Abstract
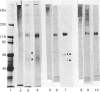
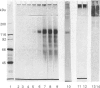
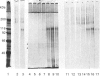
1. A specific antibody was developed against the disulphide-bound 118 kDa glycoprotein of human intestinal mucin and used to establish an e.l.i.s.a. Fourteen purified mucins [eight normal (N) and six cystic fibrosis (CF)] had the same affinity for the antibody in the e.l.i.s.a., but their relative immunoreactivities varied widely (approx. 100,000-fold). In general, CF mucins were more antigenic than N mucins. 2. Variations (approx. 10-fold) were detected in the 118 kDa glycoprotein content of both N and CF mucins (assessed from Coomassie Blue-stained polyacrylamide gels), but these did not appear to be responsible for the differences in mucin immunoreactivity. 3. Variations (approx. 6-fold) were also observed in the size of the 118 kDa peak produced by N and CF mucins on Western blots. These were mostly due to differences in the 118 kDa glycoprotein content of mucins, although a small proportion resulted from changes in the number of antigenic determinants within individual 118 kDa glycoproteins. 4. After concanavalin A affinity chromatography of four reduced mucins (two N and two CF), purified 118 kDa glycoprotein was recovered in the bound fractions from the column, specifically eluted by methyl alpha-mannoside. 5. The amounts of 118 kDa glycoprotein isolated from the four mucins varied as predicted from the size of their 118 kDa bands on Coomassie Blue-stained gels. 6. Three 118 kDa glycoproteins (one N and two CF) showed almost identical reactivity in the e.l.i.s.a.; the fourth had fewer antigenic determinants. 7. Since differences in 118 kDa glycoprotein content and in the number of antigenic determinants within the 118 kDa glycoprotein did not account for variations in the reactivity of native mucins in the e.l.i.s.a., it appeared that accessibility of the 118 kDa glycoprotein to antibody binding may be critical in determining mucin immunoreactivity. This suggests that the three-dimensional conformation of CF mucins may differ from that of N mucins, leading to increased antigenicity.
Full text
PDF










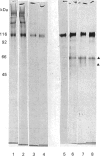
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaplin M. F. A rapid and sensitive method for the analysis of carbohydrate components in glycoproteins using gas-liquid chromatography. Anal Biochem. 1982 Jul 1;123(2):336–341. doi: 10.1016/0003-2697(82)90455-9. [DOI] [PubMed] [Google Scholar]
- Fahim R. E., Forstner G. G., Forstner J. F. Heterogeneity of rat goblet-cell mucin before and after reduction. Biochem J. 1983 Jan 1;209(1):117–124. doi: 10.1042/bj2090117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim R. E., Specian R. D., Forstner G. G., Forstner J. F. Characterization and localization of the putative 'link' component in rat small-intestinal mucin. Biochem J. 1987 May 1;243(3):631–640. doi: 10.1042/bj2430631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Oroszlan S., Konigsberg W. A micromethod for complete removal of dodecyl sulfate from proteins by ion-pair extraction. Anal Biochem. 1979 Feb;93(1):153–157. [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mantle M., Forstner G. G., Forstner J. F. Antigenic and structural features of goblet-cell mucin of human small intestine. Biochem J. 1984 Jan 1;217(1):159–167. doi: 10.1042/bj2170159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Forstner G. G., Forstner J. F. Biochemical characterization of the component parts of intestinal mucin from patients with cystic fibrosis. Biochem J. 1984 Dec 1;224(2):345–354. doi: 10.1042/bj2240345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Mantle D., Allen A. Polymeric structure of pig small-intestinal mucus glycoprotein. Dissociation by proteolysis or by reduction of disulphide bridges. Biochem J. 1981 Apr 1;195(1):277–285. doi: 10.1042/bj1950277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Thakore E. Rabbit intestinal and colonic mucins: isolation, partial characterization, and measurement of secretion using an enzyme-linked immunoassay. Biochem Cell Biol. 1988 Oct;66(10):1045–1054. doi: 10.1139/o88-121. [DOI] [PubMed] [Google Scholar]
- Morrissey S. M., Tymvios M. C. Acid mucins in human intestinal goblet cells. J Pathol. 1978 Dec;126(4):197–208. doi: 10.1002/path.1711260403. [DOI] [PubMed] [Google Scholar]
- Narasimhan S., Wilson J. R., Martin E., Schachter H. A structural basis for four distinct elution profiles on concanavalin A--Sepharose affinity chromatography of glycopeptides. Can J Biochem. 1979 Jan;57(1):83–96. doi: 10.1139/o79-011. [DOI] [PubMed] [Google Scholar]
- Neutra M. R., Trier J. S. Rectal mucosa in cystic fibrosis. Morphological features before and after short term organ culture. Gastroenterology. 1978 Oct;75(4):701–710. [PubMed] [Google Scholar]
- Pearson J. P., Allen A., Parry S. A 70000-molecular-weight protein isolated from purified pig gastric mucus glycoprotein by reduction of disulphide bridges and its implication in the polymeric structure. Biochem J. 1981 Jul 1;197(1):155–162. doi: 10.1042/bj1970155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi R., Forstner G. G., Forstner J. F. Radioimmunoassay of human intestinal goblet cell mucin. Investigation of mucus from different organs and species. J Clin Invest. 1979 Nov;64(5):1149–1156. doi: 10.1172/JCI109568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringler N. J., Selvakumar R., Woodward H. D., Simet I. M., Bhavanandan V. P., Davidson E. A. Structure of canine tracheobronchial mucin glycoprotein. Biochemistry. 1987 Aug 25;26(17):5322–5328. doi: 10.1021/bi00391a016. [DOI] [PubMed] [Google Scholar]
- Slomiany A., Slomiany B. L., Witas H., Aono M., Newman L. J. Isolation of fatty acids covalently bound to the gastric mucus glycoprotein of normal and cystic fibrosis patients. Biochem Biophys Res Commun. 1983 May 31;113(1):286–293. doi: 10.1016/0006-291x(83)90464-3. [DOI] [PubMed] [Google Scholar]
- Slomiany B. L., Laszewicz W., Slomiany A. In vitro inhibition of peptic degradation of porcine gastric mucus glycoprotein by sucralfate. Scand J Gastroenterol. 1985 Sep;20(7):857–860. doi: 10.3109/00365528509088835. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng S. C. Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology. 1985 Jun;92(6):728–733. doi: 10.1016/s0161-6420(85)33967-2. [DOI] [PubMed] [Google Scholar]
- Wesley A., Forstner J., Qureshi R., Mantle M., Forstner G. Human intestinal mucin in cystic fibrosis. Pediatr Res. 1983 Jan;17(1):65–69. doi: 10.1203/00006450-198301000-00013. [DOI] [PubMed] [Google Scholar]