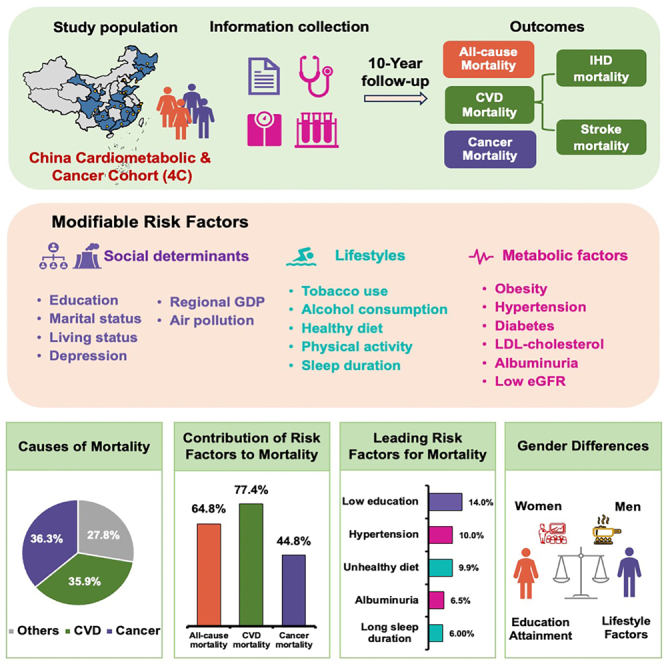
Summary
Nationwide estimates of the impact of common modifiable risk factors on mortality remain crucial. We aim to assess the influence of social determinants, lifestyle, and metabolic factors on mortality in 174,004 adults aged ≥40 years from the China Cardiometabolic Disease and Cancer Cohort (4C) Study. We reveal that 17 modifiable factors are independently associated with mortality, accounting for 64.8% of all-cause mortality, 77.4% of cardiovascular mortality, and 44.8% of cancer mortality. Low education emerges as the leading factor for both all-cause and cancer mortality, while hypertension is predominant for cardiovascular mortality. Moreover, low gross domestic product per capita and high ambient particulate matter with a diameter of <2.5 μm (PM2.5) air pollution account for 7.8% and 4.3% for all-cause mortality, respectively, using a different method. Gender-specific analyses reveal distinct patterns, with women’s mortality primarily associated with social determinants and men exhibiting stronger associations with lifestyle factors. Targeted health interventions are essential to mitigate mortality risks effectively in China.
Keywords: social determinants, lifestyle, metabolic factors, all-cause mortality, CVD mortality, cancer mortality
Graphical abstract
Highlights
-
•
Cardiovascular disease and cancer account for over 72% of all-cause mortality in China
-
•
Modifiable risk factors explain 64.8% of all-cause mortality
-
•
Low education emerges as the leading factor for both all-cause and cancer mortality
-
•
Hypertension is the predominant factor for cardiovascular mortality
Lu et al. reveal the impact of social determinants, lifestyle, and metabolic factors on mortality in China. Targeted strategies are crucial in improving metabolic health to reduce cardiovascular mortality, promoting healthy lifestyles to lower cancer mortality, and addressing lifestyles among men, as well as enhancing educational opportunities for women.
Introduction
Cardiovascular disease and cancer are rising rapidly and have recently become recognized as the predominant burden of mortality in China.1 The estimated number of deaths in China was 10.7 million in 2019. Of these, 4.6 million deaths were from cardiovascular disease and 2.7 million from cancer.2 Identification of preventable risk factors of cardiovascular and cancer mortality can aid in formulating prevention and intervention strategies during transition of disease epidemiology in countries experiencing rapid economic growth, such as China. Furthermore, targeted public health policies accounting for the disparities in risk factor distributions and contributions to health outcomes in men and women are urgently needed.
Existing studies assessing the associations between modifiable risk factors and all-cause mortality were predominantly carried out in the European ancestry.3,4,5,6,7 It is well known that there are non-negligible differences in lifestyle, metabolic features, and social determinants of health across Chinese and Europeans and between men and women.8,9 The spectrum of causes of death and the burden of mortality vary across different countries and ethnicities, with China exhibiting a higher stroke incidence rate and stroke-related deaths compared to other regions.10,11,12 However, large-scale population-based cohorts are scarce in the Chinese population, which limited the capacity of identifying important associations between modifiable risk factors and mortality in the general population and in subpopulations (e.g., in men and women). Previously, the Global Burden of Disease Study1,13,14 has comprehensively estimated the associations of a large number of risk factors with mortality in China. However, the findings from the Global Burden of Disease Study were based on data pooling from diverse sources using different modeling strategies. The subgroup analysis of Prospective Urban Rural Epidemiology study among 46,727 middle-aged Chinese adults15 identified hypertension, low education, and poor diet as three leading risk factors for all-cause mortality, and hypertension, low education, and high non-high-density lipoprotein cholesterol for cardiovascular disease, which is similar to the findings from the overall cohort and other regions.16 Other prospective cohort studies, including the China Kadoorie Biobank etc., have also explored the risk factors for mortality in the Chinese population.17,18 These studies underlie the substantial influence of metabolic factors and socioeconomic status on health outcomes. However, cardiovascular and cancer mortality were not investigated in the Prospective Urban Rural Epidemiology study, and the distinct risk factor profiles and associations with all-cause and cause-specific mortalities in subpopulations were less investigated.
To explore whether and how modifiable risk factors influenced all-cause mortality and mortalities attributed to cardiovascular disease and cancer in China, the prospective China Cardiometabolic Disease and Cancer Cohort (4C) Study was established among Chinese adults aged ≥40 years from 20 study sites across mainland China and followed up between 2011 and 2021, using standardized methods to measure risk factors and outcomes.19,20,21,22,23,24 In this analysis, we aimed to quantify the individual and overall contributions of 17 modifiable social determinants, lifestyle, and metabolic risk factors to all-cause and cause-specific mortalities in the overall population and in men and women separately in China.
Results
A total of 193,846 participants aged 40 years or older were enrolled in the 4C study between January 2011 and December 2012. After excluding 15,697 participants with cardiovascular disease or cancer at baseline, 178,149 participants were followed until November 30, 2021. During a median of 10.1 years of follow-up, 4,145 participants were lost to follow-up. The remaining 174,004 participants were included in the final analysis (Figure S1).
Baseline characteristics of social determinants, lifestyle, and metabolic risk factors of the study participants are presented in Table 1. The average age of the participants was 56.5 (standard deviation [SD] 9.1) years old, and 34.8% were men. A total of 9,274 deaths were documented, including 3,330 cardiovascular deaths (1,247 ischemic heart disease deaths, 1,355 stroke deaths, and 728 other cardiovascular deaths) and 3,365 cancer deaths.
Table 1.
Baseline characteristics of the study population
| Total (n = 174,004) | Women (n = 113,437) | Men (n = 60,567) | |
|---|---|---|---|
| Age, years | 56.5 ± 9.1 | 56.0 ± 8.9 | 57.3 ± 9.5 |
| Social determinants | |||
| Educational attainment | |||
| High school or above | 63,519 (36.5) | 38,384 (33.8) | 25,135 (41.5) |
| Middle school | 59,786 (34.4) | 37,742 (33.3) | 22,044 (36.4) |
| Primary education or less | 50,699 (29.1) | 37,311 (32.9) | 13,388 (22.1) |
| Marital status | |||
| Married | 160,298 (92.1) | 102,038 (90.0) | 58,260 (96.2) |
| Single, divorced, separated, or widowed | 13,706 (7.9) | 11,399 (10.0) | 2,307 (3.8) |
| Living alone | 7,737 (4.4) | 5,809 (5.1) | 1,928 (3.2) |
| Moderate or severe depression | 1,546 (0.9) | 1,171 (1.0) | 375 (0.6) |
| Gross domestic product per capita <36,262 (yuan) | 56,817 (32.7) | 36,642 (32.3) | 20,175 (33.3) |
| PM2.5 (μg/m3) | 47.5 ± 13.0 | 47.5 ± 12.8 | 47.6 ± 13.2 |
| Ambient PM2.5 air pollution >59.11 (μg/m3) | 49,133 (28.2) | 31,778 (28.0) | 17,355 (28.7) |
| Lifestyle factors | |||
| Tobacco use | |||
| Never | 139,201 (80.0) | 111,225 (98.1) | 27,976 (46.2) |
| Former | 8,192 (4.7) | 495 (0.4) | 7,697 (12.7) |
| Current | 26,611 (15.3) | 1,717 (1.5) | 24,894 (41.1) |
| Alcohol consumption | |||
| Never | 150,967 (86.8) | 110,977 (97.8) | 39,990 (66.0) |
| Former | 3,832 (2.2) | 484 (0.4) | 3,348 (5.5) |
| Current | |||
| Low | 4,223 (2.4) | 1,026 (0.9) | 3,197 (5.3) |
| Moderate | 7,718 (4.4) | 455 (0.4) | 7,263 (12.0) |
| High | 7,264 (4.2) | 495 (0.4) | 6,769 (11.2) |
| Diet score | 1.7 ± 0.7 | 1.7 ± 0.7 | 1.7 ± 0.7 |
| Diet score category | |||
| 3–4 | 26,744 (15.4) | 17,060 (15.0) | 9,684 (16.0) |
| 2 | 68,396 (39.3) | 44,931 (39.6) | 23,465 (38.7) |
| 0–1 | 78,864 (45.3) | 51,446 (45.4) | 27,418 (45.3) |
| Physical activity | |||
| High | 47,177 (27.1) | 30,718 (27.1) | 16,459 (27.2) |
| Moderate | 67,064 (38.6) | 44,346 (39.1) | 22,718 (37.5) |
| Low | 59,763 (34.3) | 38,373 (33.8) | 21,390 (35.3) |
| Sleep duration (hours per night) | 7.9 ± 1.4 | 7.9 ± 1.4 | 7.9 ± 1.4 |
| Sleep duration category (hours per night) | |||
| <6 | 8,945 (5.1) | 5,785 (5.1) | 3,160 (5.2) |
| 6–8 | 105,943 (60.9) | 69,424 (61.2) | 36,519 (60.3) |
| >8 | 59,116 (34.0) | 38,228 (33.7) | 20,888 (34.5) |
| Metabolic factors | |||
| Waist-to-hip ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Abdominal obesity | 47,028 (27.0) | 34,626 (30.5) | 12,402 (20.5) |
| Blood pressure | |||
| Systolic blood pressure (mmHg) | 133.2 ± 20.8 | 131.9 ± 21.0 | 135.7 ± 20.2 |
| Diastolic blood pressure (mmHg) | 78.5 ± 11.3 | 77.3 ± 11.0 | 80.8 ± 11.5 |
| Hypertension | 76,024 (43.7) | 46,469 (41.0) | 29,555 (48.8) |
| Glucose | |||
| Fasting blood glucose (mmol/L) | 6.0 ± 1.7 | 5.9 ± 1.6 | 6.1 ± 1.8 |
| 2-h postload glucose (mmol/L) | 8.2 ± 3.9 | 8.2 ± 3.7 | 8.4 ± 4.2 |
| Hemoglobin A1c (%) | 6.0 ± 1.0 | 6.0 ± 1.0 | 6.0 ± 1.1 |
| Diabetes | 40,133 (23.1) | 24,530 (21.6) | 15,603 (25.8) |
| Low-density lipoprotein cholesterol (mmol/L) | 2.9 ± 0.9 | 3.0 ± 0.9 | 2.8 ± 0.8 |
| <3.4 | 127,247 (73.1) | 80,717 (71.2) | 46,530 (76.8) |
| 3.4 to <4.9 | 43,490 (25.0) | 30,230 (26.6) | 13,260 (21.9) |
| ≥4.9 | 3,267 (1.9) | 2,490 (2.2) | 777 (1.3) |
| Urine albumin-creatinine ratio (mg/g) | 6.5 (3.9–12.8) | 7.0 (4.2–13.8) | 5.5 (3.5–10.7) |
| <30 | 155,501 (89.4) | 100,742 (88.8) | 54,759 (90.4) |
| 30 to <300 | 16,320 (9.4) | 11,288 (10.0) | 5,032 (8.3) |
| ≥300 | 2,183 (1.3) | 1,407 (1.2) | 776 (1.3) |
| Estimated glomerular filtration rate (mL/min per 1.73 m2) | 95.6 ± 13.2 | 95.6 ± 12.7 | 95.7 ± 14.0 |
| <60 | 3,107 (1.8) | 1,837 (1.6) | 1,270 (2.1) |
Values are means ± standard deviation, or number of participants (%), or the median (interquartile range). PM2.5 denotes particulate matter with a diameter of <2.5 μm. The sum of the percentages of all subgroups may not add up as 100% because of rounding.
Data are presented for the 174,004 participants after imputation. In the unimputed data, there were 5,259 (3.02%) missing values for educational attainment, 4,066 (2.34%) for marital status, 4,695 (2.70%) for living arrangements, 20,771 (11.94%) for depression status, 7,752 (4.45%) for tobacco use, 10,268 (5.90%) for alcohol consumption, 25,222 (14.50%) for diet score, 9,188 (5.28%) for physical activity, 23,934 (13.75%) for sleep duration, 5,289 (3.04%) for waist-to-hip ratio, 1,554 (0.89%) for systolic blood pressure, 1,565 (0.90%) for diastolic blood pressure, 2,008 (1.15%) for fasting blood glucose, 6,023 (3.46%) for 2-h post-load glucose, 1,391 (0.80%) for hemoglobin A1c, 529 (0.30%) for low-density lipoprotein cholesterol, 16,896 (9.71%) for urinary albumin-creatinine ratio, and 555 (0.32%) for estimated glomerular filtration rate.
For social determinants of health, 50,699 (29.1%) participants had primary school education level or less, 13,706 (7.9%) participants were single, divorced, separated, or widowed, and 7,737 (4.4%) lived alone. 1,546 (0.9%) participants reported symptoms of moderate or severe depression. For lifestyle risk factors, 34,803 (20.0%) participants were current or former smokers, and 7,264 (4.2%) participants had current high alcohol consumption (11.2% in men and 0.4% in women). Mean diet score was 1.7 (SD 0.7; a lower score indicates worse diet), and 147,260 (84.6%) participants had unhealthy diet (score 0–2). 59,763 (34.3%) participants reported low physical activity. The mean sleep duration was 7.9 (SD 1.4) hours; therefore, 8 h was used as the cutoff for sleep duration to estimate the population-attributable fractions (PAFs) in this study, which resulted in 59,116 (34.0%) participants with sleep duration >8 h. For metabolic risk factors, mean waist-to-hip ratio was 0.9 (SD 0.1), and 47,028 (27.0%) participants had abdominal obesity. Mean systolic blood pressure was 133.2 (SD 20.8) mmHg, mean hemoglobin A1c was 6.0% (SD 1.0%), mean low-density lipoprotein cholesterol (LDL-C) was 2.9 (SD 0.9) mmol/L, and median urine albumin-creatinine ratio was 6.5 (interquartile range 3.9–12.8) mg/g. A total of 76,024 (43.7%) participants had hypertension, 40,133 (23.1%) had diabetes, 46,757 (26.9%) had LDL-C ≥ 3.4 mmol/L, 18,503 (10.7%) had albuminuria, and 3,107 (1.8%) had a low estimated glomerular filtration rate (<60 mL/min per 1.73 m2).
When considering gender, baseline social determinants and lifestyle risk factors were substantially different between men and women. More women had low educational attainment than men (32.9% in women vs. 22.1% in men). For lifestyle risk factors, a larger proportion of men had current tobacco use (41.1% in men vs. 1.5% in women) or current alcohol consumption (28.5% in men vs. 1.7% in women) than women. A similar proportion of women and men had unhealthy diet habit (85.0% in women vs. 84.0% in men), low physical activity (33.8% in women vs. 35.3% in men), and long sleep duration (33.7% in women vs. 34.5% in men). For metabolic risk factors, women were more likely to have abdominal obesity (30.5% in women vs. 20.5% in men) and higher LDL-C (28.8% in women vs. 23.2% in men). By contrast, the proportions of hypertension (41.0% in women vs. 48.8% in men), diabetes (21.6% in women vs. 25.8% in men), and a low estimated glomerular filtration rate (1.6% in women vs. 2.1% in men) were higher in men than in women.
Cumulative incidence of all-cause, cardiovascular, and cancer mortalities with each risk factor using Kaplan-Meier analysis is presented in Figures S2–S4. Multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) of each of the modifiable risk factors in associations with all-cause, cardiovascular, and cancer mortalities are presented in Tables 2, 3, and 4, and the results for subtypes of cardiovascular mortality (ischemic heart disease and stroke mortality) are presented in Tables S1 and S2. Among social determinants, low educational attainment; marital status of being single, divorced, separated, or widowed; and living alone were significantly and independently associated with all-cause and cardiovascular mortality, with depression additionally associated with all-cause and cancer mortality (Tables 2, 3, and 4). Low educational attainment was additionally associated with ischemic heart disease and stroke mortality (Tables S1 and S2). Among these factors, moderate or severe depression showed the largest HRs for all-cause (HR = 1.52, 95% CI = 1.24–1.85) and cancer mortality (HR = 1.67, 95% CI = 1.21–2.30; Tables 2 and 4). Gender-specific analysis showed that low education was associated with cardiovascular and ischemic heart disease mortality more strongly in women than in men (pinteraction< 0.01; Tables 3 and S1).
Table 2.
Multivariable-adjusted hazard ratios and 95% confidence intervals for all-cause mortality in overall population and by gender using the Cox regression model
| Overall |
Women |
Men |
pinteraction of women and men | ||||
|---|---|---|---|---|---|---|---|
| Case/n (%) | HR (95% CI) | Case/n (%) | HR (95% CI) | Case/n (%) | HR (95% CI) | ||
| Social determinants | |||||||
| Educational attainment | |||||||
| High school or above | 2,195/63,519 (3.5) | 1.00 (reference) | 762/38,384 (2.0) | 1.00 (reference) | 1,433/25,135 (5.7) | 1.00 (reference) | 0.466 |
| Middle school | 2,609/59,786 (4.4) | 1.13 (1.07–1.20) | 1,002/37,742 (2.7) | 1.09 (0.99–1.20) | 1,608/22,044 (7.3) | 1.16 (1.07–1.25) | |
| Primary school or less | 4,470/50,699 (8.8) | 1.35 (1.27–1.44) | 2,577/37,311 (6.9) | 1.32 (1.20–1.44) | 1,893/13,388 (14.1) | 1.37 (1.26–1.48) | |
| Marital status | |||||||
| Married | 7,984/160,298 (5.0) | 1.00 (reference) | 3,424/102,038 (3.4) | 1.00 (reference) | 4,560/58,260 (7.8) | 1.00 (reference) | 0.922 |
| Single, divorced, separated, or widowed | 1,290/13,706 (9.4) | 1.14 (1.06–1.22) | 917/11,399 (8.1) | 1.11 (1.02–1.21) | 373/2,307 (16.2) | 1.18 (1.03–1.34) | |
| Living alone | |||||||
| No | 8,524/166,267 (5.1) | 1.00 (reference) | 3,857/107,628 (3.6) | 1.00 (reference) | 4,666/58,639 (8.0) | 1.00 (reference) | 0.975 |
| Yes | 750/7,737 (9.7) | 1.21 (1.11–1.32) | 484/5,809 (8.3) | 1.20 (1.08–1.34) | 267/1,928 (13.8) | 1.20 (1.03–1.40) | |
| Moderate or severe depression | |||||||
| No | 9,168/172,458 (5.3) | 1.00 (reference) | 4,280/112,266 (3.8) | 1.00 (reference) | 4,888/60,192 (8.1) | 1.00 (reference) | 0.807 |
| Yes | 106/1,546 (6.8) | 1.52 (1.24–1.85) | 61/1,171 (5.2) | 1.50 (1.16–1.95) | 45/375 (11.9) | 1.55 (1.14–2.11) | |
| Gross domestic product per capita (yuan)∗ | |||||||
| ≥36,262 | 5,556/117,187 (4.7) | 1.00 (reference) | 2,628/76,795 (3.4) | 1.00 (reference) | 2,928/40,392 (7.3) | 1.00 (reference) | 0.800 |
| <36,262 | 3,718/56,817 (6.5) | 1.26 (1.20–1.33) | 1,713/36,642 (4.7) | 1.28 (1.19–1.38) | 2,005/20,175 (9.9) | 1.25 (1.16–1.34) | |
| Ambient PM2.5air pollution (μg/m3)∗ | |||||||
| <59.11 | 5,912/124,871 (4.7) | 1.00 (reference) | 2,793/81,659 (3.4) | 1.00 (reference) | 3,119/43,212 (7.2) | 1.00 (reference) | 0.422 |
| ≥59.11 | 3,362/49,133 (6.8) | 1.16 (1.11–1.22) | 1,548/31,778 (4.9) | 1.13 (1.05–1.21) | 1,814/17,355 (10.5) | 1.19 (1.11–1.27) | |
| Lifestyle risk factors | |||||||
| Tobacco use | |||||||
| Never | 6,440/139,201 (4.6) | 1.00 (reference) | 4,196/111,225 (3.8) | 1.00 (reference) | 2,244/27,976 (8.0) | 1.00 (reference) | 0.870 |
| Former | 768/8,192 (9.4) | 1.20 (1.10–1.30) | 42/495 (8.5) | 1.30 (0.95–1.78) | 726/7,697 (9.4) | 1.18 (1.08–1.29) | |
| Current | 2,066/26,611 (7.8) | 1.35 (1.27–1.44) | 103/1,717 (6.0) | 1.35 (1.10–1.65) | 1,963/24,894 (7.9) | 1.34 (1.25–1.43) | |
| Alcohol consumption | |||||||
| Never | 7,525/150,967 (5.0) | 1.00 (reference) | 4,235/110,977 (3.8) | 1.00 (reference) | 3,290/39,990 (8.2) | 1.00 (reference) | 0.688 |
| Former | 393/3,832 (10.3) | 1.16 (1.04–1.30) | 27/484 (5.5) | 1.18 (0.79–1.76) | 366/3,348 (10.9) | 1.16 (1.03–1.30) | |
| Current: low | 259/4,223 (6.1) | 0.85 (0.74–0.96) | 41/1,026 (4.0) | 0.96 (0.70–1.31) | 218/3,197 (6.8) | 0.83 (0.72–0.96) | |
| Current: moderate | 522/7,718 (6.8) | 0.87 (0.79–0.96) | 19/455 (4.2) | 0.93 (0.57–1.49) | 503/7,263 (6.9) | 0.87 (0.79–0.96) | |
| Current: high | 575/7,264 (7.9) | 1.06 (0.96–1.17) | 19/495 (3.8) | 0.83 (0.52–1.33) | 556/6,769 (8.2) | 1.07 (0.97–1.18) | |
| Diet score | |||||||
| 3–4 | 1,046/26,744 (3.9) | 1.00 (reference) | 485/17,060 (2.8) | 1.00 (reference) | 562/9,684 (5.8) | 1.00 (reference) | 0.185 |
| 2 | 3,341/68,396 (4.9) | 1.09 (1.01–1.18) | 1,540/44,931 (3.4) | 1.00 (0.90–1.11) | 1,801/23,465 (7.7) | 1.17 (1.06–1.29) | |
| 0–1 | 4,886/78,864 (6.2) | 1.17 (1.08–1.26) | 2,316/51,446 (4.5) | 1.06 (0.95–1.18) | 2,570/27,418 (9.4) | 1.26 (1.14–1.39) | |
| Physical activity | |||||||
| High | 2,619/47,177 (5.6) | 1.00 (reference) | 1,182/30,718 (3.9) | 1.00 (reference) | 1,437/16,459 (8.7) | 1.00 (reference) | 0.291 |
| Moderate | 3,456/67,064 (5.2) | 1.00 (0.95–1.05) | 1,670/44,346 (3.8) | 0.99 (0.92–1.07) | 1,786/22,718 (7.9) | 1.00 (0.93–1.07) | |
| Low | 3,199/59,763 (5.4) | 1.08 (1.02–1.14) | 1,489/38,373 (3.9) | 1.04 (0.96–1.13) | 1,710/21,390 (8.0) | 1.10 (1.02–1.19) | |
| Sleep duration (hours per night) | |||||||
| 6–8 | 4,753/105,943 (4.5) | 1.00 (reference) | 2,226/69,424 (3.2) | 1.00 (reference) | 2,526/36,519 (6.9) | 1.00 (reference) | 0.691 |
| <6 | 451/8,945 (5.0) | 0.94 (0.85–1.05) | 217/5,785 (3.8) | 0.92 (0.79–1.08) | 234/3,160 (7.4) | 0.97 (0.82–1.13) | |
| >8 | 4,070/59,116 (6.9) | 1.18 (1.12–1.24) | 1,897/38,228 (5.0) | 1.18 (1.10–1.27) | 2,173/20,888 (10.4) | 1.18 (1.11–1.26) | |
| Metabolic risk factors | |||||||
| Abdominal obesity | |||||||
| No | 6,334/126,976 (5.0) | 1.00 (reference) | 2,543/78,811 (3.2) | 1.00 (reference) | 3,792/48,165 (7.9) | 1.00 (reference) | 0.532 |
| Yes | 2,940/47,028 (6.3) | 1.06 (1.01–1.11) | 17,998/34,626 (5.2) | 1.04 (0.97–1.10) | 1,141/12,402 (9.2) | 1.08 (1.01–1.15) | |
| Hypertension | |||||||
| No | 3,398/97,980 (3.5) | 1.00 (Reference) | 1,591/66,968 (2.4) | 1.00 (reference) | 1,807/31,012 (5.8) | 1.00 (reference) | 0.064 |
| Yes | 5,876/76,024 (7.7) | 1.19 (1.14–1.25) | 2,750/46,469 (5.9) | 1.22 (1.14–1.30) | 3,126/29,555 (10.6) | 1.17 (1.10–1.24) | |
| Diabetes | |||||||
| No | 5,935/133,871 (4.4) | 1.00 (reference) | 2,774/88,907 (3.1) | 1.00 (reference) | 3,161/44,964 (7.0) | 1.00 (reference) | 0.430 |
| Yes | 3,339/40,133 (8.3) | 1.31 (1.25–1.37) | 1,567/24,530 (6.4) | 1.26 (1.18–1.35) | 1,772/15,603 (11.4) | 1.35 (1.27–1.43) | |
| Low-density lipoprotein cholesterol (mmol/L) | |||||||
| <3.4 | 6,843/127,247 (5.4) | 1.00 (reference) | 2,977/80,717 (3.7) | 1.00 (reference) | 3,867/46,530 (8.3) | 1.00 (reference) | 0.245 |
| 3.4 - <4.9 | 2,233/43,490 (5.1) | 0.90 (0.86–0.95) | 1,234/30,230 (4.1) | 0.92 (0.86–0.99) | 999/13,260 (7.5) | 0.88 (0.82–0.94) | |
| ≥4.9 | 198/3,267 (6.1) | 1.04 (0.90–1.20) | 130/2,490 (5.2) | 1.03 (0.87–1.23) | 67/777 (8.7) | 1.04 (0.82–1.33) | |
| Urine albumin-creatinine ratio (mg/g) | |||||||
| <30 | 7,064/155,501 (4.5) | 1.00 (reference) | 3,127/100,742 (3.1) | 1.00 (reference) | 3,937/54,759 (7.2) | 1.00 (reference) | 0.008 |
| 30 - <300 | 1,707/16,320 (10.5) | 1.50 (1.42–1.59) | 946/11,288 (8.4) | 1.59 (1.47–1.72) | 761/5,032 (15.1) | 1.41 (1.29–1.53) | |
| ≥300 | 503/2,183 (23.0) | 2.85 (2.57–3.15) | 268/1,407 (19.0) | 3.07 (2.67–3.53) | 235/776 (30.3) | 2.63 (2.28–3.04) | |
| Estimated glomerular filtration rate <60 (mL/min per 1.73 m2) | |||||||
| No | 8,588/170,897 (5.0) | 1.00 (reference) | 4,021/111,600 (3.6) | 1.00 (reference) | 4,567/59,297 (7.7) | 1.00 (reference) | 0.335 |
| Yes | 686/3,107 (22.1) | 1.46 (1.35–1.59) | 320/1,837 (17.4) | 1.47 (1.30–1.66) | 366/1,270 (28.8) | 1.46 (1.30–1.64) | |
For the 15 individual-level risk factors (excluding gross domestic product per capita and ambient PM2.5 air pollution), each model was adjusted for age, gender, region (urban or rural), and all other individual-level risk factors. ∗For the two community-level risk factors of gross domestic product per capita and ambient PM2.5 air pollution, a separate model was employed with adjustment for age, gender, region (urban or rural), and the remaining 15 individual-level risk factors.
See also Table S4, Figures S2, and S5.
Table 3.
Multivariable-adjusted hazard ratios and 95% confidence intervals for cardiovascular mortality in overall population and by gender using the competing risk regression model
| Overall |
Women |
Men |
pinteraction of women and men | ||||
|---|---|---|---|---|---|---|---|
| Case/n (%) | HR (95% CI) | Case/n (%) | HR (95% CI) | Case/n (%) | HR (95% CI) | ||
| Social determinants | |||||||
| Educational attainment | 0.002 | ||||||
| High school or above | 655/63,519 (1.0) | 1.00 (reference) | 202/38,384 (0.5) | 1.00 (reference) | 453/25,135 (1.8) | 1.00 (reference) | |
| Middle school | 802/59,786 (1.3) | 1.11 (0.99–1.24) | 310/37,742 (0.8) | 1.18 (0.98–1.43) | 492/22,044 (2.2) | 1.09 (0.95–1.25) | |
| Primary school or less | 1,873/50,699 (3.7) | 1.46 (1.31–1.63) | 1141/37,311 (3.1) | 1.57 (1.32–1.86) | 732/13,388 (5.5) | 1.40 (1.22–1.62) | |
| Marital status | 0.300 | ||||||
| Married | 2,785/160,298 (1.7) | 1.00 (reference) | 1,272/102,038 (1.3) | 1.00 (reference) | 1,513/58,260 (2.6) | 1.00 (reference) | |
| Single, divorced, separated, or widowed | 545/13,706 (4.0) | 1.17 (1.04–1.31) | 381/11,399 (3.3) | 1.07 (0.93–1.23) | 164/2,307 (7.1) | 1.34 (1.08–1.66) | |
| Living alone | 0.517 | ||||||
| No | 3,024/166,267 (1.8) | 1.00 (reference) | 1,457/107,628 (1.4) | 1.00 (reference) | 1,568/58,639 (2.7) | 1.00 (reference) | |
| Yes | 306/7,737 (4.0) | 1.18 (1.02–1.36) | 196/5,809 (3.4) | 1.14 (0.96–1.35) | 109/1,928 (5.7) | 1.20 (0.93–1.55) | |
| Moderate or severe depression | 0.873 | ||||||
| No | 3,301/172,458 (1.9) | 1.00 (reference) | 1,635/112,266 (1.5) | 1.00 (reference) | 1,666/60,192 (2.8) | 1.00 (reference) | |
| Yes | 29/1,546 (1.9) | 1.22 (0.81–1.83) | 18/1,171 (1.6) | 1.32 (0.80–2.16) | 11/375 (3.0) | 1.10 (0.56–2.15) | |
| Gross domestic product per capita (yuan)∗ | 0.003 | ||||||
| ≥36,262 | 1,693/117,187 (1.4) | 1.00 (reference) | 813/76,795 (1.1) | 1.00 (reference) | 880/40,392 (2.2) | 1.00 (reference) | |
| <36,262 | 1,637/56,817 (2.9) | 1.46 (1.34–1.59) | 840/36,642 (2.3) | 1.60 (1.43–1.80) | 797/20,175 (4.0) | 1.34 (1.19–1.51) | |
| Ambient PM2.5air pollution (μg/m3)∗ | 0.469 | ||||||
| <59.11 | 1,743/124,871 (1.4) | 1.00 (reference) | 860/81,659 (1.1) | 1.00 (Reference) | 883/43,212 (2.0) | 1.00 (Reference) | |
| ≥59.11 | 1,587/49,133 (3.2) | 1.55 (1.43–1.67) | 793/31,778 (2.5) | 1.45 (1.30–1.62) | 794/17,355 (4.6) | 1.62 (1.45–1.81) | |
| Lifestyle risk factors | |||||||
| Tobacco use | 0.061 | ||||||
| Never | 2,447/139,201 (1.8) | 1.00 (reference) | 1,591/111,225 (1.4) | 1.00 (reference) | 856/27,976 (3.1) | 1.00 (reference) | |
| Former | 247/8,192 (3.0) | 1.05 (0.91–1.22) | 17/495 (3.5) | 1.26 (0.76–2.11) | 230/7,697 (3.0) | 1.00 (0.86–1.16) | |
| Current | 636/26,611 (2.4) | 1.21 (1.08–1.35) | 45/1,717 (2.6) | 1.54 (1.13–2.08) | 591/24,894 (2.4) | 1.13 (1.01–1.28) | |
| Alcohol consumption | 0.517 | ||||||
| Never | 2,771/150,967 (1.8) | 1.00 (reference) | 1,614/110,977 (1.5) | 1.00 (reference) | 1,156/39,990 (2.9) | 1.00 (reference) | |
| Former | 146/3,832 (3.8) | 1.26 (1.05–1.52) | 8/484 (1.7) | 0.90 (0.42–1.91) | 138/3,348 (4.1) | 1.30 (1.08–1.57) | |
| Current: low | 81/4,223 (1.9) | 0.77 (0.61–0.98) | 14/1,026 (1.4) | 0.86 (0.50–1.48) | 67/3,197 (2.1) | 0.76 (0.59–0.99) | |
| Current: moderate | 148/7,718 (1.9) | 0.72 (0.60–0.87) | 5/455 (1.1) | 0.61 (0.23–1.59) | 143/7,263 (2.0) | 0.73 (0.61–0.88) | |
| Current: high | 184/7,264 (2.5) | 0.98 (0.82–1.16) | 11/495 (2.3) | 1.21 (0.68–2.18) | 173/6,769 (2.6) | 0.99 (0.83–1.18) | |
| Diet score | 0.315 | ||||||
| 3–4 | 321/26,744 (1.2) | 1.00 (reference) | 154/17,060 (0.9) | 1.00 (reference) | 167/9,684 (1.7) | 1.00 (reference) | |
| 2 | 1,132/68,396 (1.7) | 1.16 (1.02–1.32) | 566/44,931 (1.3) | 1.10 (0.92–1.33) | 566/23,465 (2.4) | 1.20 (1.00–1.45) | |
| 0–1 | 1,877/78,864 (2.4) | 1.30 (1.14–1.48) | 933/51,446 (1.8) | 1.18 (0.98–1.41) | 944/27,418 (3.4) | 1.43 (1.19–1.71) | |
| Physical activity | 0.922 | ||||||
| High | 959/47,177 (2.0) | 1.00 (reference) | 444/30,718 (1.5) | 1.00 (reference) | 515/16,459 (3.1) | 1.00 (reference) | |
| Moderate | 1,193/67,064 (1.8) | 0.94 (0.86–1.03) | 623/44,346 (1.4) | 0.99 (0.87–1.13) | 569/22,718 (2.5) | 0.89 (0.79–1.02) | |
| Low | 1,178/59,763 (2.0) | 1.02 (0.93–1.12) | 586/38,373 (1.5) | 1.00 (0.87–1.15) | 593/21,390 (2.8) | 1.04 (0.91–1.18) | |
| Sleep duration (hours per night) | 0.089 | ||||||
| 6–8 | 1,567/105,943 (1.5) | 1.00 (reference) | 759/69,424 (1.1) | 1.00 (reference) | 808/36,519 (2.2) | 1.00 (reference) | |
| <6 | 136/8,945 (1.5) | 0.85 (0.65–1.11) | 66/5,785 (1.1) | 0.81 (0.58–1.13) | 70/3,160 (2.2) | 0.90 (0.64–1.26) | |
| >8 | 1,627/59,116 (2.8) | 1.22 (1.12–1.34) | 828/38,228 (2.2) | 1.25 (1.10–1.40) | 799/20,888 (3.8) | 1.20 (1.07–1.35) | |
| Metabolic risk factors | |||||||
| Abdominal obesity | 0.041 | ||||||
| No | 2,243/126,976 (1.8) | 1.00 (reference) | 970/78,811 (1.2) | 1.00 (reference) | 1,273/48,165 (2.6) | 1.00 (reference) | |
| Yes | 1,087/47,028 (2.3) | 1.03 (0.96–1.12) | 683/34,626 (2.0) | 0.95 (0.86–1.06) | 404/12,402 (3.3) | 1.12 (1.00–1.26) | |
| Hypertension | 0.002 | ||||||
| No | 886/97,980 (0.9) | 1.00 (reference) | 408/66,968 (0.6) | 1.00 (reference) | 478/31,012 (1.5) | 1.00 (reference) | |
| Yes | 2,444/76,024 (3.2) | 1.67 (1.54–1.82) | 1,245/46,469 (2.7) | 1.82 (1.60–2.05) | 1,199/29,555 (4.1) | 1.53 (1.36–1.72) | |
| Diabetes | 0.450 | ||||||
| No | 2,051/133,871 (1.5) | 1.00 (reference) | 1,014/88,907 (1.1) | 1.00 (reference) | 1,037/44,964 (2.3) | 1.00 (reference) | |
| Yes | 1,279/40,133 (3.2) | 1.34 (1.24–1.45) | 639/24,530 (2.6) | 1.30 (1.17–1.45) | 640/15,603 (4.1) | 1.38 (1.24–1.53) | |
| Low-density lipoprotein cholesterol (mmol/L) | 0.816 | ||||||
| <3.4 | 2,340/127,247 (1.8) | 1.00 (reference) | 1,076/80,717 (1.3) | 1.00 (reference) | 1,264/46,530 (2.7) | 1.00 (reference) | |
| 3.4 - <4.9 | 890/43,490 (2.1) | 1.04 (0.96–1.12) | 516/30,230 (1.7) | 1.06 (0.96–1.18) | 374/13,260 (2.8) | 1.00 (0.89–1.13) | |
| ≥4.9 | 100/3,267 (3.1) | 1.52 (1.24–1.87) | 61/2,490 (2.5) | 1.33 (1.03–1.72) | 39/777 (5.1) | 1.96 (1.40–2.76) | |
| Urine albumin-creatinine ratio (mg/g) | 0.066 | ||||||
| <30 | 2,322/155,501 (1.5) | 1.00 (reference) | 1,073/100,742 (1.1) | 1.00 (reference) | 1,249/54,759 (2.3) | 1.00 (reference) | |
| 30 - <300 | 767/16,320 (4.7) | 1.68 (1.54–1.84) | 447/11,288 (4.0) | 1.74 (1.53–1.98) | 320/5,032 (6.4) | 1.58 (1.38–1.82) | |
| ≥300 | 241/2,183 (11.0) | 3.15 (2.70–3.68) | 133/1,407 (9.5) | 3.25 (2.66–3.98) | 108/776 (13.9) | 2.98 (2.35–3.78) | |
| Estimated glomerular filtration rate <60 (ml/min per 1.73 m2) | 0.808 | ||||||
| No | 3,037/170,897 (1.8) | 1.00 (reference) | 1,515/111,600 (1.4) | 1.00 (reference) | 1,522/59,297 (2.6) | 1.00 (reference) | |
| Yes | 293/3,107 (9.4) | 1.34 (1.17–1.54) | 138/1,837 (7.5) | 1.30 (1.07–1.57) | 155/1,270 (12.2) | 1.41 (1.16–1.71) | |
For the 15 individual-level risk factors (excluding gross domestic product per capita and ambient PM2.5 air pollution), each model was adjusted for age, gender, region (urban or rural), and all other individual-level risk factors. ∗For the two community-level risk factors of gross domestic product per capita and ambient PM2.5 air pollution, a separate model was employed with adjustment for age, gender, region (urban or rural), and the remaining 15 individual-level risk factors.
See also Tables S1, S2, and S4 and Figures S3 and S5.
Table 4.
Multivariable-adjusted hazard ratios and 95% confidence intervals for cancer mortality in overall population and by gender using the competing risk regression model
| Overall |
Women |
Men |
pinteraction of women and men | ||||
|---|---|---|---|---|---|---|---|
| Case/n (%) | HR (95% CI) | Case/n (%) | HR (95% CI) | Case/n (%) | HR (95% CI) | ||
| Social determinants | |||||||
| Educational attainment | 0.064 | ||||||
| High school or above | 934/63,519 (1.5) | 1.00 (reference) | 346/38,384 (0.9) | 1.00 (reference) | 588/25,135 (2.3) | 1.00 (reference) | |
| Middle school | 1,057/59,786 (1.8) | 1.10 (1.00–1.20) | 413/37,742 (1.1) | 1.06 (0.92–1.23) | 644/22,044 (2.9) | 1.11 (0.99–1.25) | |
| Primary school or less | 1,374/50,699 (2.7) | 1.19 (1.08–1.31) | 711/37,311 (1.9) | 1.14 (0.98–1.32) | 662/13,388 (5.0) | 1.25 (1.10–1.42) | |
| Marital status | 0.882 | ||||||
| Married | 3,039/160,298 (1.9) | 1.00 (reference) | 1,244/102,038 (1.2) | 1.00 (reference) | 1,795/58,260 (3.1) | 1.00 (reference) | |
| Single, divorced, separated, or widowed | 326/13,706 (2.4) | 0.92 (0.80–1.05) | 226/11,399 (2.0) | 0.93 (0.79–1.10) | 100/2,307 (4.3) | 0.91 (0.72–1.16) | |
| Living alone | 0.691 | ||||||
| No | 3,159/166,267 (1.9) | 1.00 (reference) | 1,343/107,628 (1.3) | 1.00 (reference) | 1,816/58,639 (3.1) | 1.00 (reference) | |
| Yes | 206/7,737 (2.7) | 1.16 (0.98–1.36) | 127/5,809 (2.2) | 1.20 (0.97–1.48) | 79/1,928 (4.1) | 1.11 (0.85–1.43) | |
| Moderate or severe depression | 0.723 | ||||||
| No | 3,323/172,458 (1.9) | 1.00 (reference) | 1,445/112,266 (1.3) | 1.00 (reference) | 1,878/60,192 (3.1) | 1.00 (reference) | |
| Yes | 42/1,546 (2.7) | 1.67 (1.21–2.30) | 25/1,171 (2.1) | 1.72 (1.14–2.58) | 17/375 (4.5) | 1.60 (0.96–2.65) | |
| Gross domestic product per capita (yuan)∗ | 0.005 | ||||||
| ≥36,262 | 2,032/117,187 (1.7) | 1.00 (reference) | 937/76,795 (1.2) | 1.00 (reference) | 1,095/40,392 (2.7) | 1.00 (reference) | |
| <36,262 | 1,333/56,817 (2.4) | 1.28 (1.18–1.39) | 533/36,642 (1.5) | 1.19 (1.05–1.35) | 800/20,175 (4.0) | 1.35 (1.21–1.50) | |
| Ambient PM2.5air pollution (μg/m3)∗ | 0.001 | ||||||
| <59.11 | 2,243/124,871 (1.8) | 1.00 (reference) | 1,032/81,659 (1.3) | 1.00 (reference) | 1,211/43,212 (2.8) | 1.00 (reference) | |
| ≥59.11 | 1,122/49,133 (2.3) | 1.06 (0.98–1.15) | 438/31,778 (1.4) | 0.96 (0.85–1.10) | 684/17,355 (3.9) | 1.14 (1.03–1.27) | |
| Lifestyle risk factors | |||||||
| Tobacco use | 0.169 | ||||||
| Never | 2,167/139,201 (1.6) | 1.00 (reference) | 1427/111,225 (1.3) | 1.00 (reference) | 740/27,976 (2.7) | 1.00 (reference) | |
| Former | 314/8,192 (3.8) | 1.43 (1.25–1.64) | 14/495 (2.8) | 1.46 (0.85–2.50) | 300/7,697 (3.9) | 1.47 (1.28–1.69) | |
| Current | 884/26,611 (3.3) | 1.55 (1.41–1.72) | 29/1,717 (1.7) | 1.18 (0.81–1.73) | 855/24,894 (3.4) | 1.61 (1.45–1.79) | |
| Alcohol consumption | 0.473 | ||||||
| Never | 2,618/150,967 (1.7) | 1.00 (reference) | 1,429/110,977 (1.3) | 1.00 (reference) | 1,188/39,990 (3.0) | 1.00 (reference) | |
| Former | 137/3,832 (3.6) | 1.11 (0.92–1.34) | 14/484 (2.8) | 1.93 (1.13–3.33) | 124/3,348 (3.7) | 1.04 (0.86–1.27) | |
| Current: low | 108/4,223 (2.6) | 0.96 (0.79–1.17) | 15/1,026 (1.4) | 1.05 (0.62–1.79) | 93/3,197 (2.9) | 0.94 (0.76–1.17) | |
| Current: moderate | 250/7,718 (3.2) | 1.08 (0.94–1.24) | 8/455 (1.7) | 1.21 (0.58–2.54) | 242/7,263 (3.3) | 1.07 (0.93–1.23) | |
| Current: high | 252/7,264 (3.5) | 1.20 (1.04–1.39) | 4/495 (0.9) | 0.63 (0.24–1.69) | 248/6,769 (3.7) | 1.20 (1.04–1.39) | |
| Diet score | 0.258 | ||||||
| 3–4 | 449/26,744 (1.7) | 1.00 (reference) | 204/17,060 (1.2) | 1.00 (reference) | 245/9,684 (2.5) | 1.00 (reference) | |
| 2 | 1,255/68,396 (1.8) | 1.01 (0.90–1.13) | 536/44,931 (1.2) | 0.89 (0.75–1.05) | 719/23,465 (3.1) | 1.11 (0.96–1.29) | |
| 0–1 | 1,661/78,864 (2.1) | 1.03 (0.92–1.15) | 729/51,446 (1.4) | 0.93 (0.79–1.10) | 931/27,418 (3.4) | 1.12 (0.97–1.30) | |
| Physical activity | 0.276 | ||||||
| High | 927/47,177 (2.0) | 1.00 (reference) | 409/30,718 (1.3) | 1.00 (reference) | 518/16,459 (3.2) | 1.00 (reference) | |
| Moderate | 1,306/67,064 (2.0) | 1.06 (0.97–1.15) | 573/44,346 (1.3) | 0.99 (0.86–1.12) | 733/22,718 (3.2) | 1.12 (1.00–1.26) | |
| Low | 1,132/59,763 (1.9) | 1.07 (0.98–1.17) | 488/38,373 (1.3) | 1.02 (0.89–1.17) | 644/21,390 (3.0) | 1.11 (0.99–1.26) | |
| Sleep duration, hours per night | 0.381 | ||||||
| 6–8 | 1,839/105,943 (1.7) | 1.00 (reference) | 823/69,424 (1.2) | 1.00 (reference) | 1016/36,519 (2.8) | 1.00 (reference) | |
| <6 | 173/8,945 (1.9) | 0.96 (0.75–1.23) | 82/5,785 (1.4) | 0.98 (0.70–1.38) | 91/3,160 (2.9) | 0.95 (0.72–1.26) | |
| >8 | 1,353/59,116 (2.3) | 1.10 (1.02–1.19) | 565/38,228 (1.5) | 1.09 (0.96–1.23) | 788/20,888 (3.8) | 1.12 (1.01–1.24) | |
| Metabolic risk factors | |||||||
| Abdominal obesity | 0.744 | ||||||
| No | 2,361/126,976 (1.9) | 1.00 (reference) | 890/78,811 (1.1) | 1.00 (reference) | 1,471/48,165 (3.1) | 1.00 (reference) | |
| Yes | 1,004/47,028 (2.1) | 1.09 (1.01–1.18) | 580/34,626 (1.7) | 1.12 (1.01–1.25) | 424/12,402 (3.4) | 1.08 (0.97–1.21) | |
| Hypertension | 0.761 | ||||||
| No | 1,438/97,980 (1.5) | 1.00 (reference) | 664/66,968 (1.0) | 1.00 (reference) | 774/31,012 (2.5) | 1.00 (reference) | |
| Yes | 1,927/76,024 (2.5) | 1.12 (1.04–1.21) | 806/46,469 (1.7) | 1.13 (1.01–1.26) | 1,121/29,555 (3.8) | 1.13 (1.03–1.25) | |
| Diabetes | 0.357 | ||||||
| No | 2,336/133,871 (1.7) | 1.00 (reference) | 1,037/88,907 (1.2) | 1.00 (reference) | 1,299/44,964 (2.9) | 1.00 (reference) | |
| Yes | 1,029/40,133 (2.6) | 1.14 (1.05–1.23) | 433/24,530 (1.8) | 1.08 (0.96–1.22) | 596/15,603 (3.8) | 1.19 (1.07–1.31) | |
| Low-density lipoprotein cholesterol (mmol/L) | 0.551 | ||||||
| <3.4 | 2,529/127,247 (2.0) | 1.00 (reference) | 1,031/80,717 (1.3) | 1.00 (reference) | 1,498/46,530 (3.2) | 1.00 (reference) | |
| 3.4 - <4.9 | 780/43,490 (1.8) | 0.89 (0.82–0.96) | 402/30,230 (1.3) | 0.90 (0.80–1.01) | 378/13,260 (2.9) | 0.88 (0.79–0.99) | |
| ≥4.9 | 56/3,267 (1.7) | 0.86 (0.66–1.13) | 37/2,490 (1.5) | 0.91 (0.66–1.27) | 19/777 (2.5) | 0.78 (0.50–1.23) | |
| Urine albumin-creatinine ratio (mg/g) | 0.125 | ||||||
| <30 | 2,878/155,501 (1.9) | 1.00 (reference) | 1,218/100,742 (1.2) | 1.00 (reference) | 1,660/54,759 (3.0) | 1.00 (reference) | |
| 30 - <300 | 424/16,320 (2.6) | 1.03 (0.92–1.15) | 218/11,288 (1.9) | 1.12 (0.95–1.31) | 207/5,032 (4.1) | 0.96 (0.82–1.12) | |
| ≥300 | 63/2,183 (2.9) | 0.98 (0.75–1.30) | 34/1,407 (2.4) | 1.21 (0.84–1.75) | 28/776 (3.6) | 0.81 (0.54–1.21) | |
| Estimated glomerular filtration rate <60 (ml/min per 1.73 m2) | 0.186 | ||||||
| No | 3,245/170,897 (1.9) | 1.00 (reference) | 1,415/111,600 (1.3) | 1.00 (reference) | 1,831/59,297 (3.1) | 1.00 (reference) | |
| Yes | 120/3,107 (3.9) | 0.93 (0.77–1.13) | 55/1,837 (3.0) | 1.08 (0.81–1.44) | 64/1,270 (5.1) | 0.84 (0.65–1.09) | |
For the 15 individual-level risk factors (excluding gross domestic product per capita and ambient PM2.5 air pollution), each model was adjusted for age, gender, region (urban or rural), and all other individual-level risk factors. ∗For the two community-level risk factors of gross domestic product per capita and ambient PM2.5 air pollution, a separate model was employed with adjustment for age, gender, region (urban or rural), and the remaining 15 individual-level risk factors.
See also Table S4 and Figures S4 and S5.
For lifestyle risk factors, tobacco use, former alcohol consumption, unhealthy diet, low physical activity, and long sleep duration were significantly and independently associated with an increased risk of all-cause mortality (Table 2). For cardiovascular outcomes, tobacco use, former alcohol consumption, unhealthy diet, and long sleep duration were associated with cardiovascular and ischemic heart disease mortality, whereas unhealthy diet and long sleep duration were associated with stroke mortality. Tobacco use, current high alcohol consumption, and long sleep duration were associated with cancer mortality. Gender-specific analysis showed that current tobacco use had a stronger association with stroke mortality in women than in men (pinteraction = 0.037; Table S2).
For metabolic risk factors, abdominal obesity, hypertension, diabetes, microalbuminuria or macroalbuminuria, and a low estimated glomerular filtration rate were significantly and independently associated with an increased risk of all-cause mortality (Table 2). Hypertension, diabetes, microalbuminuria or macroalbuminuria, and a low estimated glomerular filtration rate were associated with an increased risk of cardiovascular, ischemic heart disease, and stroke mortality. Abdominal obesity, hypertension, and diabetes were associated with an increased risk of cancer mortality (Table 4).
It is important to highlight that our study revealed distinct impacts of LDL-C levels on cardiovascular and cancer mortality. We did not observe a significant association between LDL-C and all-cause mortality (Table 2). However, high LDL-C levels were strongly associated with cardiovascular mortality, especially with ischemic heart disease mortality (Tables 3 and S1). Furthermore, the cubic spline analysis demonstrated a consistent link between LDL-C levels above 3.4 mmol/L and an increased risk of cardiovascular mortality. Conversely, LDL-C levels below 3.4 mmol/L were associated with an elevated risk of cancer mortality (Figure S5), indicating a potential trade-off between cardiovascular health benefits and an increased risk of cancer mortality for low LDL-C levels.
Gender-specific analysis showed that albuminuria had a stronger association with all-cause mortality in women than in men (pinteraction = 0.008; Table 2), and hypertension had a stronger association with cardiovascular and ischemic heart disease mortality in women than in men (pinteraction<0.01; Tables 3 and S1).
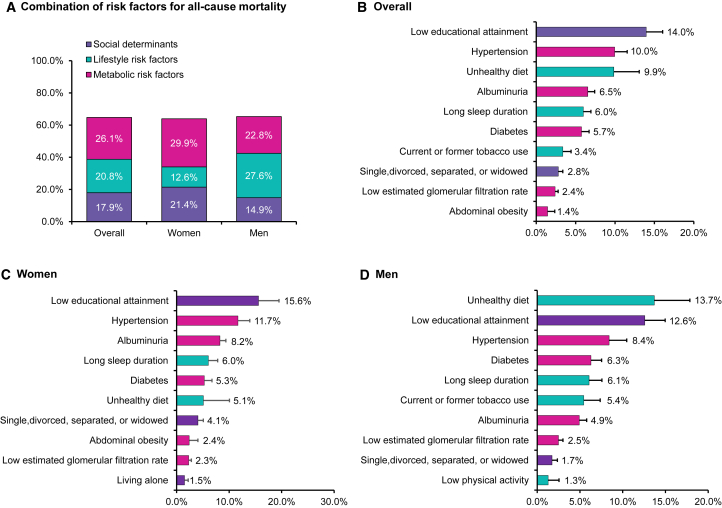
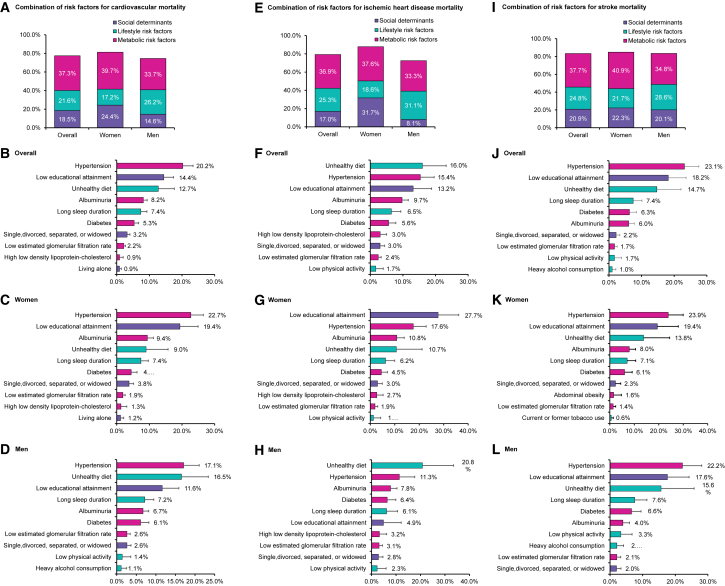
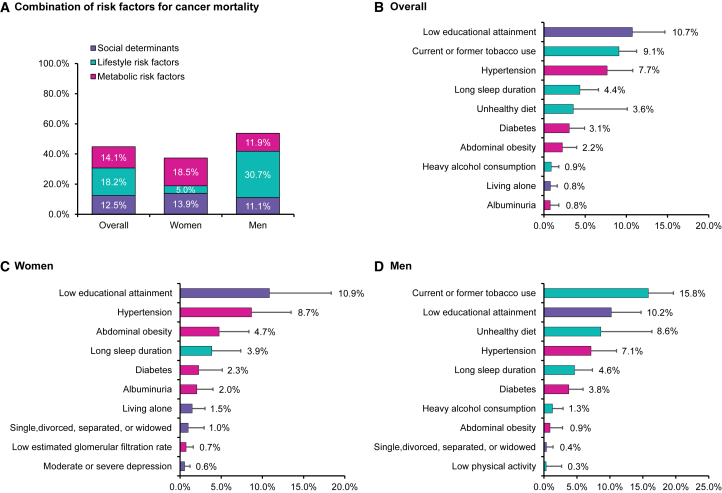
PAFs of all-cause, cardiovascular, ischemic heart disease, stroke, and cancer mortality associated with individual risk factors or combinations of risk factors are shown in Figures 1, 2, and 3. In the overall population, 64.8% of all-cause mortality was attributable to the 15 individual-level risk factors. Social determinants, lifestyle, and metabolic factors accounted for 17.9%, 20.8%, and 26.1% for all-cause mortality, respectively, with low educational attainment (14.0%) being the top leading contributor, followed by hypertension (10.0%), unhealthy diet (9.9%), albuminuria (6.5%), and long sleep duration (6.0%) (Figure 1). The 15 individual-level risk factors explained more PAFs for cardiovascular mortality than cancer mortality. Approximately 77.4% of cardiovascular mortality could be explained by the 15 risk factors (79.2% and 83.4% for ischemic heart disease and stroke mortality, respectively), whereas 44.8% of cancer mortality could be explained. Metabolic risk factors (37.3%) were the most predominant contributors to cardiovascular mortality compared to social determinants (18.5%) and lifestyle factors (21.6%). Hypertension (20.2%), low educational attainment (14.4%), unhealthy diet (12.7%), albuminuria (8.2%), and long sleep duration (7.4%) were the top five leading factors for cardiovascular mortality (Figure 2). For ischemic heart disease mortality, unhealthy diet, hypertension, low educational attainment, albuminuria, and long sleep duration were the top five leading factors (Figure 2), whereas hypertension, low educational attainment, unhealthy diet, long sleep duration, and diabetes were the top five factors for stroke mortality (Figure 2). For cancer mortality, lifestyle factors had the highest contribution (18.2%), followed by metabolic factors (14.1%) and social determinants (12.5%). Low educational attainment (10.7%), tobacco use (9.1%), hypertension (7.7%), long sleep duration (4.4%), and unhealthy diet (3.6%) were the top five leading risk factors for cancer mortality (Figure 3).
Figure 1.
Population-attributable fractions (PAFs) for all-cause mortality
(A) PAFs associated with clusters of risk factors in the overall population, women, and men.
(B–D) PAFs associated with individual risk factors in the overall population, women, and men.
Models were adjusted for age, gender, and region, and individual risk factors were mutually adjusted. We showed the top ten most contributed risk factors based on population-attributable fractions of each mortality outcome. Gross domestic product per capita and ambient PM2.5 air pollution were not included in this figure since they were community-level risk factors and analyzed using separate model. The negative population-attributable fractions were truncated at a lower limit of 0 when calculating the cluster estimates. See also Table S3.
Figure 2.
Population-attributable fractions (PAFs) for cardiovascular mortality
(A–D) PAFs for cardiovascular mortality associated with clusters or individual risk factors in the overall population, women, and men.
(E–H) PAFs for ischemic heart disease mortality associated with clusters or individual risk factors in the overall population, women, and men.
(I–L) PAFs for stroke mortality associated with clusters or individual risk factors in the overall population, women, and men.
Models were adjusted for age, gender, and region, and individual risk factors were mutually adjusted. We showed the top ten most contributed risk factors based on population-attributable fractions of each mortality outcome. Gross domestic product per capita and ambient PM2.5 air pollution were not included in this figure since they were community-level risk factors and analyzed using separate models. The negative population-attributable fractions were truncated at a lower limit of 0 when calculating the cluster estimates. See also Table S3.
Figure 3.
Population-attributable fractions (PAFs) for cancer mortality
(A) PAFs associated with clusters of risk factors in the overall population, women, and men.
(B–D) PAFs associated with individual risk factors in the overall population, women, and men.
Models were adjusted for age, gender, and region, and individual risk factors were mutually adjusted. We showed the top ten most contributed risk factors based on population-attributable fractions of each mortality outcome. Gross domestic product per capita and ambient PM2.5 air pollution were not included in this figure since they were community-level risk factors and analyzed using separate model. The negative population-attributable fractions were truncated at a lower limit of 0 when calculating the cluster estimates. See also Table S3.
Analysis in men and women separately demonstrated that social determinants (e.g., low education) contributed larger PAFs to all-cause mortality in women (21.4%) than in men (14.9%) and lifestyle risk factors (e.g., unhealthy diet and tobacco use) contributed larger PAFs to all-cause mortality in men (27.6%) than in women (12.6%), whereas metabolic factors were leading contributors in both men (22.8%) and women (29.9%). For individual risk factors, low education attainment was one of the leading contributors to all-cause mortality in both women and men (15.6% in women vs. 12.6% in men) (Figure 1). For cardiovascular mortality, hypertension was the largest contributing risk factor in both women and men (22.7% in women vs. 17.1% in men), followed by low educational attainment (19.4%) in women and unhealthy diet (16.5%) in men (Figure 2). For cancer mortality in men, tobacco use was the largest contributing risk factor (15.8%), followed by low educational attainment (10.2%) and unhealthy diet (8.6%). In women, low education was the largest contributing risk factor to cancer mortality (10.9%), followed by hypertension (8.7%) and abdominal obesity (4.7%) (Figure 3).
The associations of gross domestic product per capita and ambient particulate matter with a diameter of <2.5 μm (PM2.5) air pollution with all-cause, cardiovascular, ischemic heart disease, stroke, and cancer mortality were estimated (Tables 2, 3, 4, S1, and S2). Low gross domestic product per capita was associated with increased risks of all-cause mortality (HR = 1.26, 95% CI = 1.20–1.33), cardiovascular mortality (1.46, 1.34–1.59), ischemic heart disease mortality (1.27, 1.10–1.46), stroke mortality (1.29, 1.14–1.46), and cancer mortality (1.28, 1.18–1.39). We estimated that low gross domestic product per capita accounted for 7.8% of the PAF for all-cause mortality, 13.3% for cardiovascular mortality, 8.9% for ischemic heart disease mortality, 9.2% for stroke mortality, and 8.7% for cancer mortality. The PAF of low gross domestic product per capita for cardiovascular mortality was higher in women (16.2%) than in men (10.2%), whereas the PAF for cancer mortality was higher in men (10.7%) than in women (6.4%) (Table S3). Ambient PM2.5 air pollution was associated with increased risk of all-cause mortality (HR = 1.16, 95% CI = 1.11–1.22), cardiovascular mortality (1.55, 1.43–1.67), ischemic heart disease mortality (2.37, 2.09–2.70), and stroke mortality (1.34, 1.19–1.52). Approximately 4.3% of the PAF for all-cause mortality, 13.4% for cardiovascular mortality, 28.3% for ischemic heart disease mortality, 9.7% for stroke mortality, and 2.2% for cancer mortality were attributable to ambient PM2.5 air pollution (Table S3). It is worth mentioning that the statistical approach used to calculate the PAF for ambient air pollution and low gross domestic product per capita (as a community-level risk factor) was different from the approach used to calculate the effect of all other risk factors (which were based on individual-level data). Therefore, these two types of PAFs are not strictly comparable.
Discussion
In this study, we described the establishment of the 4C study, which is a large-scale population-based prospective cohort study with comprehensive measurements of social determinants, lifestyle, and metabolic factors during 10.1 years of follow-up. Recently, the concept of pan-risk factors for cardiovascular health and mortality has been raised by us but not been fully examined.25 Utilizing the enriched data in the 4C study, we estimated the associations between a comprehensive list of risk factors and mortality, which brings several novel insights for disease- and gender-specific risk factors for mortality. First, we showed that 72.2% of deaths in China were due to cardiovascular disease or cancer, and the identified risk factors explained 64.8% of the PAFs for all-cause mortality, 77.4% for cardiovascular mortality, and 44.8% for cancer mortality. Regarding disease-specific mortality, metabolic factors such as hypertension and albuminuria were leading contributors to all-cause and cardiovascular mortality, whereas lifestyle factors such as unhealthy diet and tobacco use were leading contributors to cancer mortality. Considering gender differences, while hypertension contributed a substantial proportion to mortality in both men and women, social determinants (e.g., low education) were leading risk factors for mortality in women, and lifestyle factors (e.g., unhealthy diet) were leading risk factors for mortality in men. Collectively, our study provides timely evidence to support the development of targeted health policies to control metabolic risk factors for the total population, control lifestyle risk factors for men, and increase social status for women. These policies may reduce the mortality rate in countries facing fast economic growth such as China.
Social determinants were known to affect a wide range of health outcomes.26,27 Several previous studies have examined the effect of a limited number of social determinants on mortality.5,28 In a meta-analysis of 48 prospective cohort studies with >1.75 million participants, low socioeconomic status (indexed by occupational position) was associated with a higher risk of all-cause mortality compared to high socioeconomic status (HR = 1.42, 95% CI = 1.38–1.45 for men; 1.34, 1.28–1.39 for women). In another study, adults with low socioeconomic status had higher risks of all-cause mortality compared with those with high socioeconomic status, in both National Health and Nutrition Examination Survey (HR = 2.13, 95% CI = 1.90–2.38) and the UK Biobank (1.96, 1.87–2.06) after adjusting for lifestyle risk factors.5 In our study, a set of social determinants also showed strong associations with all-cause and disease-specific mortalities. For factors with individual-level data, education, living arrangement, and depression were significantly associated with mortality, with education being the most important factor. For factors with community-level data, gross domestic product per capita and PM2.5 air pollution were significantly associated with mortality. As indicated by our data, increasing the entire population’s education level to middle school or above would reduce all-cause mortality by 14.0%, cardiovascular mortality by 14.4%, and cancer mortality by 10.7%, which highlighted the importance of improving the educational level in China. Although the educational attainment of the Chinese population is continuously improving during the past decades, low educational attainment remains a currently important issue in Chinese society. Apart from education, depression and living alone also exhibited associations with all-cause mortality, albeit with weaker contributions compared to education. Notably, the HR estimates for depression were stronger than those for education in relation to all-cause and cancer mortality. These findings imply that these social factors also pose significant threats to life and should receive increased attentions in future policy-making endeavors.
The associations of lifestyle and metabolic risk factors with mortality have been discussed before. Previous studies suggested that lifestyle factors interacted with social status, which increased mortality rate.5 Other studies suggested that unhealthy diet habits such as increased red meat and processed meat consumption were associated with increasing mortality rates,29 but not for dairy intake.30 The Prospective Urban Rural Epidemiology study suggested that metabolic factors were the predominant risk factors for all-cause and cardiovascular mortality, with hypertension and lipid markers as key factors.16,31 But most of these studies were conducted in participants with European ancestry. Our study moves one key step forward by identifying lifestyle and metabolic risk factors for all-cause, cardiovascular, and cancer mortality in men and women in China.
Among lifestyle risk factors, tobacco use was one of the leading contributors to cancer mortality, while unhealthy diet was the third leading factor for all-cause and cardiovascular mortality. Our study further showed that high alcohol consumption (compared with never drinkers) was not significantly associated with risks of all-cause and cardiovascular mortality but was significantly associated with a higher risk of cancer mortality. These results are consistent with the findings reported in many other studies, which showed that the results could be biased by sick quitter/healthy survivor bias.32,33 Furthermore, we found a significant association of binge drinking with a higher risk of all-cause mortality (HR = 1.21, 95% CI = 1.06–1.39) and cancer mortality (HR = 1.40, 95% CI = 1.15–1.71; Table S4).
Our findings corroborate the notion that air pollution is an increasingly important public health issue in China.34,35,36 It was estimated that 1.24 million (95% CI: 1.08–1.40 million) deaths in China were attributable to air pollution in 2017, including 0.85 million (0.71–0.99 million) from ambient PM2.5 pollution.34 To promote national-wide air pollution control, the National Air Pollution Prevention and Control Action Plan has set up a wide range of control and prevention strategies and measures since 2013.37 In our study, the annual average PM2.5 exposure (47.5 μg/m3) was much higher than the annual average PM2.5 concentration threshold (5 μg/m3) recommended by the World Health Organization Air Quality Guidelines 2021.38 Of note, due to the absence of a real control group, this study is likely to underestimate the effect of ambient PM2.5 pollution on mortality.
Among metabolic risk factors, hypertension was the leading factor for cardiovascular mortality and the second leading factor for all-cause mortality. Albuminuria was associated with a 1.5- to 3-fold higher risk of all-cause and cardiovascular mortality, which needs more attention when designing future health policies. For LDL-C, our study revealed that elevated levels of LDL-C were linked to an increased risk of cardiovascular mortality, whereas lower levels of LDL-C were associated with an elevated risk of cancer mortality. This noteworthy trade-off between cardiovascular health and increased cancer risk provides valuable insights into the potential implications for clinical management and risk assessment in the context of cardiovascular disease and cancer.
Thus far, the most comprehensive estimates of the associations between risk factors and health outcomes among Chinese adults are from the Global Burden of Disease Study by pooling data from diverse sources using modeling strategies.1,13,14 While the subgroup analysis of Prospective Urban Rural Epidemiology study in Chinese adults focused on all-cause and cardiovascular mortality,15 our study represents a significant advancement beyond existing studies. Specifically, we conducted a rigorous comparative analysis of the contributions of modifiable factors to cardiovascular and cancer mortality using centralized data and standardized statistical models. Furthermore, we delved deeper into risk factors associated with different subtypes of cardiovascular disease. Our finding revealed distinct leading risk factors for cardiovascular and cancer mortality (hypertension vs. low education, respectively), as well as different leading risk factors for specific cardiovascular disease subtypes (unhealthy diet for ischemic heart disease and hypertension for stroke mortality). These findings together with insights gained from LDL-C analysis highlighted the importance of setting up targeted prevention strategies and optimal thresholds for controlling modifiable risk factors based on individuals’ disease status.
For gender difference, the sub-study of Prospective Urban Rural Epidemiology in 47,262 Chinese previously suggested that mortality rate was higher in men compared with women, but leading risk factors (e.g., lifestyle, hypertension and education) for men and women were similar.15,30,31 Our results confirmed the findings that metabolic factors (e.g., hypertension) were leading factors for all-cause and cardiovascular mortality in both men and women. Our study also provided robust evidence to support that social determinants and lifestyle factors contributed differently to mortality in men and in women. Social determinants contributed 6.5% and 9.8% higher PAFs to all-cause and cardiovascular mortality in women than in men, respectively, whereas lifestyle factors contributed 15.0%, 9.0%, and 25.7% higher PAFs to all-cause, cardiovascular, and cancer mortality in men than in women, respectively. For instance, low education was the top leading risk factor for ischemic heart disease mortality in women (27.7%) but only the sixth-ranked factor in men (4.9%). This aligns with previous studies that showed that mortality was mainly associated with women’s social status.39,40 Our finding provides evidence to support the prioritization of policies aimed at elevating the social status of women in China, which is expected to yield significant social and health benefits in the near future. Another well-known example was the influence of tobacco use on cancer mortality in men rather than in women,41,42 which partly explained why lifestyle factors, such as smoking, were leading causes of mortality only in men.43,44 We also demonstrated that unhealthy diet was the top-ranking risk factor for all-cause, ischemic heart disease, and cancer mortality in men, but showed much lower contributions in women. This finding aligns with evidence from existing literatures.45
The strengths and uniqueness of our study stem from several key aspects. First, we completed follow-up for a large sample of 174,004 Chinese adults aged ≥40 years from 20 study sites across mainland China. Second, we conducted a 10.1-year follow-up, allowing us to assess the long-term impact of risk factors on mortality outcomes. Third, we employed standardized methods to measure risk factors and outcomes, ensuring data consistency. Last, our study simultaneously analyzed all-cause mortality, cardiovascular mortality, and cancer mortality, providing comprehensive insights into different causes of death. It also unveiled novel observations regarding the relationships between established risk factors and mortality, specifically emphasizing the heterogeneous effects of LDL-C on both cancer mortality and cardiovascular mortality. These findings offer valuable contributions to the existing knowledge in this field.
Conclusions
In conclusion, our study indicates that cardiovascular disease and cancer accounted for over 70% of all causes of death in China. The majority of all-cause and cause-specific mortalities can be attributed to the 17 modifiable risk factors examined in the current study. The leading modifiable risk factors were largely different between mortalities caused by cardiovascular disease or cancer, between different cardiovascular disease subtypes, and between women and men. This study provides timely evidence to reduce the burden of metabolic disease to control cardiovascular disease mortality, set up different control targets for LDL-C to prevent cardiovascular and cancer death, and address unhealthy diet for ischemic heart disease and hypertension for stroke. It is crucial to promote healthy lifestyle for cancer mortality, address tobacco use and unhealthy diet among men, and enhance educational opportunities for women.
Limitations of the study
There are some limitations in this study. First, data on several important social determinants, including neighborhood and physical environment, safe housing, food security, social support, stress, community engagement, healthcare coverage, and access and quality of care were not collected. In addition, data on indoor air pollution and individual economic status were not collected. Second, the 4C study was not designed to reflect a nationally representative sample by stratified multistage sampling strategy. Consequently, the presence of selection bias cannot be ruled out, and therefore the results might not be applicable to the whole Chinese population. Nevertheless, this nationwide prospective cohort study covered various geographic regions with substantial heterogeneity in social and economic circumstances, which enabled the capacity of evaluating the contributions of modifiable risk factors to mortality in the general adult population of China. Third, the interplay between age and various risk factors is substantial; thus, age-specific analyses are warranted in future studies to offer additional insights for targeted health interventions. Fourth, although the PAF calculation method in the present study offers advantages over traditional formulaic calculations by mitigating redundant contributions from multiple factors, it does not account for time variables. Fifth, premature cardiovascular and cancer deaths impose significant burdens on the majority of middle-income countries. However, our cohort primarily comprised middle to elderly participants, thereby restricting our capacity to examine premature deaths. Future studies should be conducted to explore this aspect further. Finally, since this study is observational in nature, it inherently limits the ability to infer causation from the association observed.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| SAS version 9.4 | SAS Institute | https://www.sas.com/ |
| R version 4.2.3 | R-Project | https://www.r-project.org/ |
| STAR Methods. Average PAFs analysis: R package averisk version 1.0.3 | R CRAN | https://mirrors.ustc.edu.cn/CRAN/web/packages/averisk/index.html |
| Biological samples | ||
| Serum and urinary samples of the participants | 4C BioBank | N/A |
Resource availability
Lead contact
Further information and request for resources and reagents should be directed to and will be fulfilled by the lead contact, Weiqing Wang (wqingw61@shsmu.edu.cn).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
Data generated and/or analyzed in this study, excluding identifying personal information, are available from the lead contact, Weiqing Wang (wqingw61@shsmu.edu.cn) with reasonable request to protect research participant privacy.
-
•
This paper does not report the original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and subject details
Study design and population
The 4C Study is a multicenter, population-based, prospective cohort study that was designed to investigate the risk factors of cardiovascular, cancer, and all-cause mortality in the Chinese population. In brief, a total of 20 communities from various geographic regions were selected to represent the general population in mainland China. The selection of communities considered the coverage of different geographic regions with various economic development status, as well as the feasibility of centralized sample collection and successfully achieving long-term follow-up. Based on the national criteria for coordinated regional development according to their socioeconomic development status at the time of recruitment, these 16 provinces were categorized into Eastern (Beijing, Zhejiang, Guangzhou, Fujian, Shanghai, Liaoning, Shandong), Central (Heilongjiang, Jilin, Henan, Hubei, Jiangxi), and Western regions (Guizhou, Sichuan, Gansu, Guangxi). Within each community, potential study participants were identified from the local residence registration records and must be 40 years or older. There was no restriction on gender or ethnicity. Each eligible subject was approached by trained local community workers using a door-to-door invitation method. Overall, 204,467 agreed to participate and signed informed consent, and were scheduled for a personal interview and a clinic visit within a week after the invitation. After excluding 10,621 (5.2%) individuals who did not meet age eligibility criteria or withdrew their consents, 193,846 (94.8%) participants underwent a detailed baseline examination and were enrolled in the 4C study between January 2011 and December 2012.
The study was approved by the Medical Ethics Committee of Ruijin Hospital, Shanghai Jiao-Tong University School of Medicine. All study participants provided written informed consent prior to study participation.
Baseline data collection
We used standardized methods to collect information on risk factors at baseline. The personal interview was conducted by trained study personnel using a structured questionnaire. All participants underwent an oral glucose tolerance test, and plasma glucose was obtained at zero and 2 h during the test. Blood specimens and first morning spot urine samples were collected and aliquoted into 0.5-mL Eppendorf tubes within 2 h of collection and shipped in dry ice to the central laboratory of the study located at Shanghai Institute of Endocrine and Metabolic Diseases, which is accredited by the College of American Pathologists. All biochemical tests were performed at the central clinical laboratory using validated and standardized protocols and procedures.
We collected and evaluated 15 baseline individual-level and two community-level modifiable risk factors in this study. The 17 risk factors were categorized into six social determinants (community level data of low gross domestic product per capita and high ambient PM2.5 air pollution, and individual level data of low educational attainment, marital status of being single, divorced, separated, or widowed, living alone and moderate or severe depression), five lifestyle risk factors (current or former tobacco use, former, or high current alcohol consumption, unhealthy diet, low physical activity and long sleep duration), and six metabolic risk factors (abdominal obesity, hypertension, diabetes, high low-density lipoprotein cholesterol (LDL-C), albuminuria and low estimated glomerular filtration rate). Depression, recognized as a multifaceted construct that mirrors social factors, has been classified as a social determinant in many studies,15,16 including the current study. A detailed summary of each risk factor, its measurement method, and its categorization used to calculate the PAF was available in Table S5. Spearman correlation coefficients and multicollinearity diagnosis indicated no high correlation or multicollinearity among these variables (Tables S6 and S7).
For social determinants, self-reported educational attainment was classified into three groups as primary education or less, middle school, and high school or above. Low educational attainment was defined as middle school or less education. Depression severity was assessed using the Patient Health Questionnaire-9, in which a score of ≥10 represents moderate or severe depression.46 For lifestyle factors, physical activity was assessed using the International Physical Activity Questionnaire-Short Form.47 A semi-quantitative Chinese Food Frequency Questionnaire recommended by the National Institute for Nutrition and Health of Chinese Center for Disease Control and Prevention was used to collect habitual dietary intake by asking about the consumption frequency and portion size of typical food items during the previous 12 months48 A diet score was calculated according to the recommendation of the American Heart Association and was reported to associate with health outcomes in Chinese.21,49 For metabolic factors, abdominal obesity was defined as waist-to-hip ratio ≥0.95 for men and waist-to-hip ratio ≥0.90 for women.50 Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg using the mean value of three readings or treatment with antihypertensive medications. Diabetes was defined as fasting glucose ≥7.0 mmol/L (126 mg/dL) or 2-h postload glucose ≥11.1 mmol/L (200 mg/dL) or hemoglobin A1c ≥48 mmol/mol (6.5%) or self-reported history of diabetes.51 LDL-C was divided into three subgroups: <3.4 mmol/L (<130 mg/dL), ≥3.4 and <4.9 mmol/L (130–190 mg/dL), and ≥4.9 mmol/L (≥190 mg/dL).52,53 Non-high-density lipoprotein cholesterol was also used as an alternative risk factor to LDL-C. Albuminuria was defined as microalbuminuria (urine albumin-creatinine ratio: 30-299 mg/g) or macroalbuminuria (urine albumin-creatinine ratio ≥300 mg/g).54 Estimated glomerular filtration rate was calculated using the Chronic Kidney Disease-Epidemiology Collaboration equation, with low estimated glomerular filtration rate defined as <60 mL/min per 1.73m2.55
For community-level risk factors, gross domestic product per capita of the city was obtained from government administrative data, and low gross domestic product per capita was defined as gross domestic product per capita of the city lower than the average gross domestic product per capita across China in 2011. Data on the annual average residential PM2.5 exposure in 2011 for each city were collected from the Global Annual PM2.5 Grids,56 and participants were categorized into two groups using the upper quartile of PM2.5 levels (59.11 μg/m3) in our study as a cut-off threshold.
Method details
Follow-up and outcome assessment
The follow-up was conducted till November 30, 2021 with a median follow-up duration of 10.1 years. Death was ascertained by death certificates from the local death registries of the National Disease Surveillance Point System, based on the International Classification of Diseases, 10th Revision (ICD-10) codes. ICD-10 Codes used in the 4C study for causes of death were provided in Table S8. Adjudications for cardiovascular deaths and cancer deaths were performed by the investigators from the adjudication committee of the 4C study, which was composed of cardiologists, neurologists, oncologists, and physicians of internal medicine. Each death was independently adjudicated by two members of the committee and discrepancies were discussed within the committee to make a final decision. During the adjudication, medical records including pathology reports were obtained and reviewed by the adjudicators. All members of the committee were unaware of the baseline risk factors of study participants.
Quantification and statistical analysis
Baseline characteristics of participants were summarized as mean ± SD, or number of participants and percentage, or median with interquartile range, depending on the normality distribution of continuous variables or numbers with proportions for categorical variables.
Missing data imputation
Multiple imputation by chained Equation 10 replications was conducted for all individual-level risk factors.57 Details of the imputation process for risk factors, distributions of predictors before and after multiple imputations are provided in Table S9, Figures S6 and S7. All primary analyses were performed using multiple-imputed data and SAS software (version 9.4, SAS Institute). The estimates from each of the ten imputed datasets were combined into one overall estimate with the use of Rubin’s rule.58 Given the higher incidence rate of stroke in China compared to other countries,10,11,12 we separated cardiovascular mortality into two subgroups, ischemic heart disease mortality and stroke mortality, and conducted statistical analyses separately.
Cox proportional hazards models
Cox proportional hazards models were used to calculate HRs and 95% CIs of individual risk factors associated with all-cause mortality, while competing risk regression models were employed for cause-specific mortality, including cardiovascular, ischemic heart disease, stroke and cancer mortality. The analyses were conducted by using SAS software (version 9.4, SAS Institute). The proportional hazards assumption was not violated according to the tests of the interaction terms of variables and follow-up duration. A single multivariable model was utilized to account for the mutually adjusted effects of the 15 individual-level risk factors, in addition to age, gender, and area of residence (urban or rural). Since the information on gross domestic product per capita and ambient air pollution was at community level, HRs were calculated in separate models after adjusting for age, gender, area of residence (urban or rural), and 15 individual-level risk factors. We also estimated the HR of each risk factor with mortality in men and women separately. Multiplicative interactions of gender with risk factors were examined by including the product term (e.g., gender × risk factor) in the models to assess whether the associations of risk factors with outcomes vary across different gender groups. Multiple testing was not performed as the risk factors were mutually adjusted in the regression model. In these time-to-event analyses, participants were censored at either the date of death or the date of last follow-up till November, 2021, whichever occurred first.
Average PAF calculation
Average PAFs of all-cause, cardiovascular, ischemic heart disease, stroke and cancer mortality related to individual and clusters of social determinants, lifestyle, and metabolic risk factors were calculated using the approach described by Eide and Gefeller.59,60 PAFs quantify the proportional reduction in disease incidence that would be achieved if the risk factor is theoretically removed from the population. PAFs were calculated with adjustment for age, gender, area of residence (urban or rural), and 15 individual-level risk factors simultaneously, which enables to avoid the overlapping contributions of multiple factors to the same outcome. Since the information on gross domestic product per capita and ambient air pollution was at the community level, the PAFs for these two factors were calculated separately, using the fully adjusted HRs and the exposure distributions based on the aforementioned formula: PAF = p(HR-1)/(1 + p(HR-1)), where p is the population prevalence of the risk factor. Average PAF was calculated by using the R package 'averisk' (version 1.0.3) on R version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
Acknowledgments
This study was supported by the National Key Research and Development Program of China (grant nos. 2022YFC2505202, 2022YFC2505201, 2023YFC2506700, and 2021YFA1301103), National Natural Science Foundation of China (grant nos. 82370810, 91857205, 92157204, 82088102, and 82170819), Science and Technology Commission of Shanghai Municipality (grant nos. 23JS1400900, 23Y11908400, and 23XD1422400), Shanghai Municipal Health Commission (202340084), and an innovative research team of high-level local universities in Shanghai.
Author contributions
G.N., Y.B., and W.W. conceived and designed the study. J.L., M.L., R.Z., C.H., and Q.C. did the statistical analysis. All authors contributed to acquisition, analysis, or interpretation of data. J.L., J.H., Y.B., Y.X., J. Zheng, and M.L. contributed to drafting the manuscript and the revisions. G.N., Y.B., and W.W. supervised the study.
Declaration of interests
The authors declare no competing interests.
Published: July 26, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2024.101656.
Contributor Information
Yufang Bi, Email: byf10784@rjh.com.cn.
Weiqing Wang, Email: wqingw61@shsmu.edu.cn.
Supplemental information
References
- 1.Zhou M., Wang H., Zeng X., Yin P., Zhu J., Chen W., Li X., Wang L., Wang L., Liu Y., et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394:1145–1158. doi: 10.1016/S0140-6736(19)30427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results. 2020:1736–1788. https://vizhub.healthdata.org/gbd-results/; [Google Scholar]
- 3.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y.B., Chen C., Pan X.F., Guo J., Li Y., Franco O.H., Liu G., Pan A. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: two prospective cohort studies. BMJ. 2021;373 doi: 10.1136/bmj.n604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celis-Morales C.A., Lyall D.M., Anderson J., Iliodromiti S., Fan Y., Ntuk U.E., Mackay D.F., Pell J.P., Sattar N., Gill J.M. The association between physical activity and risk of mortality is modulated by grip strength and cardiorespiratory fitness: evidence from 498 135 UK-Biobank participants. Eur. Heart J. 2017;38:116–122. doi: 10.1093/eurheartj/ehw249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anand S.S., Yusuf S., Vuksan V., Devanesen S., Teo K.K., Montague P.A., Kelemen L., Yi C., Lonn E., Gerstein H., et al. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE) Lancet. 2000;356:279–284. doi: 10.1016/s0140-6736(00)02502-2. [DOI] [PubMed] [Google Scholar]
- 9.Kataoka M., Venn B.J., Williams S.M., Te Morenga L.A., Heemels I.M., Mann J.I. Glycaemic responses to glucose and rice in people of Chinese and European ethnicity. Diabet. Med. 2013;30:e101–e107. doi: 10.1111/dme.12080. [DOI] [PubMed] [Google Scholar]
- 10.Ma Q., Li R., Wang L., Yin P., Wang Y., Yan C., Ren Y., Qian Z., Vaughn M.G., McMillin S.E., et al. Temporal trend and attributable risk factors of stroke burden in China, 1990-2019: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2021;6:e897–e906. doi: 10.1016/S2468-2667(21)00228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GBD 2019 Stroke Collaborators Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou M., Wang H., Zhu J., Chen W., Wang L., Liu S., Li Y., Wang L., Liu Y., Yin P., et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387:251–272. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 14.Cai Z., Chen M., Ye P., Yip P.S.F. Socio-economic determinants of suicide rates in transforming China: A spatial-temporal analysis from 1990 to 2015. Lancet Reg. Health. West. Pac. 2022;19 doi: 10.1016/j.lanwpc.2021.100341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S., Liu Z., Joseph P., Hu B., Yin L., Tse L.A., Rangarajan S., Wang C., Wang Y., Islam S., et al. Modifiable risk factors associated with cardiovascular disease and mortality in China: a PURE substudy. Eur. Heart J. 2022;43:2852–2863. doi: 10.1093/eurheartj/ehac268. [DOI] [PubMed] [Google Scholar]
- 16.Yusuf S., Joseph P., Rangarajan S., Islam S., Mente A., Hystad P., Brauer M., Kutty V.R., Gupta R., Wielgosz A., et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395:795–808. doi: 10.1016/S0140-6736(19)32008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xing A., Tian X., Wang Y., Chen S., Xu Q., Xia X., Zhang Y., Zhang X., Wang A., Wu S. Life's Essential 8' cardiovascular health with premature cardiovascular disease and all-cause mortality in young adults: the Kailuan prospective cohort study. Eur. J. Prev. Cardiol. 2023;30:593–600. doi: 10.1093/eurjpc/zwad033. [DOI] [PubMed] [Google Scholar]
- 18.Sun Q., Yu D., Fan J., Yu C., Guo Y., Pei P., Yang L., Chen Y., Du H., Yang X., et al. Healthy lifestyle and life expectancy at age 30 years in the Chinese population: an observational study. Lancet Public Health. 2022;7:e994–e1004. doi: 10.1016/S2468-2667(22)00110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu J., He J., Li M., Tang X., Hu R., Shi L., Su Q., Peng K., Xu M., Xu Y., et al. Predictive Value of Fasting Glucose, Postload Glucose, and Hemoglobin A(1c) on Risk of Diabetes and Complications in Chinese Adults. Diabetes Care. 2019;42:1539–1548. doi: 10.2337/dc18-1390. [DOI] [PubMed] [Google Scholar]
- 20.Lu J., Wang W., Li M., Bi Y., Xu Y., Chen L., Zhao J., Mu Y., DeFronzo R.A., Ning G. Associations of Hemoglobin A(1c) With Cardiovascular Disease and Mortality in Chinese Adults With Diabetes. J. Am. Coll. Cardiol. 2018;72:3224–3225. doi: 10.1016/j.jacc.2018.09.062. [DOI] [PubMed] [Google Scholar]
- 21.Wang T., Lu J., Su Q., Chen Y., Bi Y., Mu Y., Chen L., Hu R., Tang X., Yu X., et al. Ideal Cardiovascular Health Metrics and Major Cardiovascular Events in Patients With Prediabetes and Diabetes. JAMA Cardiol. 2019;4:874–883. doi: 10.1001/jamacardio.2019.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang T., Lu J., Shi L., Chen G., Xu M., Xu Y., Su Q., Mu Y., Chen L., Hu R., et al. Association of insulin resistance and β-cell dysfunction with incident diabetes among adults in China: a nationwide, population-based, prospective cohort study. Lancet Diabetes Endocrinol. 2020;8:115–124. doi: 10.1016/s2213-8587(19)30425-5. [DOI] [PubMed] [Google Scholar]
- 23.Zheng R., Xu Y., Li M., Gao Z., Wang G., Hou X., Chen L., Huo Y., Qin G., Yan L., et al. Data-driven subgroups of prediabetes and the associations with outcomes in Chinese adults. Cell Rep. Med. 2023;4 doi: 10.1016/j.xcrm.2023.100958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S., Li M., Lin H., Wang G., Xu Y., Zhao X., Hu C., Zhang Y., Zheng R., Hu R., et al. Amino acids, microbiota-related metabolites, and the risk of incident diabetes among normoglycemic Chinese adults: Findings from the 4C study. Cell Rep. Med. 2022;3 doi: 10.1016/j.xcrm.2022.100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng R., Xu Y., Li M., Lu J., Xu M., Wang T., Zhao Z., Wang S., Lin H., Zhang X., et al. Pan-risk factor for a comprehensive cardiovascular health management. J. Diabetes. 2022;14:179–191. doi: 10.1111/1753-0407.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marmot M., Friel S., Bell R., Houweling T.A., Taylor S., Commission on Social Determinants of Health Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008;372:1661–1669. doi: 10.1016/s0140-6736(08)61690-6. [DOI] [PubMed] [Google Scholar]
- 27.Hales C.N., Barker D.J. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/bf00400248. [DOI] [PubMed] [Google Scholar]
- 28.Stringhini S., Carmeli C., Jokela M., Avendaño M., Muennig P., Guida F., Ricceri F., d'Errico A., Barros H., Bochud M., et al. Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1·7 million men and women. Lancet. 2017;389:1229–1237. doi: 10.1016/s0140-6736(16)32380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Y., Li Y., Satija A., Pan A., Sotos-Prieto M., Rimm E., Willett W.C., Hu F.B. Association of changes in red meat consumption with total and cause specific mortality among US women and men: two prospective cohort studies. BMJ. 2019;365 doi: 10.1136/bmj.l2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding M., Li J., Qi L., Ellervik C., Zhang X., Manson J.E., Stampfer M., Chavarro J.E., Rexrode K.M., Kraft P., et al. Associations of dairy intake with risk of mortality in women and men: three prospective cohort studies. BMJ. 2019;367 doi: 10.1136/bmj.l6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walli-Attaei M., Rosengren A., Rangarajan S., Breet Y., Abdul-Razak S., Sharief W.A., Alhabib K.F., Avezum A., Chifamba J., Diaz R., et al. Metabolic, behavioural, and psychosocial risk factors and cardiovascular disease in women compared with men in 21 high-income, middle-income, and low-income countries: an analysis of the PURE study. Lancet. 2022;400:811–821. doi: 10.1016/S0140-6736(22)01441-6. [DOI] [PubMed] [Google Scholar]
- 32.Xi B., Veeranki S.P., Zhao M., Ma C., Yan Y., Mi J. Relationship of Alcohol Consumption to All-Cause, Cardiovascular, and Cancer-Related Mortality in U.S. Adults. J. Am. Coll. Cardiol. 2017;70:913–922. doi: 10.1016/j.jacc.2017.06.054. [DOI] [PubMed] [Google Scholar]
- 33.Ding C., O'Neill D., Bell S., Stamatakis E., Britton A. Association of alcohol consumption with morbidity and mortality in patients with cardiovascular disease: original data and meta-analysis of 48,423 men and women. BMC Med. 2021;19:167. doi: 10.1186/s12916-021-02040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin P., Brauer M., Cohen A.J., Wang H., Li J., Burnett R.T., Stanaway J.D., Causey K., Larson S., Godwin W., et al. The effect of air pollution on deaths, disease burden, and life expectancy across China and its provinces, 1990-2017: an analysis for the Global Burden of Disease Study 2017. Lancet Planet. Health. 2020;4:e386–e398. doi: 10.1016/s2542-5196(20)30161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang C., Moran A.E., Coxson P.G., Yang X., Liu F., Cao J., Chen K., Wang M., He J., Goldman L., et al. Potential Cardiovascular and Total Mortality Benefits of Air Pollution Control in Urban China. Circulation. 2017;136:1575–1584. doi: 10.1161/circulationaha.116.026487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu C., Chen R., Sera F., Vicedo-Cabrera A.M., Guo Y., Tong S., Coelho M., Saldiva P.H.N., Lavigne E., Matus P., et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N. Engl. J. Med. 2019;381:705–715. doi: 10.1056/NEJMoa1817364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The General Office of the State Council of China On the publication of the Action Plan against Atmospheric Pollution. 2013. http://www.gov.cn/zwgk/2013-09/12/content_2486773.htm
- 38.Hoffmann B., Boogaard H., de Nazelle A., Andersen Z.J., Abramson M., Brauer M., Brunekreef B., Forastiere F., Huang W., Kan H., et al. WHO Air Quality Guidelines 2021–Aiming for Healthier Air for all: A Joint Statement by Medical, Public Health, Scientific Societies and Patient Representative Organisations. Int. J. Publ. Health. 2021;66 doi: 10.3389/ijph.2021.1604465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu H., Bragg F., Yang L., Du H., Guo Y., Jackson C.A., Zhu S., Yu C., Luk A.O.Y., Chan J.C.N., et al. Sex differences in the association between socioeconomic status and diabetes prevalence and incidence in China: cross-sectional and prospective studies of 0.5 million adults. Diabetologia. 2019;62:1420–1429. doi: 10.1007/s00125-019-4896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross S., Armas Rojas N., Sawatzky J., Varona-Perez P., Burrett J.A., Calderon Martinez M., Lorenzo-Vazquez E., Bess Constanten S., Sherliker P., Morales Rigau J.M., et al. Educational inequalities and premature mortality: the Cuba Prospective Study. Lancet Public Health. 2022;7:e923–e931. doi: 10.1016/S2468-2667(22)00237-7. [DOI] [PubMed] [Google Scholar]
- 41.Millwood I.Y., Walters R.G., Mei X.W., Guo Y., Yang L., Bian Z., Bennett D.A., Chen Y., Dong C., Hu R., et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500 000 men and women in China. Lancet. 2019;393:1831–1842. doi: 10.1016/S0140-6736(18)31772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan K.H., Wright N., Xiao D., Guo Y., Chen Y., Du H., Yang L., Millwood I.Y., Pei P., Wang J., et al. Tobacco smoking and risks of more than 470 diseases in China: a prospective cohort study. Lancet Public Health. 2022;7:e1014–e1026. doi: 10.1016/S2468-2667(22)00227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen W., Xia C., Zheng R., Zhou M., Lin C., Zeng H., Zhang S., Wang L., Yang Z., Sun K., et al. Disparities by province, age, and sex in site-specific cancer burden attributable to 23 potentially modifiable risk factors in China: a comparative risk assessment. Lancet Global Health. 2019;7:e257–e269. doi: 10.1016/S2214-109X(18)30488-1. [DOI] [PubMed] [Google Scholar]
- 44.Islami F., Goding Sauer A., Miller K.D., Siegel R.L., Fedewa S.A., Jacobs E.J., McCullough M.L., Patel A.V., Ma J., Soerjomataram I., et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA A Cancer J. Clin. 2018;68:31–54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 45.Shan Z., Guo Y., Hu F.B., Liu L., Qi Q. Association of Low-Carbohydrate and Low-Fat Diets With Mortality Among US Adults. JAMA Intern. Med. 2020;180:513–523. doi: 10.1001/jamainternmed.2019.6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Craig C.L., Marshall A.L., Sjostrom M., Bauman A.E., Booth M.L., Ainsworth B.E., Pratt M., Ekelund U., Yngve A., Sallis J.F., Oja P. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 48.Zhao W.-H., Huang Z.-P., Zhang X., He L., Willett W., Wang J.-L., Hasegawa K., Chen J.-S. Reproducibility and Validity of a Chinese Food Frequency Questionnaire. Biomed. Environ. Sci. 2010;23:1–38. doi: 10.1016/s0895-3988(11)60014-7. [DOI] [Google Scholar]
- 49.Bi Y., Jiang Y., He J., Xu Y., Wang L., Xu M., Zhang M., Li Y., Wang T., Dai M., et al. Status of cardiovascular health in Chinese adults. J. Am. Coll. Cardiol. 2015;65:1013–1025. doi: 10.1016/j.jacc.2014.12.044. [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization . World Health Organization; 2011. Waist Circumference and Waist-hip Ratio. Report of a WHO Expert Consultation. [Google Scholar]
- 51.American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42:S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 52.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., Chapman M.J., De Backer G.G., Delgado V., Ference B.A., et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 53.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., Himmelfarb C.D., Khera A., Lloyd-Jones D., McEvoy J.W., et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019;74:1376–1414. doi: 10.1016/j.jacc.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu Y., Li M., Qin G., Lu J., Yan L., Xu M., Wang T., Zhao Z., Dai M., Zhang D., et al. Cardiovascular Risk Based on ASCVD and KDIGO Categories in Chinese Adults: A Nationwide, Population-Based, Prospective Cohort Study. J. Am. Soc. Nephrol. 2021;32:927–937. doi: 10.1681/asn.2020060856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., CKD-EPI Chronic Kidney Disease Epidemiology Collaboration. Feldman H.I., 3rd, Kusek J.W., Eggers P., Van Lente F., et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hammer M.S., van Donkelaar A., Li C., Lyapustin A., Sayer A.M., Hsu N.C., Levy R.C., Garay M.J., Kalashnikova O.V., Kahn R.A., et al. NASA Socioeconomic Data and Applications Center (SEDAC); 2022. Global Annual PM2.5 Grids from MODIS, MISR and SeaWiFS Aerosol Optical Depth (AOD), 1998-2019, V4.GL.03. [Google Scholar]
- 57.Resche-Rigon M., White I.R. Multiple imputation by chained equations for systematically and sporadically missing multilevel data. Stat. Methods Med. Res. 2018;27:1634–1649. doi: 10.1177/0962280216666564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raghunathan T.E. What do we do with missing data? Some options for analysis of incomplete data. Annu. Rev. Publ. Health. 2004;25:99–117. doi: 10.1146/annurev.publhealth.25.102802.124410. [DOI] [PubMed] [Google Scholar]
- 59.Eide G.E., Gefeller O. Sequential and average attributable fractions as aids in the selection of preventive strategies. J. Clin. Epidemiol. 1995;48:645–655. doi: 10.1016/0895-4356(94)00161-i. [DOI] [PubMed] [Google Scholar]
- 60.Ferguson J., Alvarez-Iglesias A., Newell J., Hinde J., O'Donnell M. Estimating average attributable fractions with confidence intervals for cohort and case-control studies. Stat. Methods Med. Res. 2018;27:1141–1152. doi: 10.1177/0962280216655374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data generated and/or analyzed in this study, excluding identifying personal information, are available from the lead contact, Weiqing Wang (wqingw61@shsmu.edu.cn) with reasonable request to protect research participant privacy.
-
•
This paper does not report the original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.