Abstract
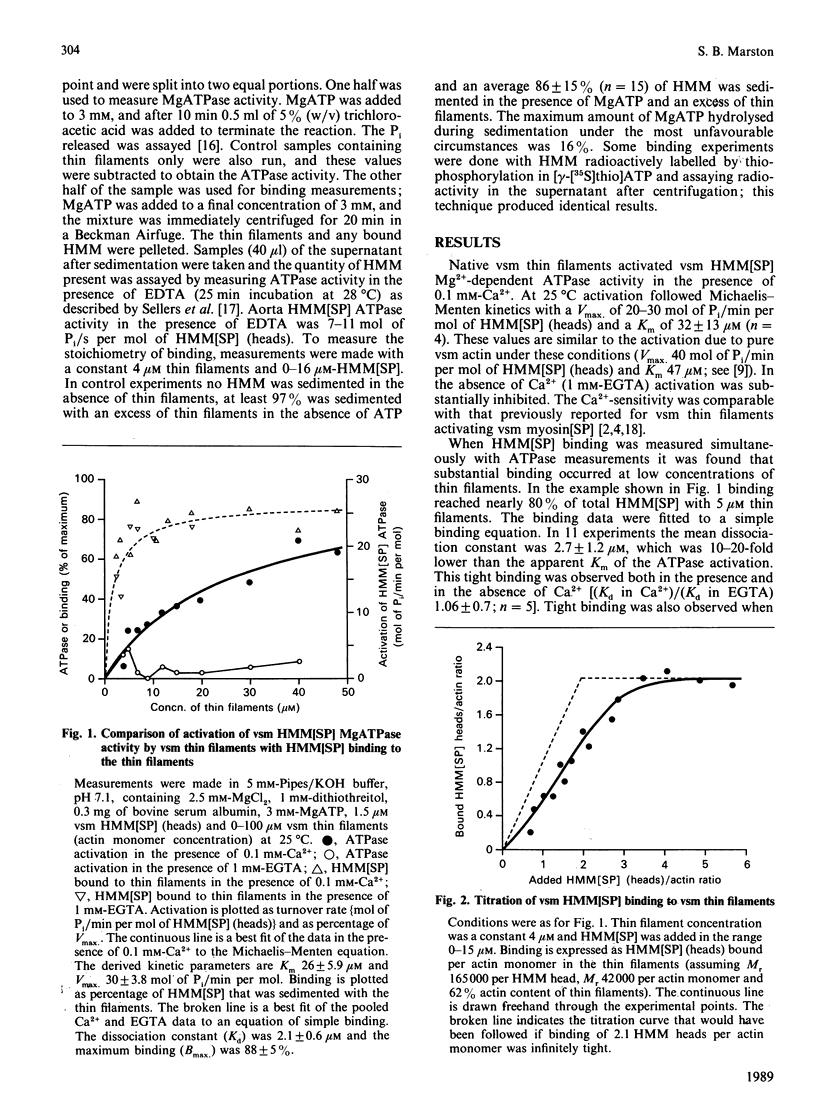
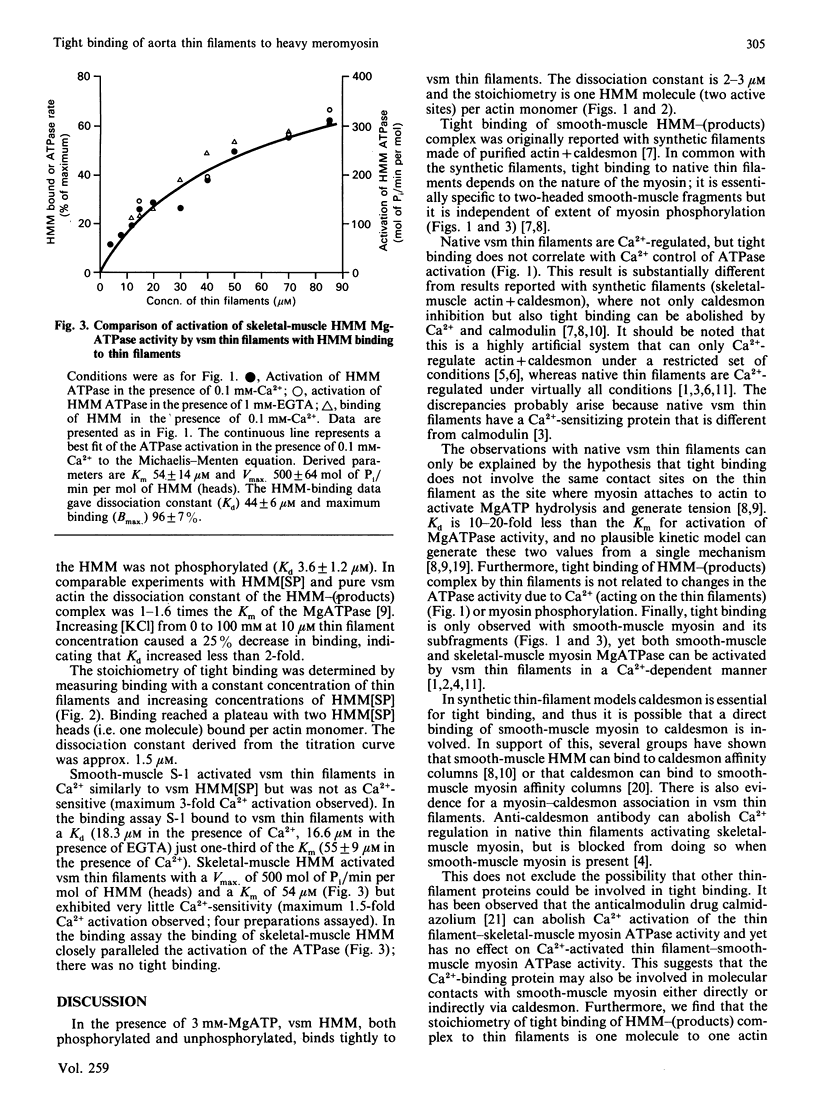
The binding of the Ca2+-regulated native thin filaments from vascular smooth muscle to vascular smooth-muscle heavy meromyosin was measured in the presence of 3 mM-MgATP. At 25 degrees C and I 0.25 binding had an affinity of 1 X 10(-6)-0.3 X 10(-6) M-1 with a stoichiometry of one molecule bound to one actin monomer. The Km for the activation of heavy-meromyosin ATPase was 20-50 microM. Thin filament-heavy meromyosin binding was not altered by Ca2+ (pCa 9-4) or the extent of myosin phosphorylation. With skeletal-muscle heavy meromyosin affinity was 0.023 X 10(6) M-1 in parallel with activation of the ATPase (Km 54 microM). It is concluded that tight binding is specific to smooth-muscle proteins and that it is not related to the ATPase activation site.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clarke M. L., Tregear R. T. Tension maintenance and crossbridge detachment. FEBS Lett. 1982 Jul 5;143(2):217–219. doi: 10.1016/0014-5793(82)80102-6. [DOI] [PubMed] [Google Scholar]
- Dillon P. F., Aksoy M. O., Driska S. P., Murphy R. A. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science. 1981 Jan 30;211(4481):495–497. doi: 10.1126/science.6893872. [DOI] [PubMed] [Google Scholar]
- Heaslip R. J., Chacko S. Effects of Ca2+ and Mg2+ on the actomyosin adenosine-5'-triphosphatase of stably phosphorylated gizzard myosin. Biochemistry. 1985 May 21;24(11):2731–2736. doi: 10.1021/bi00332a020. [DOI] [PubMed] [Google Scholar]
- Hemric M. E., Chalovich J. M. Effect of caldesmon on the ATPase activity and the binding of smooth and skeletal myosin subfragments to actin. J Biol Chem. 1988 Feb 5;263(4):1878–1885. [PubMed] [Google Scholar]
- Ikebe M., Reardon S. Binding of caldesmon to smooth muscle myosin. J Biol Chem. 1988 Mar 5;263(7):3055–3058. [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Lash J. A., Sellers J. R., Hathaway D. R. The effects of caldesmon on smooth muscle heavy actomeromyosin ATPase activity and binding of heavy meromyosin to actin. J Biol Chem. 1986 Dec 5;261(34):16155–16160. [PubMed] [Google Scholar]
- Marston S. B. Ca2+ can control vascular smooth-muscle thin filaments without caldesmon phosphorylation. Biochem J. 1986 Jul 15;237(2):605–607. doi: 10.1042/bj2370605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. B., Lehman W. Caldesmon is a Ca2+-regulatory component of native smooth-muscle thin filaments. Biochem J. 1985 Nov 1;231(3):517–522. doi: 10.1042/bj2310517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. B., Redwood C. S., Lehman W. Reversal of caldesmon function by anti-caldesmon antibodies confirms its role in the calcium regulation of vascular smooth muscle thin filaments. Biochem Biophys Res Commun. 1988 Aug 30;155(1):197–202. doi: 10.1016/s0006-291x(88)81068-4. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Smith C. W. Purification and properties of Ca2+-regulated thin filaments and F-actin from sheep aorta smooth muscle. J Muscle Res Cell Motil. 1984 Oct;5(5):559–575. doi: 10.1007/BF00713261. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Smith C. W. The thin filaments of smooth muscles. J Muscle Res Cell Motil. 1985 Dec;6(6):669–708. doi: 10.1007/BF00712237. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Taylor E. W. Comparison of the myosin and actomyosin ATPase mechanisms of the four types of vertebrate muscles. J Mol Biol. 1980 Jun 5;139(4):573–600. doi: 10.1016/0022-2836(80)90050-9. [DOI] [PubMed] [Google Scholar]
- Marston S. Aorta caldesmon inhibits actin activation of thiophosphorylated heavy meromyosin Mg2+-ATPase activity by slowing the rate of product release. FEBS Lett. 1988 Sep 26;238(1):147–150. doi: 10.1016/0014-5793(88)80245-x. [DOI] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol. 1984 Jun;351:155–167. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard K., Marston S. B. Ca2+-calmodulin binding to caldesmon and the caldesmon-actin-tropomyosin complex. Its role in Ca2+ regulation of the activity of synthetic smooth-muscle thin filaments. Biochem J. 1989 Feb 1;257(3):839–843. doi: 10.1042/bj2570839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Takuwa Y., Park S. Protein kinase C in the regulation of smooth muscle contraction. FASEB J. 1987 Sep;1(3):177–185. [PubMed] [Google Scholar]
- Sellers J. R., Eisenberg E., Adelstein R. S. The binding of smooth muscle heavy meromyosin to actin in the presence of ATP. Effect of phosphorylation. J Biol Chem. 1982 Dec 10;257(23):13880–13883. [PubMed] [Google Scholar]
- Sellers J. R., Pato M. D., Adelstein R. S. Reversible phosphorylation of smooth muscle myosin, heavy meromyosin, and platelet myosin. J Biol Chem. 1981 Dec 25;256(24):13137–13142. [PubMed] [Google Scholar]
- Smith C. W., Pritchard K., Marston S. B. The mechanism of Ca2+ regulation of vascular smooth muscle thin filaments by caldesmon and calmodulin. J Biol Chem. 1987 Jan 5;262(1):116–122. [PubMed] [Google Scholar]
- Sutherland C., Walsh M. P. Phosphorylation of caldesmon prevents its interaction with smooth muscle myosin. J Biol Chem. 1989 Jan 5;264(1):578–583. [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Taylor E. W. Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Crit Rev Biochem. 1979;6(2):103–164. doi: 10.3109/10409237909102562. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]


