ABSTRACT
Background
Obesity increases the risk of atrial fibrillation (AF). We hypothesize that ‘obese’ epicardial adipose tissue (EAT) is, regardless of comorbidities, associated with markers of AF vulnerability
Methods
Patients >40y of age undergoing bariatric surgery and using <2 antihypertensive drugs and no insulin were prospectively included. Study investigations were conducted before and 1y after surgery. Heart rhythm and p-wave duration were measured through ECGs and 7-d-holters. EAT-volume and attenuation were determined on non-enhanced CT scans. Serum markers were quantified by ELISA.
Results
Thirty-seven patients underwent surgery (age: 52.1 ± 5.9y; 27 women; no AF). Increased p-wave duration correlated with higher BMI, larger EAT volumes, and lower EAT attenuations (p < 0.05). Post-surgery, p-wave duration decreased from 109 ± 11 to 102 ± 11ms. Concurrently, EAT volume decreased from 132 ± 49 to 87 ± 52ml, BMI from 43.2 ± 5.2 to 28.9 ± 4.6kg/m2, and EAT attenuation increased from –76.1 ± 4.0 to −71.7 ± 4.4HU (p <0.001). Adiponectin increased from 8.7 ± 0.8 to 14.2 ± 1.0 μg/ml (p <0.001). However, decreased p-wave durations were not related to changed EAT characteristics, BMI or adiponectin.
Conclusion
In this explorative study, longer p-wave durations related to higher BMIs, larger EAT volume, and lower EAT attenuations. P-wave duration and EAT volume decreased, and EAT attenuation increased upon drastic weightloss. However, there was no relation between decreased p-wave duration and changed BMI or EAT characteristics.
KEYWORDS: Adipokines, (epicardial) adipose tissue, adipose remodelling, bariatric surgery, atrial fibrillation
Introduction
Atrial fibrillation (AF) is the most common arrhythmia, and its prevalence is projected to grow at least 2-fold by 2050 [1]. Obesity, defined as a body mass index (BMI)>30 kg/m2, is a worldwide growing pandemic and increases the risk of onset and progression of AF [2,3]. Conversely, weight loss reduces the duration and number of AF episodes and has been shown to reduce AF recurrences in outpatient settings [4,5]. Obesity may induce AF through systemic changes such as adiposopathy, i.e. remodelling of adipose tissue (AT) towards a low-grade inflammatory state [6]. This is reflected by increased secretion of inflammatory and decreased abundance of anti-inflammatory proteins (hereafter adipokines), such as adiponectin [7]. During obesity, changed levels of these adipokines can also be measured in the serum [8]. Inflammatory proteins can affect atrial (or myocardial) fibrosis and ion-channel functioning, promoting AF vulnerability [9]. In addition to altered haemodynamics and sympathetic tone [10,11], obesity may induce AF vulnerability through metabolic syndrome, a cluster of diseases such as diabetes and hypertension [7,12]. Indeed, the risk of AF onset is 20% higher in metabolically healthy, and even 40% higher in metabolically unhealthy obese versus non-obese individuals [13].
Especially, visceral adiposity plays a role in AF [14,15], potentially through its higher inflammatory potential compared to subcutaneous AT (SAT) [10]. Especially, the epicardial adipose tissue (EAT) has been associated with AF [15]. Interestingly, the secretome of EAT, but not of SAT, facilitated re-entrant arrhythmias in cultured neonatal rat ventricular myocytes [16,17]. Beyond volume, EAT density, measured by computed tomography (CT) attenuation, is associated with inflammation and has gained significant interest as a marker for inflammatory EAT.
It remains largely unknown how metabolically healthy obesity and AF vulnerability are related and how these relationships may change upon drastic weight loss. We therefore performed an explorative study whereby we assessed 1. How markers of AF vulnerability, i.e. p-wave duration and LA size, relate to BMI, CT-EAT characteristics, and circulating pro- and anti-inflammatory adipokines and 2. How these markers change upon drastic weight loss induced by bariatric surgery.
Methods
Patient inclusion
Prior to undergoing Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy at Onze Lieve Vrouwe Gasthuis West Hospital in Amsterdam, patients underwent standard eligibility screening [18]. Specific to the current analysis, the primary inclusion criterion was an age of 40 years or older. Key exclusion criteria were the use of insulin, two or more antihypertensive medications, prior myocardial infarction or other cardiac diseases, active systemic inflammation disorders, and the intake of lipid-lowering medications such as statins and/or fibrates. A detailed list of the inclusion and exclusion criteria is displayed in supplementary table 1.
All participating patients had a study visit scheduled between 4 and 1 weeks prior to surgery and at 1 year after surgery. During these visits, patients underwent cardiovascular history assessments, including the evaluation of AF symptoms, as well as physical examinations that included anthropometric measurements. Additionally, patients received a non-enhanced ECG-triggered CT scan, serum sample collection, and an ECG. Rhythm monitoring was performed using 7-d Holters. This comprehensive assessment was repeated 1 year after the surgery. At the time of surgical intervention, a 1 ml biopsy of omental visceral adipose tissue (VAT) was excised. The Weight Loss AF study (NL6205601817) conformed to the principles of the Declaration of Helsinki and was approved by the local Medical Ethics Review Committee (METC). All participating patients provided written informed consent.
EAT assessment through CT-scan analysis
A study-specific scan protocol for optimal low-dose EAT visualization was developed using a phantom pilot study. For image acquisition, prospective non-enhanced ECG-gated high-pitch cardiac CT in end-diastole was acquired with a third-generation dual-source CT scanner (Somatom Force, Siemens Healthineers, Erlangen, Germany), at baseline and at 1 year of follow-up. We consistently applied the scans with fixed beam energy at 120 kVp with dosis modulation based on a Quality reference (Qref.) of 30 mAs for all patients. Images were reconstructed with a slice thickness of 3 mm and increment of 1.5 mm, with a Br36 kernel and iterative reconstruction strength ADMIRE on level 3.
Total EAT was quantified with QFAT version 2.0, Cedars-Sinai Medical Center, with a built-in deep learning algorithm [19]. After delineating the epicardium, EAT was defined as pixels within an HU range from −190 to −30 situated between the myocardium and the visceral layer of the epicardium. The superior and inferior limits of the pericardium were identified as the bifurcation of the pulmonary trunk and the most caudal point of the pericardial sac, respectively. We confirmed the automatically determined epicardial contours and manually adapted them if necessary.
Collection of blood serum and visceral fat secretome
Serum was collected to assess the circulatory levels of selected pro- and anti-inflammatory adipokines myeloperoxidase and adiponectin in relation to anthropometrics and CT-EAT characteristics. After serum withdrawal, serum samples were centrifuged and then stored at −80°C. Secondarily, we aimed to assess the relation between peripherally drawn serum and visceral AT adipokine secretion. During surgery, the omental VAT secretome was collected as a proxy for the EAT secretome for practical reasons, as the pericardium was not opened. Specimens of VAT were directly placed in phosphate-buffered saline (PBS) and cut into cubes of approximately 1 mm3 (±20 cubes/sample) for secretome collection. These cubes were washed three times for 5 minutes to remove serum and other contaminants. Individual cubes were incubated in 100 µl PBS on a thermo-shaker at 250 rotations per minute at 37°C for 1 hour and subsequently centrifuged to remove cell and tissue debris. The secretome was harvested and snap-frozen in liquid nitrogen and stored at −80°C.
ELISA on serum and visceral fat secretome samples
To assess the protein count in serum and VAT secretome, we utilized two Human Adiponectin/Acrp30 Quantikine ELISA kits (Catalog#: DRP300) and two Human Total Myeloperoxidase Quantikine ELISA kits (Catalog#: DMYE00B), which are obtained from BioTeche, RnD Systems. All patients with complete baseline and follow-up samples were included for the serum analysis. For the secretome analysis, 16 randomly selected samples were chosen from patients who had complete serum samples available.
The ELISA experiments were conducted in duplicate, adhering to the manufacturer’s protocol. The maximum allowable coefficient of variation for the duplicates was set at ≤ 20%.
Markers of AF vulnerability
P-wave duration and LA area on CT were employed as surrogate markers for electrical and structural remodelling [20,21]. Both were determined before and 1-year post-surgery. P-wave duration was determined in lead II from the 12-lead electrocardiograms (ECGs) by two researchers independently (E.R.M., M.M.T). Per ECG, the duration of three subsequent p-waves was manually measured in ImageJ and averaged. For the evaluation of the LA size, the LA area at the mitral valve level (one slice) in axial view CT scans served as a proxy and was delineated in ITK-SNAP version 3.4.0 [20] by two researchers independently (E.R.M., P.Z). Subsequently, the slice-based 3D volume calculation (mm3) was converted to a surface area (mm2) by the open source program MeshLab.
Statistics
Data normality was examined through the visual interpretation of the data distribution histogram and Q-Q plots, along with the application of the Shapiro-Wilk test. Associations between EAT characteristics (volume and attenuation), as well as BMI and serum proteins (adiponectin and myeloperoxidase abundance), and AF vulnerability markers were evaluated using linear mixed effect models. Both preoperative and follow-up measures were combined for these analyses; linear mixed models were correct for the potential correlation between these measures. Depending on data normality, differences in pre- versus post-surgical values were tested by either the paired sample t-test or the Wilcoxon signed-rank test. Pearson’s correlation or Spearman’s rank correlation coefficient was used to assess the relation between changes in these variables upon surgery. To assess inter-person variability for p-wave duration and LA area, intraclass correlations (ICC) were calculated. Statistical analyses were carried out using the R lme4 package (1.1–35.1) and all other statistics with SPSS version 28.0. Normally- and non-normally distributed variables were presented as value±standard deviation (SD) and value[interquartile range (IQR)], respectively.
Results
Clinical characteristics before and after surgery
From 2018 to 2020, a total of 40 patients were initially enrolled in the study. Three of them later decided not to undergo bariatric surgery and were excluded from further follow-up. Thirty-six patients successfully completed the follow-up. Due to the impact of the COVID-19 pandemic, for 11 patients, serum withdrawal at follow-up, requiring an extra visit, could not be performed, resulting in the absence of complete baseline-follow-up serum data for those patients.
Table 1 displays the baseline characteristics of the study cohort. Patients were on average 52.1 ± 5.9 years old, 27 (73%) were female, 11 (30%) used antihypertensive medication, 2 (5%) had diabetes, and none had AF. At follow-up, three patients (8%) continued with antihypertensives, and none used diabetes medications.
Table 1.
Patient characteristics.
| Variable | Before surgery (n = 37) | 12 months after surgery (n = 36) |
|---|---|---|
| Age, y ± SD | 52 ± 6 | 53 ± 8 |
| Female sex n (%) | 27 (73) | 26 (72) |
| Anthropometrics | ||
| BMI, kg/m2 ± SD | 43.2 ± 5.1 | 28 ± 4.5 |
| weight (kg) ± SD | 127 ± 20 | 84.3 ± 19 |
| waist (cm) ± SD | 128 ± 15 | 96.6 ± 15 |
| hip (cm) ± SD | 137 ± 13 | 111 ± 12 |
| Fat percentage ± SD | 46.0 ± 5.3 | 34.9 ± 7.1 |
| Cardiovascular (co)morbidities | ||
| Atrium fibrillation (holter) n (%) | 0 (0) | 0 (0) |
| Congestive heart disease n (%) | 0 (0) | 0 (0) |
| Hypertension n (%) | 11 (30) | 3 (8) |
| Systolic RR ± SD | 131 ± 8 | 129 ± 22 |
| Diastolic RR [IQR] | 81 [72-90] | 75 [62-88] |
| Mean RR [IQR] | 95[88-103] | 91[70-112] |
| Diabetes mellitus n (%) | 2 (5) | 0 (0) |
| Stroke n (%) | 0 (0) | 0 (0) |
| Vascular disease n (%) | 0 (0) | 0 (0) |
| Sleep apnoea n (%) | 28 (76) | not available |
| Lab | ||
| Cholesterol ± SD | 5.1 (0.8) | 4.2 (0.7) |
| LDL ± SD | 3.5 (0.8) | 2.6 (0.7) |
| HDL ± SD | 1.3 (0.2) | 1.6 (0.3) |
| totHDL_chol ± SD | 4.1 (1.0) | 2.7 (0.5) |
| Medication | ||
| Antihypertensive drugs, all (%) | 11 (27) | 4 (11) |
| β-Blocker n (%) | 3 (8) | 1 (3) |
| Ca-blocker n (%) | 3 (8) | 1 (3) |
| ACE-remmers n (%) | 2 (5) | 0 (0) |
| diuretics n (%) | 1 (3) | 1 (3) |
| ARBs n (%) | 3 (8) | 1 (3) |
| alfa-blockers n (%) | 1 (3) | 1 (3) |
| anti-diabetic drugs, all n (%) | 2 (5) | 0 (0) |
| Metformine n (%) | 1 (3) | 0 (0) |
| Gliclazide n (%) | 1 (3) | 0 (0) |
| Statins n (%) | 0 (0) | 1 (3) |
ARB, angiotensin receptor blockers; BMI, body mass index; RR, blood pressure, LDL, low density lipid; HDL, high density lipid.
Markers of AF vulnerability upon drastic weight loss
P-wave duration decreased from 109.2 ± 11.1 to 102.2 ± 10.6 ms, (p = 0.002), and LA area remained unchanged, 25.1 ± 4.2 to 25.0 ± 6.1 cm2, (p = 0.93), at follow-up. ICC for p-wave duration was 0.75, CI: 0.57 to 0.84, (p < 0.001), and for LA area 0.76, CI: 0.60 to 0.86, (p < 0.001).
Changing anthropometrics and CT-EAT characteristics upon drastic weight loss
Following weight loss surgery, there was a significant decrease in BMI from 43.2 ± 5.2 to 28.9 ± 4.6 kg/m2, (p < 0.001). Waist circumference, reflecting abdominal VAT adiposity, decreased from 128 ± 15 to 96 ± 15 cm, (p < 0.001). EAT volume decreased from 130 [87] to 79 [54] ml, and EAT attenuation increased from −76.1 ± 4.0 to −71.7 ± 4.4 HU, (p < 0.001).
Markers of AF vulnerability in relation to BMI and CT-EAT characteristics
Next, we investigated AF vulnerability markers in relation to BMI and CT-EAT characteristics (Table 2). A longer p-wave duration was associated with a larger EAT volume (19.4 ml/10 ms p = 0.005, Figure 1a), a lower EAT attenuation (−1.7HU/10 ms p = 0.002, Figure 1b), and a higher BMI (2.4 kg/m2/10 ms p = 0.02, Figure 1c). There was a trend towards an association between p-wave duration and LA area (0.4 cm2/10 ms, p = 0.07). Larger LA areas were associated with larger EAT volumes (p = 0.03) but not with EAT attenuations (p = 0.19). The decrease in p-wave duration was neither associated with the decrease in BMI (r = 0.07, p = 0.69) nor with the decrease in EAT volume (r = −0.02, p = 0.92) or the increase in attenuation (r = 0.16, p = 0.41). Thus, although there was a strong relation between BMI, EAT volume or EAT attenuation, and p-wave duration, their respective changes did not correlate.
Table 2.
AF vulnerability markers in relation to BMI and CT-EAT characteristics.
|
p-wave duration (ms) |
Serum adiponectin (ng/ml) |
|||||
|---|---|---|---|---|---|---|
| Adipose tissue (AT) depot | Change AT variable per 10 ms longer p-wave duration | std. error | p-value | Change AT variable per 5.5 μg higher adiponectin | std. error | p-value |
| EAT volume (ml) | 19.4 ml higher | 7.0 | 0.005 | 27 ml lower | 4.2 | <0.001 |
| EAT attenuation (HU) | 1.7HU lower | 0.6 | 0.002 | 2.9HU higher | 1.2 | <0.001 |
| BMI (kg/m2) | 2.4 kg/m2 higher | 1.0 | 0.02 | 4.1 kg/m2 lower | 1.1 | 0.004 |
Figure 1.
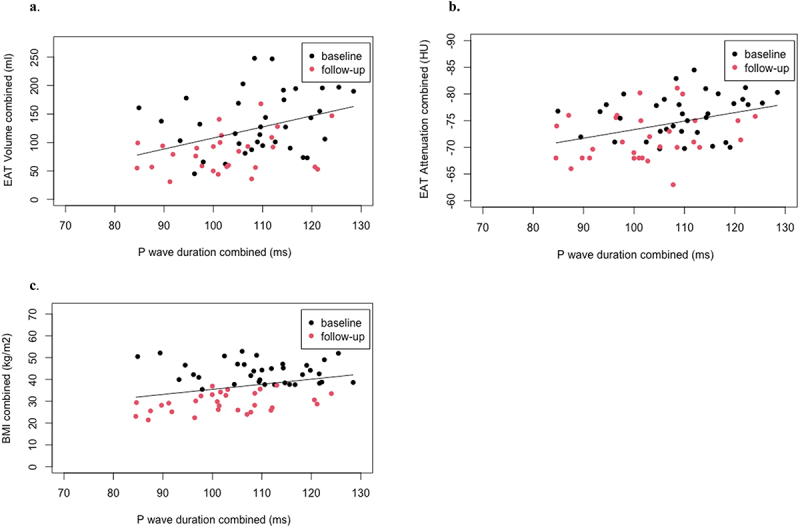
Figure 1 shows the relation between p-wave duration on the x-axes and EAT characteristics and BMI on the y-axes. Analyses were performed with linear mixed models: 10 ms increase in p-wave relates to 1a: a 19.4 ml larger volume of EAT (p = 0.005), 1b: −1.7HU units lower EAT attenuation (p = 0.002), and 1c: a 2.4 kg/m2 higher BMI (p = 0.02).
Adiponectin and myeloperoxidase in relation to BMI, EAT characteristics, and AF vulnerability
Next, we assessed how BMI and EAT characteristics relate to the anti- and proinflammatory adipokines adiponectin and myeloperoxidase. A higher serum adiponectin was associated with lower EAT volumes (−27 ml/5.5 μg p < 0.001, Figure 2a), a higher EAT attenuation (2.9 HU/5.5 μg p < 0.001, Figure 2b), and a lower BMI (4.1 kg/m2 p = 0.004, Figure 2c). Adiponectin concentration was not associated with p-wave duration (Figure 2d).
Figure 2.
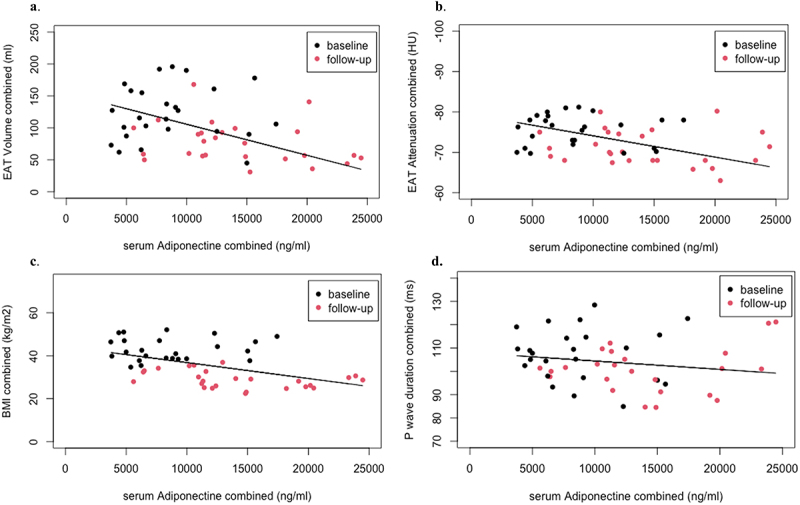
Figure 2 shows the relation between serum adiponectin on the x-axes and EAT characteristics and BMI on the y-axes. Analyses were performed with linear mixed models: 5.5 μg (5532 ng) higher serum adiponectin relates to 2a: the decrease of 27 ml of EAT (p < 0.001), 2b: the increase of 2.9HU units EAT attenuation (p < 0.001), 2c: the decrease of 4.1 kg/m2 BMI (p = 0.004), and 2d: unchanged p-wave duration (−2.0 ms, p = 0.22).
There was a trend towards an inverse association between the concentration of adiponectin in serum and in VAT secretome (n = 16, r = −0.46, p = 0.08). Adiponectin increased significantly after weight loss from 8.7 ± 0.8 to 14.2 ± 1.0 μg/ml (p < 0.001). The mean adiponectin concentration in VAT secretome was 0.006 ± 0.005 μg/ml. Serum myeloperoxidase remained unchanged upon weight loss: 0.3 ± 0.2 to 0.3 ± 0.1 μg/ml (p = 0.24) and was not associated with VAT secretome levels (n = 13, r = 0.37, p = 0.29). VAT secretome levels of adiponectin and myeloperoxidase were not related to CT-EAT characteristics or AF vulnerability markers.
Upon weight loss, the decrease in EAT volume showed a trend towards association, and the increase in EAT attenuation was significantly associated with the increase in serum adiponectin concentration (r = −0.38, p = 0.10 and r = 0.47, p = 0.02, respectively). EAT characteristics were not associated with myeloperoxidase concentrations. Adiponectin and myeloperoxidase changes were not associated with alterations in p-wave duration or LA area.
Impact of age and sex on CT-EAT characteristics and markers of AF vulnerability
There was a trend towards a positive relation between age at baseline and EAT volumes (r = 0.31, p = 0.06). Age was significantly associated with lower EAT attenuations (r = −0.35, p = 0.03).
In terms of sex differences, baseline EAT volumes were smaller in women (123 ± 47) compared to men (188 ± 81 ml). Despite the lesser decrease in EAT volume upon weight loss in women than in men, EAT volume persisted to be lower in women (76 [44]) compared to men (101 [55]ml, p = 0.01) at follow-up. Meanwhile, baseline EAT attenuation was −75.7 ± 3.9 in women and −77.6 ± 4.9HU in men (p = 0.2). After weight loss, attenuation increased to −72.0 ± 4.4 for women versus −70.5 ± 4.7 for men (p = 0.43). Women had a smaller baseline waist circumference (123 ± 12) than men (140 ± 14 cm [2], p < 0.001) which decreased after weight loss to 93 ± 11 vs 107 ± 18 cm2 (p = 0.01), respectively. Nevertheless, BMI was similar between sexes both at baseline (women: 42.9 ± 4.8 vs men: 44.2 ± 6.3 kg/m2) and at follow-up (women: 28.8 ± 4.3, men: 29.4 ± 5.5 kg/m2, (p = 0.76)). P-wave duration at baseline was 108 ± 10 for women and 114 ± 112 ms for men (p = 0.15) and decreased to significantly lower duration in women than in men at follow-up (to 100 ± 10 and 112 ± 8 ms, p = 0.01), respectively. LA areas were similar between sexes.
Discussion
In this cohort of metabolically healthy obese individuals, we demonstrated that a longer p-wave duration was associated with a higher BMI, larger EAT volumes, and lower EAT attenuations on CT. Similarly, the LA area was associated with EAT volume. Importantly, except for the LA area, all of these markers significantly changed after substantial weight loss. This suggests that weight loss may (in part) reverse atrial remodelling and mitigate AF vulnerability. However, the absence of a relation between the decrease in p-wave duration and changes in CT-EAT or circulating EAT markers implies that either peripherally measured adipokines do not reflect local EAT adipokine secretion and effect or that alterations of EAT characteristics are not the primary drivers of reduced AF vulnerability after weight loss, at least not within this metabolically healthy cohort. Alternatively, the adaptation of p-wave duration follows a different time course that of BMI or EAT characteristics on CT. Furthermore, changed haemodynamics and increased sympathetic tone may contribute to the change in p-wave duration [10,11].
Reversed atrial remodelling upon weight loss: a role for EAT?
Both the relation between increased p-wave duration and obesity and the decrease in p-wave duration after bariatric surgery were shown previously [21]. In line with our findings, Friedman et al. showed that CT-EAT volume positively correlated with p-wave duration [22]. In a different study with a healthy obese cohort comparable to our study, including 20 patients, p-wave duration decreased and LA size remained unchanged upon bariatric surgery, also similar to our findings [23]. Differently, these authors showed that the decrease in EAT volume and p-wave duration upon weight loss were interrelated. The discrepancy may be explained by their more precise ECG acquisition at the same time of the day before and 12 months after surgery, excluding potential circadian changes in the ECG from their analysis. However, these authors also reported that p-wave duration was quantified in a non-blinded fashion and by only one assessor, leaving the possibility of bias in this relatively small study. In our study, p-wave duration was assessed by two experienced ECG readers independently. Whether drastic weight loss reverses electrical remodelling through EAT should be further investigated in a prospective, large-scale study, blinded for a moment of visit. Preferably, this also includes local EAT biopsies and more extensive electrophysiological measurements.
While the decrease in EAT volume upon significant weight loss is a well-known phenomenon, the reported data on EAT attenuation in obesity and its change upon drastic weight loss are limited. EAT attenuation reflects tissue density. Both high and low attenuation have been associated with cardiovascular outcomes [24,25]. Increased attenuation has been associated with inflammatory cell infiltration and oedema and decreased attenuation with adipocyte hypertrophy, both related to a proinflammatory status of EAT [26]. In our study, lower EAT attenuations were associated with longer p-wave durations and decreased adiponectin levels. EAT attenuation increased after 1 year of follow-up, irrespective of advancing age. An older age at baseline was associated with a lower EAT attenuation. Furthermore, the interrelated increase in EAT attenuations and adiponectin levels may be indicative of an improved metabolic profile of the EAT after weight loss (which could manifest, among other things, in changes in secretome). The absence of interrelation between changes in p-wave duration and EAT characteristics in this study implicates that mechanisms other than EAT volume or density also affect p-wave duration changes upon drastic weight loss. Alternatively, adipokines measured peripherally do not necessarily reflect adipokine levels in EAT. MPO is abundantly present in EAT [17]. Locally applied MPO caused both arrhythmogenic structural and electrical remodelling in neonatal rat ventricular myocyte monolayers [27]. EAT vesicles carry a major proportion of the secreted adipokines. Upon secretion, the directly attached myocardium is exposed to these vesicles. The concentration in the EAT secretome is up to 31 times larger than the SAT secretome [28]. Therefore, the concentration and inherent effect of local EAT adipokines such as myeloperoxidase may be difficult to detect peripherally where adipokine levels are a reflection of a mixed, whole-body fat vesicle secretion. Another explanation would be that p-wave and EAT characteristics change with a different time constant following weight loss. LA area remained unchanged at 1 year after bariatric surgery. Our findings are in line with Henry et al. who studied geometric cardiac changes in CMR after bariatric surgery [29]. Interestingly, they found that LA size primarily decreased after surgery and suggest that this is due to the initial reduction in cardiac output and a reduction in EAT (leaving more space for LA). Towards 1 year and later of follow-up, LA size increased again to the pre-surgical size and there was a reversal of obesity-induced myocardial hypertrophy. Henry et al. suggest that this late reversal was related to a decrease in blood pressure and normalization of insulin and leptin, which are inducers of myocyte hypertrophy [30,31]. Another study showed that all components of the LA strain significantly improved, 1 year after bariatric surgery, suggestive of improved atrial function independent from atrial size [32]. These findings may support a different temporal change in LA anatomical and electrical remodelling and form an explanation for our findings in this study.
Potential clinical implications of alterations in EAT attenuation
Low CT-EAT attenuations relate to cardiovascular (CV) risk factors and outcomes, including coronary artery disease, in patients without AF [24,33,34]. Also, it was shown that lower CT-EAT attenuations were associated with higher CT calcium scores in men but not in women, independent of EAT volume and BMI [35]. Lower EAT attenuation is generally interpreted as a pathological marker for CV outcomes, especially for men. Here, we show that, upon weight loss, men seem to have a larger increase in EAT attenuation than women, while BMI decreased similarly between sexes. In the context of CV risk, men may benefit more from weight loss surgery. These suggestions should be interpreted with caution, as the number of male patients in our cohort was limited.
Conversely, in studies comparing AF to non-AF patients, attenuation was increased instead of decreased in AF (Meulendijks et al., submitted [36]). This may suggest that AF imposes a different or additional pathological process in the EAT compared to other CV diagnoses. Furthermore, it may be that in AF, EAT composition is affected by the mechanical effect of the myocardium itself. Interestingly, the EAT directly adjacent to the myocardium contains smaller adipocytes compared to EAT closer to the epicardium, which may be the consequence of myocardial effects on EAT [37]. Moreover, the current study was performed in patients with a significantly younger age compared to patients cohorts with AF. This, as well as heterogeneity in scan settings and quantification tools, complicates the interpretation of attenuations among different studies [38].
Anti- and proinflammatory adipokines
Higher EAT volumes and lower EAT attenuations were associated with lower serum adiponectin concentrations, in line with Goeller et al. [24]. It was also shown previously that adiponectin levels increase after weight loss [39]. Adiponectin is an adipocyte-enriched hormone and has demonstrated insulin-sensitizing, anti-inflammatory, and cardioprotective properties in experimental studies [40]. Adiponectin inhibits AngII-ROS-induced cardiomyocyte remodelling (MMP expression), in rat ventricular myocytes [41]. Also, after inducing myocardial ischaemia and subsequent reperfusion, infarct size and formation of nitric oxide, superoxide, and their cytotoxic product were significantly higher in cardiac tissue obtained from adiponectin-/- than from wild-type mice. This was largely normalized upon applying adiponectin [42]. Furthermore, adiponectin decreases inflammation induced by oxidized low-density lipoprotein [43,44]. Myeloperoxidase is a peroxidase associated with inflammation and oxidative stress and has been associated with AF, both locally in atrial EAT and in the circulation [17]. However, the relation between myeloperoxidase and obesity or weight loss is heterogeneous [45–48] and potentially mediated by cardiovascular and other comorbidities. In our study, myeloperoxidase concentration did not change upon weight loss nor did it correlate with EAT characteristics. It may be that in these metabolically healthy obese patients with presumable limited oxidative stress, myeloperoxidase was present at baseline only at very low concentrations, which may have limited to potential to further decrease after drastic weight loss. Therefore, although physiologically plausible, we cannot demonstrate a direct relation between circulating MPO and change in AF markers upon weight loss. Additionally, this study is limited to the electrophysiological variable p-wave duration, extracted from ECGs. More elaborate electrophysiological investigation of the patients before and after surgery may add new, valuable insight into the impact of myeloperoxidase on AF risk upon bariatric surgery.
Limitations
Our study cohort consists of relatively young patients. It is conceivable that despite the obese state, atrial remodelling had not fully emerged at the time of surgery, and therefore, reverse remodelling would only be possible to a limited extent.
There is a possibility that haemodynamic and pericardial constraints contributed to the increased AF vulnerability in obesity. In the current study, we used CT scans rather than echocardiography for being better able to delineate the EAT volumes and attenuation. However, dynamic echocardiographic data could have added to establish this.
Conditions such as hypertension may induce EAT adiposopathy, exacerbating the relationship between EAT inflammation and AF vulnerability. The absence of these comorbid conditions may be responsible for the lack of interrelations between changing circulating adipokines and AF vulnerability upon bariatric surgery. The study’s sample size is modest but adequate and sufficient for our explorative objectives. While the COVID-19 situation limited our available serum samples, the vast majority of patients had sequential serum samplings and the number of patients was sufficient for the explorative nature of our study to determine correlations. Women were overrepresented in this study. Despite this, our sample size remains adequate to highlight differences between sexes. Our study lacks a true AF group for direct comparison. We intentionally selected individuals with healthy obesity to assess the specific effects of adiposity without other comorbidities. Additionally, accessing a healthy AF population undergoing bariatric surgery is inherently challenging.
While being from the same embryonic source, omental VAT may differ from EAT. Therefore, the relations between EAT secretome adipokines and AF vulnerability may be different than described here.
Conclusion
Longer p-wave durations are associated with larger EAT volumes, lower EAT attenuations, and lower circulating levels of the anti-inflammatory adipokine adiponectin. Importantly, all of these markers significantly changed after substantial weight loss, but the decrease in p-wave duration was not associated with the change in BMI or EAT characteristics. This suggests that drastic weight loss (in part) reverses atrial remodelling and mitigates AF vulnerability in relatively healthy morbid obese patients. Our findings also suggest that alterations of EAT characteristics may either not be the primary drivers of reduced AF vulnerability after weight loss or may follow a different time course than markers of atrial reverse remodelling.
Supplementary Material
Funding Statement
Dr. E. Meulendijks has been supported by an MD-PhD 2016 grant from the University of Amsterdam. Prof. de Groot has been supported by research grants from Abbott, AtriCure, Boston Scientific, Bayer, Daiichi Sankyo, Johnson & Johnson, and Medtronic Servier and has received consultancy fees from AtriCure, Bayer, Daiichi Sankyo, Johnson & Johnson, and Medtronic (outside the submitted work). Dr. Tim A.C. de Vries reports nonfinancial support from Daiichi Sankyo and speaker fees from Bristol Myers Squibb, both outside the submitted work.None of the above funders were involved in this submitted work.Abbott Fund AtriCure Boston Scientific Corporation Daiichi Sankyo Europe Johnson and Johnson Medtronic Europe.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
EM: conception and design, analysis and interpretation of the data, drafting paper, and intellectual content.
CJT: analysis and interpretation of the data.
EH: patient inclusions, analysis and interpretation of the data.
NL: collection and postprocessing of CT data.
PZ: analysis and interpretation of the data.
MT: analysis and interpretation of the data.
RW: analysis and interpretation of the data.
TdV: analysis and interpretation of the data.
RaS: analysis and interpretation of the data.
RvV: provided the biopsies, analysis and interpretation of the data.
SdC: provided the biopsies, analysis and interpretation of the data.
CdV: patient inclusions, analysis and interpretation of the data.
LN: patient inclusions, analysis and interpretation of the data.
RP: analysis and interpretation of the data.
SK: conception and design, analysis and interpretation of the data, drafting paper, and intellectual content.
JdG: conception and design, analysis and interpretation of the data, drafting paper, and intellectual content.
All authors have read and approved the final version of the manuscript.
Abbreviations
- AF
atrial fibrillation
- AT
adipose tissue
- BMI
body mass index
- CT
Computed tomography
- EAT
epicardial adipose tissue
- SAT
Subcutaneous adipose tissue
- VAT
Visceral adipose tissue
- LA
left atrium
- PBS
Phosphate-buffered saline
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, E.R.M. The data are not publicly available due to information that could compromise the privacy of research participants.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21623945.2024.2395565
References
- [1].Naccarelli GV, Varker H, Lin J, et al. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104(11):1534–11. doi: 10.1016/j.amjcard.2009.07.022 [DOI] [PubMed] [Google Scholar]
- [2].Ncdrf C. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tsang TS, Barnes ME, Miyasaka Y, et al. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J. 2008;29(18):2227–2233. doi: 10.1093/eurheartj/ehn324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pathak RK, Middeldorp ME, Meredith M, et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol. 2015;65(20):2159–2169. doi: 10.1016/j.jacc.2015.03.002 [DOI] [PubMed] [Google Scholar]
- [5].Donnellan E, Wazni OM, Elshazly M, et al. Impact of bariatric surgery on atrial fibrillation type. Circ Arrhythm Electrophysiol. 2020;13(2):e007626. doi: 10.1161/CIRCEP.119.007626 [DOI] [PubMed] [Google Scholar]
- [6].Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol. 2011;57(25):2461–2473. doi: 10.1016/j.jacc.2011.02.038 [DOI] [PubMed] [Google Scholar]
- [7].Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29(1):415–445. doi: 10.1146/annurev-immunol-031210-101322 [DOI] [PubMed] [Google Scholar]
- [8].Meulendijks ER, Krul SPJ, Baalman SW, et al. Circulating adipose tissue proteins involved in atrial fibrillation: an explorative scoping review. Trends Cardiovasc Med. 2022;24(Supplement_1). doi: 10.1016/j.tcm.2022.12.004 [DOI] [PubMed] [Google Scholar]
- [9].Lazzerini PE, Capecchi PL, El-Sherif N, et al. Emerging arrhythmic risk of autoimmune and inflammatory cardiac channelopathies. J Am Heart Assoc. 2018;7(22):e010595. doi: 10.1161/JAHA.118.010595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gawalko M, Saljic A, Li N, et al. Adiposity-associated atrial fibrillation: molecular determinants, mechanisms, and clinical significance. Cardiovasc Res. 2023;119(3):614–630. doi: 10.1093/cvr/cvac093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nattel S. Atrial fibrillation and body composition: Is it fat or lean that ultimately determines the risk? J Am Coll Cardiol. 2017;69(20):2498–2501. doi: 10.1016/j.jacc.2017.03.566 [DOI] [PubMed] [Google Scholar]
- [12].Lavie CJ, Pandey A, Lau DH, et al. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol. 2017;70(16):2022–2035. doi: 10.1016/j.jacc.2017.09.002 [DOI] [PubMed] [Google Scholar]
- [13].Lee H, Choi EK, Lee SH, et al. Atrial fibrillation risk in metabolically healthy obesity: a nationwide population-based study. Int J Cardiol. 2017;240:221–227. doi: 10.1016/j.ijcard.2017.03.103 [DOI] [PubMed] [Google Scholar]
- [14].Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American heart association. Circulation. 2021;143(21):e984–e1010. doi: 10.1161/CIR.0000000000000973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wong CX, Sun MT, Odutayo A, et al. Associations of Epicardial, abdominal, and overall adiposity with atrial fibrillation. Circ Arrhythm Electrophysiol. 2016;9(12). doi: 10.1161/CIRCEP.116.004378 [DOI] [PubMed] [Google Scholar]
- [16].Ernault AC, Verkerk AO, Bayer JD, et al. Secretome of atrial epicardial adipose tissue facilitates reentrant arrhythmias by myocardial remodeling. Heart Rhythm. 2022;19(9):1461–1470. doi: 10.1016/j.hrthm.2022.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Meulendijks ER, Al-Shama RFM, Kawasaki M, et al. Atrial epicardial adipose tissue abundantly secretes myeloperoxidase and activates atrial fibroblasts in patients with atrial fibrillation. J Transl Med. 2023;21(1):366. doi: 10.1186/s12967-023-04231-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].de Vries Cee, Tsangaris E, Makarawung DJS, et al. Validation of the Dutch version of the BODY-Q measuring appearance, health-related quality of life, and experience of healthcare in patients undergoing bariatric and body contouring surgery. Aesthet Surg J. 2023;43(5):569–579. doi: 10.1093/asj/sjac311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Commandeur F, Goeller M, Razipour A, et al. Fully automated CT quantification of epicardial adipose tissue by deep learning: a multicenter study. Radiol Artif Intell. 2019;1(6):e190045. doi: 10.1148/ryai.2019190045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mahabadi AA, Lehmann N, Sonneck NC, et al. Left atrial size quantification using non-contrast-enhanced cardiac computed tomography – association with cardiovascular risk factors and gender-specific distribution in the general population: the heinz nixdorf recall study. Acta Radiol. 2014;55(8):917–925. doi: 10.1177/0284185113507446 [DOI] [PubMed] [Google Scholar]
- [21].Russo V, Ammendola E, De Crescenzo I, et al. Severe obesity and P-wave dispersion: the effect of surgically induced weight loss. Obes Surg. 2008;18(1):90–96. doi: 10.1007/s11695-007-9340-7 [DOI] [PubMed] [Google Scholar]
- [22].Friedman DJ, Wang N, Meigs JB, et al. Pericardial fat is associated with atrial conduction: the framingham heart study. J Am Heart Assoc. 2014;3(2):e000477. doi: 10.1161/JAHA.113.000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fernandes-Cardoso A, Santos-Furtado M, Grindler J. Effects of epicardial Fat reduction on P-wave duration of morbidly obese patients submitted to bariatric surgery: an observational study. J Card Arrhtythmias. 2019;32(2):82–88. doi: 10.24207/jca.v32n2.009_IN [DOI] [Google Scholar]
- [24].Goeller M, Achenbach S, Marwan M, et al. Epicardial adipose tissue density and volume are related to subclinical atherosclerosis, inflammation and major adverse cardiac events in asymptomatic subjects. J Cardiovasc Comput Tomogr. 2018;12(1):67–73. doi: 10.1016/j.jcct.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Antonopoulos AS, Sanna F, Sabharwal N, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9(398). doi: 10.1126/scitranslmed.aal2658 [DOI] [PubMed] [Google Scholar]
- [26].Fitzgibbons TP, Czech MP. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J Am Heart Assoc. 2014;3(2):e000582. doi: 10.1161/JAHA.113.000582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Al-Shama R, Ernault AC, Meulendijks ER, et al. Myeloperoxidase causes both arrhythmogenic structural and electrical remodelling in neonatal rat ventricular myocyte monolayers. Europace. 2023;25. doi: 10.1093/europace/euad122.016 [DOI] [Google Scholar]
- [28].Ernault AC, de Winter R, Fabrizi B, et al. MicroRNAs in extracellular vesicles released from epicardial adipose tissue promote arrhythmogenic conduction slowing. Heart Rhythm O2. 2023;4(12):805–814. doi: 10.1016/j.hroo.2023.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Henry JA, Abdesselam I, Deal O, et al. Changes in epicardial and visceral adipose tissue depots following bariatric surgery and their effect on cardiac geometry. Front Endocrinol (Lausanne). 2023;14:1092777. doi: 10.3389/fendo.2023.1092777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Levelt E, Mahmod M, Piechnik SK, et al. Relationship between left ventricular structural and metabolic remodeling in type 2 diabetes. Diabetes. 2016;65(1):44–52. doi: 10.2337/db15-0627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Athithan L, Gulsin GS, Gp M, et al. Diabetic cardiomyopathy: pathophysiology, theories and evidence to date. World J Diabetes. 2019;10(10):490–510. doi: 10.4239/wjd.v10.i10.490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Strzelczyk J, Kalinowski P, Zieniewicz K, et al. The influence of surgical weight reduction on left atrial strain. Obes Surg. 2021;31(12):5243–5250. doi: 10.1007/s11695-021-05710-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hanley C, Shields KJ, Matthews KA, et al. Associations of cardiovascular fat radiodensity and vascular calcification in midlife women: the SWAN cardiovascular fat ancillary study. Atherosclerosis. 2018;279:114–121. doi: 10.1016/j.atherosclerosis.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pandey NN, Sharma S, Jagia P, et al. Epicardial fat attenuation, not volume, predicts obstructive coronary artery disease and high risk plaque features in patients with atypical chest pain. Br J Radiol. 2020;93(1114):20200540. doi: 10.1259/bjr.20200540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Franssens BT, Nathoe HM, Visseren FL, et al. Relation of epicardial adipose tissue radiodensity to coronary artery calcium on cardiac computed tomography in patients at high risk for cardiovascular disease. Am J Cardiol. 2017;119(9):1359–1365. doi: 10.1016/j.amjcard.2017.01.031 [DOI] [PubMed] [Google Scholar]
- [36].Meulendijks Bf ER, Bruns S, Eringa EC, et al. Neutrophil infiltration in atrial epicardial adipose tissue is higher in persistent than paroxysmal atrial fibrillation and is associated with CT attenuation. Submitted. [Google Scholar]
- [37].Ishii Y, Abe I, Kira S, et al. Detection of fibrotic remodeling of epicardial adipose tissue in patients with atrial fibrillation: imaging approach based on histological observation. Heart Rhythm O2. 2021;2(4):311–323. doi: 10.1016/j.hroo.2021.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chan J, Thakur U, Tan S, et al. Inter-software and inter-scan variability in measurement of epicardial adipose tissue: a three-way comparison of a research-specific, a freeware and a coronary application software platform. Eur Radiol. 2023;33(12):8445–8453. doi: 10.1007/s00330-023-09878-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Askarpour M, Alizadeh S, Hadi A, et al. Effect of bariatric surgery on the circulating level of Adiponectin, Chemerin, plasminogen activator inhibitor-1, leptin, resistin, and Visfatin: a systematic review and meta-analysis. Horm Metab Res. 2020;52(4):207–215. doi: 10.1055/a-1129-6785 [DOI] [PubMed] [Google Scholar]
- [40].Karmazyn M, Purdham DM, Rajapurohitam V, et al. Signalling mechanisms underlying the metabolic and other effects of adipokines on the heart. Cardiovasc Res. 2008;79(2):279–286. doi: 10.1093/cvr/cvn115 [DOI] [PubMed] [Google Scholar]
- [41].Essick EE, Ouchi N, Wilson RM, et al. Adiponectin mediates cardioprotection in oxidative stress-induced cardiac myocyte remodeling. Am J Physiol Heart Circ Physiol. 2011;301(3):H984–993. doi: 10.1152/ajpheart.00428.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tao L, Gao E, Jiao X, et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115(11):1408–1416. doi: 10.1161/CIRCULATIONAHA.106.666941 [DOI] [PubMed] [Google Scholar]
- [43].Harun NH, Froemming GRA, Nawawi HM, et al. Inflammation and vascular calcification causing effects of oxidized HDL are attenuated by Adiponectin in human vascular smooth muscle cells. Int J Mol Cell Med. 2019;8(1):39–55. doi: 10.22088/IJMCM.BUMS.8.1.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pandey GK, Vadivel S, Raghavan S, et al. High molecular weight adiponectin reduces glucolipotoxicity-induced inflammation and improves lipid metabolism and insulin sensitivity via APPL1-AMPK-GLUT4 regulation in 3T3-L1 adipocytes. Atherosclerosis. 2019;288:67–75. doi: 10.1016/j.atherosclerosis.2019.07.011 [DOI] [PubMed] [Google Scholar]
- [45].Boesing F, Moreira EA, Wilhelm-Filho D, et al. Roux-en-Y bypass gastroplasty: markers of oxidative stress 6 months after surgery. Obes Surg. 2010;20(9):1236–1244. doi: 10.1007/s11695-010-0196-x [DOI] [PubMed] [Google Scholar]
- [46].Andrade VL, Petruceli E, Belo VA, et al. Evaluation of plasmatic MMP-8, MMP-9, TIMP-1 and MPO levels in obese and lean women. Clin Biochem. 2012;45(6):412–415. doi: 10.1016/j.clinbiochem.2012.01.008 [DOI] [PubMed] [Google Scholar]
- [47].Netto BD, Moreira EA, Patino JS, et al. Influence of roux-en-Y gastric bypass surgery on vitamin C, myeloperoxidase, and oral clinical manifestations: a 2-year follow-up study. Nutr Clin Pract. 2012;27(1):114–121. doi: 10.1177/0884533611431462 [DOI] [PubMed] [Google Scholar]
- [48].da Silva Vr, Moreira EA, Wilhelm-Filho D, et al. Proinflammatory and oxidative stress markers in patients submitted to roux-en-Y gastric bypass after 1 year of follow-up. Eur J Clin Nutr. 2012;66(8):891–899. doi: 10.1038/ejcn.2012.17 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, E.R.M. The data are not publicly available due to information that could compromise the privacy of research participants.


