Abstract
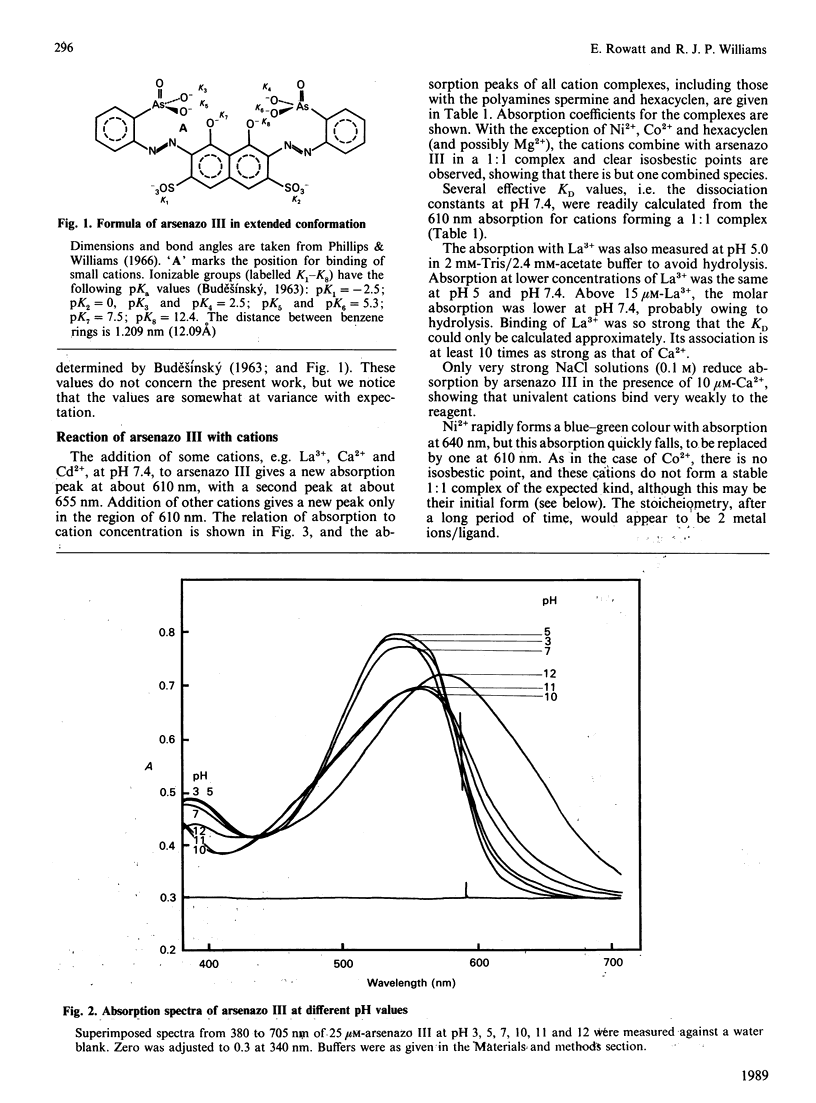
1. The dye arsenazo III combines with a selection of cations to give an altered absorption spectrum. 2. Large metal cations such as Ca2+, La3+ and quadrivalent cations give a 1:1 complex with two new absorption peaks at about 610 nm and 655 nm and a KD of about 10(-6) M. 3. Aliphatic polyamines and complex cobalt ions give a 1:1 complex, with one absorption peak at about 610 nm and a KD from 10(-6) to 10(-3) M. 4. Small metal cations finally form a 2:1 complex and also have one absorption peak at about 610 nm, but with a KD of 10(-5)-10(-4) M. 5. The absorption peak at 610 nm is similar to that formed at high pH in the absence of bivalent cations and is due to ionization of phenolic groups with the dye molecule in an extended form. 6. The peak at 655 nm with 1:1 complex can be explained as a change in orientation of the diazo bonds caused by a conformational change of the molecule when it wraps around the single atom of Ca2+ or other large cation.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Durham A. C., Walton J. M. A survey of the available colorimetric indicators for Ca2+ and Mg2+ ions in biological experiments. Cell Calcium. 1983 Feb;4(1):47–55. doi: 10.1016/0143-4160(83)90048-9. [DOI] [PubMed] [Google Scholar]
- Rowatt E., Williams R. J. The effect of multivalent ions on the inactivation of bacteriophage phi X174 by lipopolysaccharide from Escherichia coli C. Biochem J. 1985 Nov 1;231(3):765–768. doi: 10.1042/bj2310765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowatt E., Williams R. J. The inhibition of infectivity of bacteriophage phi X174 by high-valency metal cations and cyclic polyamines. Biochem J. 1987 Aug 1;245(3):641–647. doi: 10.1042/bj2450641. [DOI] [PMC free article] [PubMed] [Google Scholar]


