ambient storage.
3.5.3. E-tongue analysis
Notably, the umami, bitterness, and astringency of fish surpassed the tasteless point (Fig. 6B), which meant they were effective taste indicators of postmortem grass carp flesh. The initial response signal of umami was the highest, implying that the primary taste of the flesh was predominantly umami during the early postmortem period. As the postmortem duration extended, the taste profile of the flesh underwent progressive decline, with sourness, richness, astringency, aftertaste-astringency, bitterness and aftertaste-bitterness all increasing to varying extents, while saltiness and umami decreased. Collectively, the predominant taste of fish flesh transitioned from umami to bitterness and then to astringency throughout the postmortem storage period. These findings aligned with those of Li et al. (2023). The postmortem changes in taste values may be attributed to shifts in the concentration of taste compounds and electrolyte loss in fish flesh caused by quality deterioration during storage (Ismail et al., 2020). When juxtaposed with the aforementioned FAAs, ATP-associated compounds, and K values results, it can be deduced that fish exhibiting bitterness and astringency were likely in a state of deterioration. This suggested that it was feasible to adopt the signal response values from E-tongue sensors to characterize the freshness of grass carp flesh.
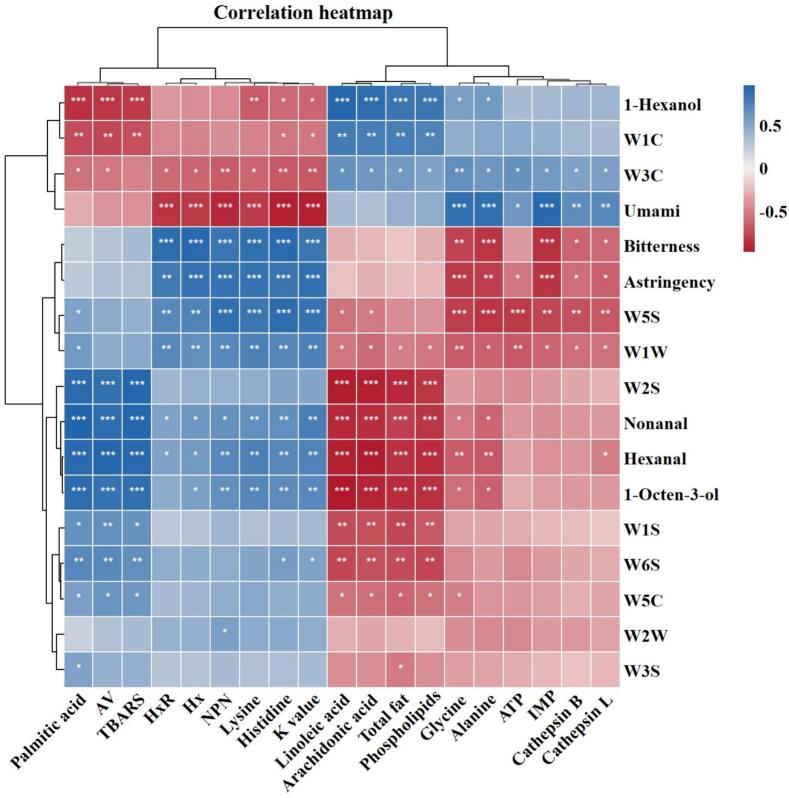
3.6. Correlations between muscle degradation and flavor evolution, and insights into mechanisms underlying the postmortem quality changes
To further figure out the impact of muscle degradation on flavor evolution under postmortem refrigerated conditions, characteristic VOCs (hexanal, nonanal, 1-hexanol, and 1-octen-3-ol), FFAs (palmitic, linoleic, and arachidonic acid), and FAAs (glycine, alanine, lysine, and histidine) were selected to construct a correlation matrix. The results are presented in Fig. 7. It was observed that the characteristic VOCs were highly correlated with the characteristic FFAs, AV, TBARS, phospholipid, and total fat. Previous research has proved that phospholipids and UFAs (oleic, linoleic, and arachidonic acids, etc.) are susceptible to oxidation due to their double bonds. This process leads to the formation of peroxides, which subsequently decompose into aldehydes, ketones, alcohols, esters, acids, and hydrocarbons, thereby generating VOCs (Tu et al., 2022). These findings suggest that the characteristic VOCs primarily originate from the secondary oxidation of lipids. Likewise, the response values of certain E-nose sensors can be traced back to their corresponding sources in the correlation matrix. It was determine that lipolysis-oxidation had the most significant effect on the generation of broad-alcohols, aldehydes, and ketones (W2S), followed by aroma component (W1C), hydrogen (W6S), and methyl (W1S). Meanwhile, protein degradation and ATP breakdown were found to have the highest correlation with the formation of nitrogen oxides (W5S), followed by inorganic sulfides (W1W) and ammonium hydroxide and aroma component (W3C). Regarding representative tastes such as umami, bitterness, and astringency, they were found to be closely associated with protein degradation-related histidine, lysine, alanine, glycine, and NPN and ATP breakdown-related IMP, HxR, Hx, and K value. From this, it was determined metabolites of protein and nucleotide serve as the primary precursors for taste-active substances in postmortem grass carp muscle.
Fig. 7.
Correlation map between muscle degradation and flavor deterioration. Blue represents a positive correlation, and red represents a negative correlation. * p < 0.05, ** p < 0.01, *** p < 0.001.
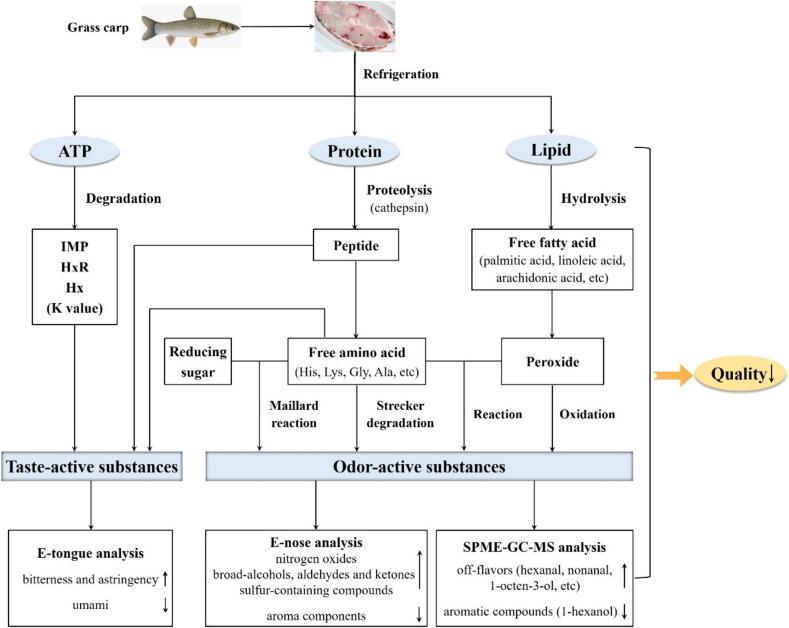
Factually, the mechanisms underlying postmortem flesh quality changes are highly intricate. Herein, from the perspectives of muscle degradation and flavor evolution, the possible mechanisms driving the alterations in grass carp muscle during postmortem refrigeration is depicted in Fig. 8. As the duration of postmortem refrigeration increases, a series of intricate chemical reactions involving flavor precursors, intermediates, and degradation products contribute to the production of flesh flavor. The primary sources of taste-active substances were protein hydrolysis and ATP degradation. Meanwhile, the formation of odor-active substances was multifaceted and primarily involved reaction pathways such as lipolysis-oxidation, Maillard reaction, Strecker degradation, and interactions between peroxides and amino acids (Zhang et al., 2019). In the Maillard reaction, amino acids and reducing sugars within the flesh interact to form various flavor compounds like pyrazine, pyrrole, sulfur compounds, etc. Strecker degradation involved oxidation and degradation of α-amino acids and α-dicarbonyl compounds to produce Strecker aldehydes, which played a significant role in flesh flavor formation (Deng, Luo, Wang, & Zhao, 2015). Additionally, FAAs could undergo oxidation by peroxides, resulting in final products such as aldehydes, ammonia, and carbon dioxide (Wang et al., 2022). Moreover, Maillard reaction products may interact with lipid oxidation products, including aldehydes, ketones, alcohols, and alkanes. This interaction led to the formation of new heterocyclic VOCs, specifically thiophenes and mercaptans, which subsequently influenced the flavor of fish flesh (Bleicher, Ebner, & Bak, 2022). Nevertheless, the associated mechanisms of lipolysis-oxidation, protein hydrolysis, nucleotide metabolism, and flavor formation still require further investigation and validation.
Fig. 8.
The mechanisms underlying the postmortem quality changes in grass carp muscle.
4. Conclusion
This study initially investigated the dynamic changes in muscle degradation of grass carp during postmortem refrigerated and ambient storage. It was revealed that the muscular chemical compositions, encompassing lipid, protein, and ATP, underwent progressive degradation, with their associated biochemical indicators also deteriorating to varying degrees during storage processes. These changes largely contributed to the disruption of microstructural integrity in fish flesh. Subsequently, the study investigated flavor evolution, revealing that postmortem storage at different temperatures and durations resulted in significant changes in odor substances (carbonyls, alcohols, nitrogen oxides, sulfur-containing compounds) and taste attributes (astringency, bitterness, and umami). Furthermore, correlation analysis demonstrated that the flavor profile exhibited excellent correlations with lipolysis-oxidation, protein hydrolysis, and ATP degradation. These findings facilitate further understanding of the relationship between component decomposition, microstructure transformation, and flavor evolution in fish muscle during postmortem storage. This knowledge may guide the identification of potential freshness indicators and the development of strategies for quality improvement. The purpose of this paper is to give a broad overview of postmortem muscle changes in fish and the potential mechanisms involved.
CRediT authorship contribution statement
Hui Li: Writing – original draft, Methodology, Conceptualization. Na Deng: Visualization, Resources. Yongjian Cai: Resources, Formal analysis. Jing Yang: Methodology, Data curation. Fangfang Ouyang: Methodology, Data curation. Miao Liu: Validation, Software. Jianhui Wang: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (32302188), Science and Technology Innovation Program of Hunan Province (2023RC1056), Research and Development Program in Key Areas of Hunan Province (2024JK2154, 2024JK2156, 2021NK2015), Hunan Provincial Science Fund for Distinguished Young Scholars (2021JJ10007), and Dongting Laboratory Cultivation Project of Hunan Yuelushan Center for Industrial Innovation (2024-DTPY-006).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101751.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- AOCS . Phosphorus; Reapproved: 2009. AOCS Official Method Ca 12–55; p. 2009. [Google Scholar]
- Bahuaud D., Mørkøre T., Langsrud Ø., Sinnes K., Veiseth E., Ofstad R., Thomassen M.S. Effects of −1.5 °C Super-chilling on quality of Atlantic salmon (Salmo salar) pre-rigor Fillets: Cathepsin activity, muscle histology, texture and liquid leakage. Food Chemistry. 2008;111(2):329–339. doi: 10.1016/j.foodchem.2008.03.075. [DOI] [PubMed] [Google Scholar]
- Bi S., Xue C., Wen Y., Du X., Xue Q., Li Z., Liu H. Effects of cooling rates during depuration on the quality of Pacific oysters (Crassostrea gigas) at anhydrous preservation stage. Food Chemistry: X. 2023;17(100):606. doi: 10.1016/j.fochx.2023.100606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicher J., Ebner E.E., Bak K.H. Formation and analysis of volatile and odor compounds in meat—A review. Molecules. 2022;27:6703. doi: 10.3390/molecules27196703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Mei J., Xie J. Analysis of key volatile compounds and quality properties of tilapia (Oreochromis mossambicus) fillets during cold storage: Based on thermal desorption coupled with gas chromatography–mass spectrometry (TD-GC–MS) LWT. 2023;184(115):051. doi: 10.1016/j.lwt.2023.115051. [DOI] [Google Scholar]
- Chéret R., Delbarre-Ladrat C., Lamballerie-Anton M., Verrez-Bagnis V. Calpain and cathepsin activities in post mortem fish and meat muscles. Food Chemistry. 2007;101(4):1474–1479. doi: 10.1016/j.foodchem.2006.04.023. [DOI] [Google Scholar]
- Chu Y., Ding Z., Wang J., Xie J. Exploration of the evolution and production of volatile compounds in grouper (Epinephelus coioides) during cold storage. Food Bioscience. 2023;52(102):496. doi: 10.1016/j.fbio.2023.102496. [DOI] [Google Scholar]
- Chu Y., Ding Z., Wang J., Xie J., Ding Y. Factors affecting the quality of frozen large yellow croaker (Pseudosciaena crocea) in cold chain logistics: Retention time and temperature fluctuation. Food Chemistry: X. 2023;18(100):742. doi: 10.1016/j.fochx.2023.100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y., Mei J., Xie J. Exploring the effects of lipid oxidation and free fatty acids on the development of volatile compounds in grouper during cold storage based on multivariate analysis. Food Chemistry: X. 2023;20(100):968. doi: 10.1016/j.fochx.2023.100968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y., Mei J., Xie J. Integrated volatile compounds and non-targeted metabolomics analysis reveal the characteristic flavor formation of proteins in grouper (Epinephelus coioides) during cold storage. Food Research International. 2023;172(113):145. doi: 10.1016/j.foodres.2023.113145. [DOI] [PubMed] [Google Scholar]
- Deng Y., Luo Y., Wang Y., Zhao Y. Effect of different drying methods on the myosin structure, amino acid composition, protein digestibility and volatile profile of squid fillets. Food Chemistry. 2015;171:168–176. doi: 10.1016/j.foodchem.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Dou L., Liu C., Yang Z., Su R., Chen X., Hou Y., Jin Y. Effects of oxidative stability variation on lamb meat quality and flavor during postmortem aging. Journal of Food Science. 2022;87(6):2578–2594. doi: 10.1111/1750-3841.16138. [DOI] [PubMed] [Google Scholar]
- Gherbawy Y.A., Thabet M.A., Sultan S. Genetic and morphological characterization of a new genotype of nervous necrosis virus circulating among Nile tilapia in the south of Egypt. International Microbiology. 2023;27(2):559–569. doi: 10.1007/s10123-023-00406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Chen C., Ma G., Yu Q., Zhang L. LF-NMR determination of water distribution and its relationship with protein- related properties of yak and cattle during postmortem aging. Food Chemistry: X. 2023;20(100):891. doi: 10.1016/j.fochx.2023.100891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H., Regenstein J.M., Luo Y. The importance of ATP-related compounds for the freshness and flavor of post-mortem fish and shellfish muscle: A review. Critical Reviews in Food Science and Nutrition. 2017;57(9):1787–1798. doi: 10.1080/10408398.2014.1001489. [DOI] [PubMed] [Google Scholar]
- Ismail I., Hwang Y., Joo S. Low-temperature and long-time heating regimes on non-volatile compound and taste traits of beef assessed by the electronic tongue system. Food Chemistry. 2020;320(126):656. doi: 10.1016/j.foodchem.2020.126656. [DOI] [PubMed] [Google Scholar]
- Li H., Wang Y., Zhang J., Li X., Wang J., Yi S., Li J. Prediction of the freshness of horse mackerel (Trachurus japonicus) using E-nose, E-tongue, and colorimeter based on biochemical indexes analyzed during frozen storage of whole fish. Food Chemistry. 2023;402(134):325. doi: 10.1016/j.foodchem.2022.134325. [DOI] [PubMed] [Google Scholar]
- Li X., Luan A., Li X., Wang F., Huang Y., Li A., Liu Y. Protein degradation and aggregation in silver carp (Hypophthalmichthys molitrix) muscle during hot air drying. LWT. 2022;163(113):540. doi: 10.1016/j.lwt.2022.113540. [DOI] [Google Scholar]
- Limbo S., Sinelli N., Torri L., Riva M. Freshness decay and shelf life predictive modelling of European sea bass (Dicentrarchus labrax) applying chemical methods and electronic nose. LWT - Food Science and Technology. 2009;42(5):977–984. doi: 10.1016/j.lwt.2008.12.011. [DOI] [Google Scholar]
- Liu D., Liang L., Xia W., Regenstein J.M., Zhou P. Biochemical and physical changes of grass carp (Ctenopharyngodon idella) fillets stored at −3 and 0 °C. Food Chemistry. 2013;140(1–2):105–114. doi: 10.1016/j.foodchem.2013.02.034. [DOI] [PubMed] [Google Scholar]
- Liu J., Shen S., Xiao N., Jiang Q., Shi W. Effect of glycation on physicochemical properties and volatile flavor characteristics of silver carp mince. Food Chemistry. 2022;386(132):741. doi: 10.1016/j.foodchem.2022.132741. [DOI] [PubMed] [Google Scholar]
- Liu J., You M., Zhu X., Shi W. Characterization of aroma characteristics of silver carp mince glycated with different reducing sugars. Food Chemistry: X. 2024;22(101):335. doi: 10.1016/j.fochx.2024.101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Mei J., Xie J. Effect of locust bean gum‑sodium alginate coatings incorporated with daphnetin emulsions on the quality of Scophthalmus maximus at refrigerated condition. International Journal of Biological Macromolecules. 2021;170:129–139. doi: 10.1016/j.ijbiomac.2020.12.089. [DOI] [PubMed] [Google Scholar]
- Matsui K., Sasahara S., Akakabe Y., Kajiwara T. Linoleic acid 10-hydroperoxide as an intermediate during formation of 1-octen-3-ol from linoleic acid in Lentinus decadetes. Bioscience Biotechnology & Biochemistry. 2003;67(10):2280–2282. doi: 10.1271/bbb.67.2280. [DOI] [PubMed] [Google Scholar]
- Pongsetkul J., Yongsawatdigul J., Boonanuntanasarn S., Benjakul S. Development of flavor and taste components of sous-vide-cooked Nile tilapia (Oreochromis niloticus) fillet as affected by various conditions. Foods. 2022;11(22):3681. doi: 10.3390/foods11223681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng X., Yan L., Peng L., Zhao L., Dai F., Chen F., Raghavan V. Effect of plasma-activated lactic acid on microbiota composition and quality of puffer fish (Takifugu obscurus) fillets during chilled storage. Food Chemistry: X. 2024;21(101):129. doi: 10.1016/j.fochx.2024.101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie H., Jiang W., Feng L., Wu P., Liu Y., Kuang S., Zhou X. Dietary nucleotides in the diets of on-growing grass carp (Ctenopharyngodon idella) suppress Aeromonas hydrophila induced intestinal inflammation and enhance intestinal disease-resistance via NF-κB and TOR signaling. Aquaculture. 2021;533(736):075. doi: 10.1016/j.aquaculture.2020.736075. [DOI] [Google Scholar]
- Tie H., Yu D., Yang F., Jiang Q., Xu Y., Xia W. Postmortem grass carp (Ctenopharyngodon idella) muscle towards the disruption of integrity: a likely cause of abnormal regulation of tight junction and decreased antioxidant capacity. International Journal of Food Science & Technology. 2022;57(11):7222–7232. doi: 10.1111/ijfs.16069. [DOI] [Google Scholar]
- Tu C., Qi X., Shui S., Lin H., Benjakul S., Zhang B. Investigation of the changes in lipid profiles induced by hydroxyl radicals in whiteleg shrimp (Litopenaeus vannamei) muscle using LC/MS-based lipidomics analysis. Food Chemistry. 2022;369(130):925. doi: 10.1016/j.foodchem.2021.130925. [DOI] [PubMed] [Google Scholar]
- Wang Y., Xie J., Zhang C., Xu Y., Yang X. Effect of lipid on formation of Maillard and lipid-Maillard meaty flavor compounds in heated cysteine-xylose-methyl linoleate system. Flavor and Fragrance Journal. 2022;37(5):274–284. doi: 10.1002/ffj.3710. [DOI] [Google Scholar]
- Wu H., Forghani B., Abdollahi M., Undeland I. Five cuts from herring (Clupea harengus): Comparison of nutritional and chemical composition between co-product fractions and fillets. Food Chemistry: X. 2022;16(100):488. doi: 10.1016/j.fochx.2022.100488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao N., Zhang Q., Xu H., Zheng C., Yin Y., Liu S., Shi W. Effect of Lactobacillus plantarum and flavourzyme on protein degradation and flavor development in grass carp during fermentation. Food Chemistry: X. 2024;22(101):439. doi: 10.1016/j.fochx.2024.101439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Yin Y., Zhao H., Li Q., Yi S., Li X., Li J. Effects of cinnamaldehyde combined with ultrahigh pressure treatment on the flavor of refrigerated Paralichthys olivaceus fillets. RSC Advances. 2020;10(21) doi: 10.1039/d0ra01020k. 12573–12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Xia W., Zhang X., Xu Y., Jiang Q. A comparison of endogenous and microbial proteolytic activities during fast fermentation of silver carp inoculated with Lactobacillus plantarum. Food Chemistry. 2016;207:86–92. doi: 10.1016/j.foodchem.2016.03.049. [DOI] [PubMed] [Google Scholar]
- Yarnpakdee S., Benjakul S., Nalinanon S., Kristinsson H.G. Lipid oxidation and fishy odor development in protein hydrolysate from Nile tilapia (Oreochromis niloticus) muscle as affected by freshness and antioxidants. Food Chemistry. 2012;132(4):1781–1788. [Google Scholar]
- Yi L., An Y., Liu R., Hu Y., Xiong S. Purification and comparison of enzymatic properties of endogenous transglutaminase between silver carp and black carp. Food & Machinery. 2023;39(10):4–12. [Google Scholar]
- Zhang J., Cao J., Pei Z., Wei P., Xiang D., Cao X., Li C. Volatile flavor components and the mechanisms underlying their production in golden pompano (Trachinotus blochii) fillets subjected to different drying methods: A comparative study using an electronic nose, an electronic tongue and SDE-GC–MS. Food Research International. 2019;123:217–225. doi: 10.1016/j.foodres.2019.04.069. [DOI] [PubMed] [Google Scholar]
- Zhang M., Wang D., Huang W., Liu F., Zhu Y., Xu W., Cao J. Apoptosis during postmortem conditioning and its relationship to duck meat quality. Food Chemistry. 2013;138(1):96–100. doi: 10.1016/j.foodchem.2012.10.142. [DOI] [PubMed] [Google Scholar]
- Zhao C., Benjakul S., Eun J. Changes in protein compositions and textural properties of the muscle of skate fermented at 10 °C. International Journal of Food Properties. 2019;22(1):173–185. doi: 10.1080/10942912.2019.1575396. [DOI] [Google Scholar]
- Zhao D., Fang Y., Wei Z., Duan W., Chen Y., Zhou X., Xiao C., Chen W. Proteomics reveals the mechanism of protein degradation and its relationship to sensorial and texture characteristics in dry-cured squid during processing. Food Chemistry: X. 2024;22(101):409. doi: 10.1016/j.fochx.2024.101409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D., Hu J., Chen W. Analysis of the relationship between microorganisms and flavor development in dry-cured grass carp by high-throughput sequencing, volatile flavor analysis and metabolomics. Food Chemistry. 2022;368(130):889. doi: 10.1016/j.foodchem.2021.130889. [DOI] [PubMed] [Google Scholar]
- Zhao J., Li J., Wang J., Lv W. Applying different methods to evaluate the freshness of large yellow croacker (Pseudosciaena crocea) fillets during chilled storage. Journal of Agricultural and Food Chemistry. 2012;60(45) doi: 10.1021/jf303439p. 11387–11394. [DOI] [PubMed] [Google Scholar]
- Zou B., Yu Q., Shao L., Sun Y., Li X., Dai R. Alteration of mitochondrial lipidome and its potential effect on apoptosis, mitochondrial reactive oxygen species production, and muscle oxidation in beef during early postmortem. Journal of Agricultural and Food Chemistry. 2022;70(26):8064–8074. doi: 10.1021/acs.jafc.2c02519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.