Abstract
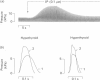
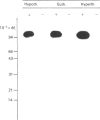
The effects of beta-adrenergic stimulation on the relaxation rate and the Ca2+-transport rate in sarcoplasmic reticulum of hypothyroid, euthyroid and hyperthyroid rat hearts were studied. Administration of isoproterenol (0.1 microM) to perfused, electrically stimulated hearts (5 Hz) caused a decrease in the half-time of relaxation (RT 1/2) the extent of which depended on the thyroid status, i.e. hypothyroid (-24%), euthyroid (-19%) or hyperthyroid (-8%). A similar decreasing effect was found for the stimulation of Ca2+ transport in isolated SR by cyclic AMP and protein kinase, i.e. hypothyroid (75%), euthyroid (37%) and hyperthyroid (20%). These alterations were not due to differences in endogenous protein kinase activity or cyclic AMP production. Estimations of Ca2+-ATPase and phospholamban (PL) content of the sarcoplasmic reticulum were obtained by measurement of the phosphorylated forms of Ca2+-ATPase (E-P) and phospholamban (PL-P) followed by electrophoresis and autoradiography. A 3-fold decrease of PL-P, accompanied by a 2-fold increase of E-P per mg of protein was observed in sarcoplasmic reticulum preparations in the direction hypothyroid----hyperthyroid. Consequently the E-P/PL-P ratio increased from 0.32 (hypothyroid), through 0.81 (euthyroid) to 1.69 (hyperthyroid). In spite of certain limitations inherent to quantification of Ca2+-ATPase and phospholamban by their phosphorylated products, these data provide strong evidence that during thyroid-hormone mediated cardiac hypertrophy, with concomitant proliferation of the sarcoplasmic reticulum, the relative amount of phospholamban decreases with respect to Ca2+-ATPase. This could provide an explanation for the observed gradual diminishment of the beta-adrenergic effect on the relaxation rate when cardiac tissue is exposed to increasing amounts of thyroid hormone.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beekman R. E., van Hardeveld C., Simonides W. S. Effect of thyroid state on cytosolic free calcium in resting and electrically stimulated cardiac myocytes. Biochim Biophys Acta. 1988 Apr 2;969(1):18–27. doi: 10.1016/0167-4889(88)90083-3. [DOI] [PubMed] [Google Scholar]
- Bidlack J. M., Ambudkar I. S., Shamoo A. E. Purification of phospholamban, a 22,000-dalton protein from cardiac sarcoplasmic reticulum that is specifically phosphorylated by cyclic AMP-dependent protein kinase. J Biol Chem. 1982 Apr 25;257(8):4501–4506. doi: 10.2172/5008458. [DOI] [PubMed] [Google Scholar]
- Entam M. L., Kanike K., Goldstein M. A., Nelson T. E., Bornet E. P., Futch T. W., Schwartz A. Association of gylcogenolysis with cardiac sarcoplasmic reticulum. J Biol Chem. 1976 May 25;251(10):3140–3146. [PubMed] [Google Scholar]
- Fujii J., Lytton J., Tada M., MacLennan D. H. Rabbit cardiac and slow-twitch muscle express the same phospholamban gene. FEBS Lett. 1988 Jan 18;227(1):51–55. doi: 10.1016/0014-5793(88)81412-1. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Guarnieri T., Filburn C. R., Beard E. S., Lakatta E. G. Enhanced contractile response and protein kinase activation to threshold levels of beta-adrenergic stimulation in hyperthyroid rat heart. J Clin Invest. 1980 Apr;65(4):861–868. doi: 10.1172/JCI109738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harigaya S., Schwartz A. Rate of calcium binding and uptake in normal animal and failing human cardiac muscle. Membrane vesicles (relaxing system) and mitochondria. Circ Res. 1969 Dec;25(6):781–794. doi: 10.1161/01.res.25.6.781. [DOI] [PubMed] [Google Scholar]
- Ianuzzo D., Patel P., Chen V., O'Brien P., Williams C. Thyroidal trophic influence on skeletal muscle myosin. Nature. 1977 Nov 3;270(5632):74–76. doi: 10.1038/270074a0. [DOI] [PubMed] [Google Scholar]
- Inui M., Kadoma M., Tada M. Purification and characterization of phospholamban from canine cardiac sarcoplasmic reticulum. J Biol Chem. 1985 Mar 25;260(6):3708–3715. [PubMed] [Google Scholar]
- Jones L. R., Simmerman H. K., Wilson W. W., Gurd F. R., Wegener A. D. Purification and characterization of phospholamban from canine cardiac sarcoplasmic reticulum. J Biol Chem. 1985 Jun 25;260(12):7721–7730. [PubMed] [Google Scholar]
- Jorgensen A. O., Jones L. R. Localization of phospholamban in slow but not fast canine skeletal muscle fibers. An immunocytochemical and biochemical study. J Biol Chem. 1986 Mar 15;261(8):3775–3781. [PubMed] [Google Scholar]
- Kranias E. G., Garvey J. L., Srivastava R. D., Solaro R. J. Phosphorylation and functional modifications of sarcoplasmic reticulum and myofibrils in isolated rabbit hearts stimulated with isoprenaline. Biochem J. 1985 Feb 15;226(1):113–121. doi: 10.1042/bj2260113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lichtner R., Wolf H. U. Dodecyl sulphate/polyacrylamide-gel electrophoresis at low pH values and low temperatures. Biochem J. 1979 Sep 1;181(3):759–761. doi: 10.1042/bj1810759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limas C. J. Calcium transport ATPase of cardiac sarcoplasmic reticulum in experimental hyperthyroidism. Am J Physiol. 1978 Dec;235(6):H745–H752. doi: 10.1152/ajpheart.1978.235.6.H745. [DOI] [PubMed] [Google Scholar]
- Limas C. J. Enhanced phosphorylation of myocardial sarcoplasmic reticulum in experimental hyperthyroidism. Am J Physiol. 1978 Apr;234(4):H426–H431. doi: 10.1152/ajpheart.1978.234.4.H426. [DOI] [PubMed] [Google Scholar]
- Lindemann J. P., Jones L. R., Hathaway D. R., Henry B. G., Watanabe A. M. beta-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem. 1983 Jan 10;258(1):464–471. [PubMed] [Google Scholar]
- Louis C. F., Maffitt M., Jarvis B. Factors that modify the molecular size of phospholamban, the 23,000-dalton cardiac sarcoplasmic reticulum phosphoprotein. J Biol Chem. 1982 Dec 25;257(24):15182–15186. [PubMed] [Google Scholar]
- McNeill J. H., Muschek L. D., Brody T. M. The effect of triiodothyronine on cyclic AMP, phosphorylase, and adenyl cyclase in rat heart. Can J Physiol Pharmacol. 1969 Nov;47(11):913–916. doi: 10.1139/y69-149. [DOI] [PubMed] [Google Scholar]
- Morkin E., Flink I. L., Goldman S. Biochemical and physiologic effects of thyroid hormone on cardiac performance. Prog Cardiovasc Dis. 1983 Mar-Apr;25(5):435–464. doi: 10.1016/0033-0620(83)90004-x. [DOI] [PubMed] [Google Scholar]
- Müntener M., van Hardeveld C., Everts M. E., Heizmann C. W. Analysis of the Ca2+-binding parvalbumin in rat skeletal muscles of different thyroid states. Exp Neurol. 1987 Dec;98(3):529–541. doi: 10.1016/0014-4886(87)90262-7. [DOI] [PubMed] [Google Scholar]
- Rodgers R. L., Black S., Katz S., McNeill J. H. Thyroidectomy of SHR: effects on ventricular relaxation and on SR calcium uptake activity. Am J Physiol. 1986 May;250(5 Pt 2):H861–H865. doi: 10.1152/ajpheart.1986.250.5.H861. [DOI] [PubMed] [Google Scholar]
- Rohrer D., Dillmann W. H. Thyroid hormone markedly increases the mRNA coding for sarcoplasmic reticulum Ca2+-ATPase in the rat heart. J Biol Chem. 1988 May 25;263(15):6941–6944. [PubMed] [Google Scholar]
- Simonides W. S., van Hardeveld C. The effect of hypothyroidism on sarcoplasmic reticulum in fast-twitch muscle of the rat. Biochim Biophys Acta. 1985 Feb 21;844(2):129–141. doi: 10.1016/0167-4889(85)90083-7. [DOI] [PubMed] [Google Scholar]
- Suko J. The calcium pump of cardiac sarcoplasmic reticulum. Functional alterations at different levels of thyroid state in rabbits. J Physiol. 1973 Feb;228(3):563–582. doi: 10.1113/jphysiol.1973.sp010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Inui M. Regulation of calcium transport by the ATPase-phospholamban system. J Mol Cell Cardiol. 1983 Sep;15(9):565–575. doi: 10.1016/0022-2828(83)90267-5. [DOI] [PubMed] [Google Scholar]
- Van Hardeveld C., Clausen T. Effect of thyroid status on K+-stimulated metabolism and 45Ca exchange in rat skeletal muscle. Am J Physiol. 1984 Oct;247(4 Pt 1):E421–E430. doi: 10.1152/ajpendo.1984.247.4.E421. [DOI] [PubMed] [Google Scholar]
- Wegener A. D., Jones L. R. Phosphorylation-induced mobility shift in phospholamban in sodium dodecyl sulfate-polyacrylamide gels. Evidence for a protein structure consisting of multiple identical phosphorylatable subunits. J Biol Chem. 1984 Feb 10;259(3):1834–1841. [PubMed] [Google Scholar]