Abstract
Background
Streptococcus pneumoniae is a common pathogen associated with bloodstream infections, respiratory infections, peritonitis, infective endocarditis, and meningitis. Literature assessing duration of antibiotic therapy for a S pneumoniae bacteremia secondary to common infection is scarce, leading to variability in practice. Therefore, this study evaluated the effectiveness of short (5–10 days) versus long (11–16 days) antibiotic durations for S pneumoniae bacteremia.
Methods
This retrospective, single-center cohort study assessed hospitalized patients with S pneumoniae–positive blood cultures, who received active antibiotics within 48 hours of first positive blood culture collection and achieved clinical stability by day 10 of the first positive blood culture collection. Exclusion criteria included treatment duration <5 or >16 days, death before completion of 10 days of therapy, polymicrobial bloodstream infection, and invasive infection. Rates of clinical failure (composite of 30-day hospital readmission, bacteremia recurrence, and mortality) were compared between the groups.
Results
A total of 162 patients were included, with 51 patients in the short- and 111 patients in the long-duration group. Pneumonia was the suspected source of bacteremia in 90.1% of patients. Rates of clinical failure were not significantly different between the 2 groups. Patients received a median antibiotic course of 7 days in the short group compared to 14 days in the long group; however, there was no significant difference observed in the median hospital length of stay, median intensive care unit length of stay, or rate of Clostridioides difficile infection.
Conclusions
Shorter antibiotic courses may be appropriate in patients with S pneumoniae bacteremia secondary to community-acquired pneumonia.
Keywords: antimicrobial stewardship, bloodstream infection, duration of treatment, pneumonia, Streptococcus pneumoniae
Rates of clinical failure were no different when comparing short antibiotic courses (5–10 days) versus long antibiotic courses (11–16 days) for Streptococcus pneumoniae bacteremia. Most patients in this cohort had pneumonia as the source of bacteremia.
Streptococcus pneumoniae bacteremia ranks among the top 10 most common bloodstream infection (BSI) pathogens and is associated with high morbidity and mortality worldwide [1, 2]. S pneumoniae is typically a cause of respiratory infections such as community-acquired pneumonia but can also cause nonrespiratory infections such as peritonitis, infective endocarditis, and meningitis [3]. Current guidelines recommend a short course (5–7 days) of antibiotics for community-acquired pneumonia treatment; however, they do not specifically address duration of therapy for secondary BSI [3].
Shorter antibiotic durations have been effective in several types of infections, but there is limited data available for gram-positive cocci or S pneumoniae BSI specifically. Because of the lack of randomized clinical trials on the efficacy and safety of short versus long antibiotic treatment durations, notably in patients with S pneumoniae BSI, variability in clinical practice exists. These discrepancies in practice can potentially lead to increased adverse events such as Clostridioides difficile infection and antimicrobial resistance. Therefore, this study evaluated the effectiveness of short (≤10 days) versus long (>10 days) durations of therapy for S pneumoniae bacteremia.
METHODS
This single health system, retrospective, observational study examined patients with S pneumoniae bacteremia admitted to a Methodist Health System hospital from 1 April 2017 to 31 May 2023. Methodist Health System comprises 6 hospitals in the Dallas-Fort Worth metroplex, equating to approximately 1700 beds in total. The protocol was approved by the Methodist Health System institutional review board. A retrospective chart review was completed for all admitted patients to evaluate the effectiveness of short versus long antibiotic treatment duration. Patients were identified for study inclusion via a query of the electronic medical record (EMR). Data acquired during the EMR query included age, gender, hospital length of stay (LOS), intensive care unit (ICU) LOS, Charlson Comorbidity Index score, Acute Physiology and Chronic Health Evaluation (APACHE) II score, Pitt bacteremia score, and central venous catheter placement. Additional demographic, clinical management, and laboratory data were collected via manual chart review, such as blood culture results with associated date, discharge disposition, start and stop dates of all inpatient and outpatient antibiotics, white blood cell count, serum creatinine level, and other applicable vital signs at the time of positive blood cultures and at day 10 of therapy. Admission and discharge summaries were reviewed to determine immunosuppression status (eg, solid organ transplantation, active malignancy, HIV) and pneumococcal vaccination status.
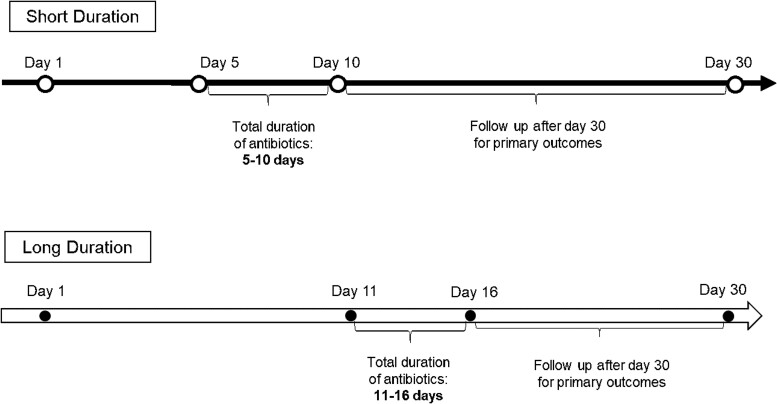
Patients were included if they were ≥18 years of age, had S pneumoniae isolated in at least 1 bottle out of a set of blood cultures, received active antibiotic therapy within 48 hours of the first positive blood culture result, and achieved clinical stability by day 10 of the first positive blood culture collection (Figure 1). Clinical stability was defined as a temperature of <100.4 °F for ≥48 hours and at most 1 sign of clinical instability (eg, heart rate >100 beats per minute, systolic blood pressure <90 mm Hg, oxygen saturation <90%, partial pressure of oxygen <60 mm Hg on room air). Exclusion criteria included treatment duration <5 or >16 days, death before completion of 10 days of therapy, a polymicrobial BSI, and any invasive infection caused by S pneumoniae (ie, endocarditis, meningitis, and lung abscess).
Figure 1.
Timeline of short versus long duration groups.
A threshold of 10 days for determining short versus long therapy was adopted a priori based on previous literature in gram-negative BSIs [4]. Moreover, this cutoff value allowed for dissection of the cohort into relatively equal distributed comparator groups and allowed for commonly used durations to be accommodated (eg, 7, 10, 14 days of treatment). Patients receiving <5 days of antibiotic therapy were excluded to fairly assess the efficacy of a shorter duration of antibiotics by ensuring the primary infection was adequately treated, which in most cases would be a minimum of 5 days of active therapy. The requirement of clinical stability by day 10 was to ensure that patients had the opportunity to be treated for the shorter duration because it would not be expected that antibiotic treatment would be stopped in an unstable patient.
The primary objective was to assess the clinical efficacy of a shorter antibiotic duration compared to a longer antibiotic duration for treatment of S pneumoniae bacteremia. Clinical failure was defined as a composite of all-cause hospital readmission, bacteremia recurrence with S pneumoniae following completion of initial antibiotic therapy, and all-cause mortality within 30 days of the first positive blood culture. Secondary objectives included examining the impact of differing treatment durations on hospital LOS, ICU LOS, rate of C difficile infections, and rate of central venous catheter placement.
Blood cultures were collected on all patients at the time of hospital admission with signs of infection, such as elevation in white blood cell count >11 × 109/L, elevation in temperature >100.4 °F, or signs of clinical instability as previously defined. Pathogen identification and antimicrobial susceptibility testing was standardized and was determined using a MicroScan Walkaway system (Beckman Coulter; Brea, CA, USA) until June 2021 when a matrix-assisted laser desorption/ionization–time of flight mass spectrometer began being used for pathogen identification. The minimum inhibitory concentration breakpoints and interpretative criteria used were up to date and concordant with the Clinical and Laboratory Standards Institute M100 document [5]. All classifications of antibiotic susceptibilities were based on in vitro susceptibility testing using these established Clinical and Laboratory Standards Institute breakpoints.
Data analyses were performed using SPSS statistics software (IBM SPSS Statistics, version 22.0; Chicago, IL, USA). Descriptive analysis was performed for continuous variables, which included baseline characteristics, culture results, and imaging results. Mean and standard deviation were used to present normally distributed variables, and median and interquartile range (IQR) were used to analyze nonnormally distributed variables. Count and proportions were included for all categorical variables. Categorical variables were analyzed using chi-square or Fisher exact test as appropriate. Continuous data were analyzed using Mann-Whitney U test as appropriate. Variables identified in univariate analysis as being associated with clinical failure with P values <.20 were entered into a multivariable logistic regression analysis. P values <.05 were considered statistically significant.
Inverse probability of treatment weighting (IPTW) was used to reduce prescriber bias because of patients being nonrandomly assigned to shorter or longer antibiotic duration of therapy. A logistic regression model was used for assessment of clinical failure. The covariates selected for inclusion were the use of combination antibiotics, patient age ≥70 years, immunocompromised patients (solid organ transplant, bone marrow transplant, hematologic malignancy, use of prednisone >20 mg equivalent for 4 weeks or longer, asplenia, HIV infection, hypogammaglobinemia, or active chemotherapy within the past 3 months), APACHE II score, Pitt bacteremia score (on day 1 of therapy), specific hospital in the health system, pneumonia as the source of bacteremia, need for vasopressor (norepinephrine) support, liver disease, and Charlson Comorbidity Index.
Patients were given a weight that was determined by the inverse of the percent of patients in the group that had been exposed to the selected covariate. This technique transforms the data so that the measured covariates are approximately random because of the formation of a pseudo-population that balances the covariates. These variables would then impact the outcome without impacting the choice of therapy.
Patient Consent Statement
This retrospective review of the EMR was approved and informed consent requirements were waived by the Methodist Health System Institutional Review Board (Dallas, Texas).
RESULTS
From 1 April 2017 to 31 May 2023, 439 patients were admitted to a Methodist Health System hospital and had a positive blood culture for S pneumoniae during their admission. Of these, 162 patients were included in the study (Supplementary Figure 1, Supplementary Table 1). The median (IQR) patient age at hospitalization was 59 (48–71) years and 50% (n = 81) of the cohort was male. The most commonly observed comorbidities were chronic obstructive pulmonary disease and congestive heart failure with additional comorbidities within the population summarized in Table 1.
Table 1.
Baseline Demographics and Characteristics of the Study Cohort
| Short Duration N = 51 |
Long Duration N = 111 |
P Value | |
|---|---|---|---|
| Age, y, median (IQR) | 59 (47–71) | 59 (49–71) | NS |
| Male sex, n (%) | 29 (56.9) | 52 (46.8) | NS |
| Charlson Comorbidity Index score, median (IQR) | 6 (4–10) | 5 (3–8) | .033 |
| Comorbid conditions, n (%) | |||
| Active chemotherapy within last 90 d | 0 (0) | 0 (0) | NS |
| Autoimmune disorder | 2 (5.7) | 3 (2.4) | NS |
| Bone marrow transplant | 0 (0) | 0 (0) | NS |
| Congestive heart failure | 15 (29.4) | 37 (33.3) | NS |
| Chronic kidney disease | 14 (27.5) | 29 (26.1) | NS |
| COPD | 20 (39.2) | 41 (36.9) | NS |
| Connective tissue disorder | 2 (5.7) | 7 (6.3) | NS |
| CVA | 4 (7.8) | 10 (9) | NS |
| Diabetes | 15 (29.4) | 32 (28.8) | NS |
| Hematologic malignancy | 1 (2) | 5 (4.5) | NS |
| HIV infection | 7 (13.7) | 8 (7.2) | NS |
| Hypogammaglobinemia | 0 (0) | 0 (0) | NS |
| Mild liver diseasea | 17 (33.3) | 21 (18.9) | NS |
| Prior splenectomy | 1 (2) | 1 (0.9) | NS |
| Severe liver diseasea | 7 (13.7) | 9 (8.1) | NS |
| Solid organ transplant | 1 (2) | 1 (0.9) | NS |
| Allergies, n (%) | |||
| Penicillin allergy | 12 (23.5) | 12 (10.8) | NS |
| Non-penicillin antibiotic allergy | 5 (9.8) | 12 (10.8) | NS |
| Severity, n (%) | |||
| Met SIRS criteria | 24 (47.1) | 61 (55) | NS |
| ICU admission/transfer | 12 (23.5) | 34 (30.6) | NS |
| APACHE II score, median (IQR) | 6 (4–7) | 7 (4–13) | NS |
| Pitt bacteremia score ≥1, n (%) | 9 (17.6) | 11 (9.9) | NS |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; COPD, chronic obstructive pulmonary disease; CVA, cerebral vascular accident; ICU, intensive care unit; NS, not significant; SIRS, systemic inflammatory response syndrome.
aMild liver disease, chronic hepatitis, or cirrhosis without portal hypertension; severe liver disease, cirrhosis, and portal hypertension with variceal bleeding history or cirrhosis and portal hypertension in the absence of variceal bleeding history.
Fifty-one (31%) patients received a shorter duration of therapy, and 111 (69%) patients received a longer duration. The median (IQR) durations of antibiotic therapy in the short and long groups were 7 (5–9) days and 14 (13–15) days, respectively. There was a statistically significant difference in the Charlson Comorbidity Index between the short and long duration groups (6 [4–10] versus 5 [3–8]; P = .033). The Pitt bacteremia score on the date of blood culture was comparable between the 2 groups.
The source of bacteremia was identified as pneumonia in 50 (98%) patients in the short-duration group and 96 (86.5%) patients in the long-duration group (P = .023) (Table 2). A combination of beta-lactam and macrolide therapy was the most common antibiotic regimen initiated in both the short- (19 patients, 37.3%) and long- (43 patients, 38.7%) duration groups. The median (IQR) days of inpatient antibiotic therapy in the short- and long-duration groups were 6 (5–7) and 7 (4–9) days, respectively. At discharge, beta-lactam monotherapy was most frequently chosen for continued therapy for 8 (53.3%) patients in the short-duration group and 65 (79.3%) patients in the long-duration group (Table 2). Fluoroquinolones were the second most frequently chosen antibiotic, with 6 (40%) patients in the short-duration group compared to 13 (15.9%) in the long-duration group being prescribed this antibiotic class at discharge. The patients in the short-duration group received outpatient antibiotics for a median of 2 (0–4) days, whereas the patients in the long-duration group received a median of 7 (5–10) days. Antibiotic resistance was most frequently seen for penicillin (23.5% [short] and 18.9% [long]) and azithromycin (25.5% [short] and 29.7% [long]) in both groups.
Table 2.
Hospitalization Patient Characteristics and Antibiotic Choices
| Short Duration N = 51 |
Long Duration N = 111 |
P Value | |
|---|---|---|---|
| Infection type, n (%) | |||
| Pneumonia | 50 (98) | 96 (86.5) | .023 |
| Peritonitis | 0 (0) | 5 (4.5) | NS |
| Otitis media | 0 (0) | 4 (3.6) | NS |
| SSTI | 1 (0) | 3 (2.4) | NS |
| Endovascular | 0 (0) | 1 (0.9) | NS |
| Unknown etiology | 0 (0) | 2 (1.8) | NS |
| Initial inpatient antibiotic choice, n (%) | |||
| Beta-lactam | 10 (19.6) | 16 (14.4) | NS |
| Fluoroquinolone | 2 (3.9) | 3 (2.7) | NS |
| Vancomycin | 5 (9.8) | 8 (7.2) | NS |
| Combination beta-lactam and macrolide | 19 (37.3) | 43 (38.7) | NS |
| Combination beta-lactam and vancomycin | 10 (19.6) | 33 (29.7) | NS |
| Combination beta-lactam, vancomycin, and macrolide | 5 (9.8) | 8 (7.2) | NS |
| Initial outpatient antibiotic choice (monotherapy), n (%) | |||
| Beta-lactam | 8 (53.3) | 65 (79.3) | <.001 |
| Macrolide | 1 (6.7) | 4 (4.9) | NS |
| Fluoroquinolone | 6 (40) | 13 (15.9) | NS |
| Antibiotic resistance, n (%) | |||
| Azithromycin resistance | 13 (25.5) | 33 (29.7) | NS |
| Ceftriaxone resistance | 1 (2) | 6 (5.4) | NS |
| Fluoroquinolone resistance | 0 (0) | 0 (0) | NS |
| Penicillin resistance | 12 (23.5) | 21 (18.9) | NS |
| Tetracycline resistance | 2 (3.9) | 9 (8.1) | NS |
| Antibiotic duration, days, median (IQR) | |||
| Total | 7 (5–9) | 14 (13–15) | <.001 |
| Inpatient | 5 (5–7) | 5 (4–9) | .301 |
| Outpatient | 1 (0–4) | 7 (5–10) | <.001 |
Abbreviations: IQR, interquartile range; NS, not significant; SSTI, skin and soft tissue infection.
The primary composite outcome of clinical failure was met in 35 patients, including 14 (27.5%) patients in the short-duration group and 21 (18.9%) patients in the long-duration group (P = .225). Any differences among the individual clinical outcomes of 30-day all-cause hospital readmission, bacteremia recurrence, or all-cause mortality were not statistically significant (Table 3). Though not statistically different, there was a higher percentage of all-cause hospital readmission in the short group than the long group (27.5% vs 18.2%; P = .214). A post hoc analysis was used to evaluate the differences in these hospital readmission rates. Following a 2-physician adjudication process, it was noted that 1 patient from the short-duration group was likely readmitted in association with the original S pneumoniae bacteremia diagnosis for a postpneumatic cough and 1 patient possibly associated with the original diagnosis with C difficile infection. The remaining 12 patients were deemed to be readmitted for a diagnosis unlikely to be related to their initial S pneumoniae bacteremia admission (Supplementary Figure 2).
Table 3.
Primary and Secondary Outcomes
| Short Duration N = 51 |
Long Duration N = 111 |
P Value | |
|---|---|---|---|
| Primary outcomea—clinical failure, n (%) | 14 (27.5) | 21 (18.9) | .225 |
| All-cause hospital readmission | 14 (27.5) | 20 (18.2) | .214 |
| Bacteremia recurrence | 1 (2) | 1 (0.9) | .532 |
| All-cause mortality | 1 (2) | 1 (0.9) | .532 |
| Secondary outcomes | |||
| Hospital LOS, days, median (IQR) | 5 (4–7) | 5(4–9) | .860 |
| ICU LOS, days, median (IQR) | 4 (1–6) | 2 (2–5) | .529 |
| C difficile infection, n (%) | 1 (2) | 1 (0.9) | .532 |
| Central venous catheter placement, n (%) | 2 (3.9) | 5 (4.5) | .614 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; LOS, length of stay.
aWithin 30 days of positive blood culture.
Results of the IPTW for predicted probability of clinical failure between the shorter and longer groups were not significantly different when stratified by use of combination antibiotics, patient age ≥70 years, immunocompromised status, APACHE II score, Pitt bacteremia score (on day 1 of therapy), specific hospital in the health system, pneumonia as the source of bacteremia, need for vasopressor support, liver disease, and Charlson Comorbidity Index (adjusted odds ratio: 1.33; 95% confidence interval, .59–3.00; P = .885) (Table 4). When analyzing each variable individually, liver disease posed a significant risk for clinical failure (adjusted odds ratio: 2.86; 95% confidence interval, 1.27–6.43; P = .029).
Table 4.
Inverse Probability of Treatment Weighting for Composite Clinical Failure
| Adjusted OR | 95% Confidence Interval | P Value | |
|---|---|---|---|
| Short duration | 1.33 | .59–3.00 | .885 |
| APACHE II score | 1.02 | .98–1.05 | .342 |
| Age ≥70 y | 1.38 | .63–3.03 | .307 |
| Charlson Comorbidity Index score | 1.11 | .98–1.27 | .098 |
| Combination antibiotics | 1.14 | .49–2.68 | .522 |
| Immunocompromised | 2.19 | .97–4.90 | .059 |
| Liver disease | 2.86 | 1.27–6.43 | .029 |
| MDMC versus other site | 1.16 | .54–2.72 | .557 |
| Pitt bacteremia score | 1.79 | .16–20.40 | .322 |
| Pneumonia (source) | 2.04 | .44–9.45 | .184 |
| Vasopressor requirement | 0.58 | .12–2.47 | .322 |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; MDMC, Methodist Dallas Medical Center; OR, odds ratio.
DISCUSSION
In this study, there was no association between clinical failure and shorter duration of antibiotic therapy for S pneumoniae bacteremia. The shorter and longer antibiotic duration groups had similar rates of all-cause hospital readmission, bacteremia recurrence, and all-cause mortality. There was also no significant difference between hospital LOS, ICU LOS, rate of C difficile occurrence, or central venous catheter placement in either group.
There is a growing movement toward shorter courses of treatment for many common infectious diseases, including BSIs [6–8]. Yet, there is limited evidence addressing the effectiveness of shorter antibiotic durations for S pneumoniae bacteremia. A multicenter retrospective cohort study by Hojat et al (2020) evaluated shorter duration of antibiotic treatment in pneumonia, urinary tract infections, and acute bacterial skin and skin structure infection-associated BSIs [9]. Of 408 patients, 123 received a shorter duration of antibiotic therapy (median: 8 days [IQR, 7–9]) and 285 patients received a longer duration (13 days [IQR, 11–14]). In patients with gram-positive bacteremia, 38.2% of patients were treated with shorter courses of antibiotics and 32.6% of patients were treated with longer courses of antibiotic therapy. Although it was not defined how many patients had S pneumoniae bacteremia specifically, there were no findings suggesting increased risk of antibiotic failure when using shorter versus longer durations of therapy. One notable difference observed between the results of this study and ours was an increased probability of patients being restarted on antibiotics for the same infection within a 30-day period (13.5% vs 8.8%, P = .046) when using a shorter duration of antibiotics.
Boulos et al (2021) similarly evaluated duration of therapy for streptococcal (all species types except S pneumoniae) BSIs in a retrospective, multicenter observational cohort. A total of 176 patients were included, with 35 receiving a shorter (≤10 days) and 141 receiving a longer (>10 days) duration of antibiotic therapy [10]. The most common infectious pathogen was viridians group streptococci (28%), with skin and soft tissue infections being the most common source (40%). Overall, the shorter duration group was treated a median of 8 days compared to 15 days for the longer duration group, and there was no statistically significant difference in recurrent bacteremia, hospital readmission, or all-cause mortality within 30 days of completing therapy. Although none of the studies specifically analyzed S pneumoniae bacteremia-associated duration of antibiotic therapy, they all concluded that mortality or recurrence does not significantly increase in cases of uncomplicated bacteremia secondary to community-acquired infections treated with a shorter duration of antibiotic therapy.
Multiple studies have reviewed intravenous to oral stepdown therapy in uncomplicated bacteremia. These studies suggested treatment with shorter duration of antibiotic therapy is reasonable [11, 12]. Ramos-Otero et al (2022) analyzed oral antibiotic stepdown in uncomplicated streptococcal BSI (51 patients in the intravenous to oral group and 47 patients in the intravenous-only group) [11]. Of the included patients, 29 patients had S pneumoniae bacteremia. The mean total antibiotic duration was 11.8 days in patients switched to oral therapy and 14 days in patients continued on intravenous therapy with no association of worse clinical outcomes. Although different durations of antibiotics were used for treatment overall in the studies, no differences in clinical outcomes related to duration of therapy or infectious pathogen (eg, S pneumoniae) were identified. Similar to our findings, the majority of patients were discharged on a beta-lactam antibiotic, which may reflect that prescribers felt more comfortable using this drug class to mitigate potential toxicity with other classes such as fluoroquinolones. The current clinical evidence available supports our finding of no difference in outcomes in regard to treatment duration for S pneumoniae BSI.
Limitations of the current study should be recognized, including the expected limitations of a retrospective observational study design. Additionally, rates of recurrence could be underrepresented because of patients presenting to health systems that were not included in Epic Care Everywhere (EMR technology used for eHealth exchange between community and hospital centers). Second, prescribing bias may have played a role in duration of antibiotics received. We attempted to mitigate such disparities through utilization of IPTW but the potential for impact of unmeasured variables exists. Third, we counted both intravenous and oral antibiotic therapy equally in determining total duration. The assumption that oral therapy is similar in effectiveness to intravenous is reasonable based on contemporary clinical data; however, the potential for an impact of this difference on outcomes could exist. Fourth, the majority of our patients had pneumonia as the source of their bacteremia. Although this was expected because of S pneumoniae being a leading cause of pneumonia, the generalizability of our study is limited when S pneumoniae BSI is secondary to another source of infection. Fifth, this study took place within a single healthcare system in the United States and, although the health system represents variety in setting (eg, teaching and nonteaching, urban and rural), applicability in other settings may be vary. Sixth, there were no means to assess antibiotic adherence in the outpatient setting. With more than half of the cohort being sent home on an antibiotic regimen, variability in adherence could have influenced rates of clinical failure.
CONCLUSION
In the cohort of patients being treated with short versus long duration of antibiotics for S pneumoniae bacteremia, there was no difference in overall clinical efficacy when evaluating all-cause hospital readmission, bacteremia recurrence, or all-cause mortality. Secondary outcomes regarding hospital LOS, ICU LOS, and adverse drug events are also not statistically different between the groups. Further research should be conducted to determine the appropriateness of shorter antibiotic durations as well as potential benefits associated with reducing antibiotic exposure in this setting.
Supplementary Data
Supplementary materials are available at the Journal of The Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Matthew Crotty, Department of Pharmacy, Methodist Dallas Medical Center, Dallas, Texas, USA.
Hadley Devall, Department of Pharmacy, Methodist Dallas Medical Center, Dallas, Texas, USA.
Natalie Cook, Department of Pharmacy, Methodist Dallas Medical Center, Dallas, Texas, USA.
Francis Fischer, Department of Internal Medicine, Methodist Dallas Medical Center, Dallas, Texas, USA.
Julie Alexander, Department of Internal Medicine, Methodist Dallas Medical Center, Dallas, Texas, USA.
Leigh Hunter, Department of Internal Medicine, Methodist Dallas Medical Center, Dallas, Texas, USA.
Edward Dominguez, Organ Transplant Infectious Diseases, Methodist Transplant Specialists, Methodist Dallas Medical Center, Dallas, Texas, USA.
Notes
Acknowledgments. Dr. Anne Murray of the Clinical Research Institute at Methodist Health System (Dallas, TX, USA) for providing editorial support. Mingyang Cui, MSc, of the Clinical Research Institute at Methodist Health System (Dallas, TX, USA) for providing statistical support.
Author Contributions. M.C.: Conceptualization, Methodology, Investigation, Data Curation, Writing—Original Draft, Writing—Review & Editing, Visualization. H.D.: Conceptualization, Methodology, Investigation, Data Curation, Writing—Original Draft, Writing—Review & Editing, Visualization. N.C.: Conceptualization, Methodology, Writing—Original Draft, Writing—Review & Editing. F.F.: Conceptualization, Methodology, Investigation, Writing—Review & Editing. J.A.: Conceptualization, Methodology, Writing—Review & Editing. L.H.: Conceptualization, Methodology, Writing—Review & Editing. E.D.: Conceptualization, Methodology, Writing—Review & Editing.
Financial support. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Diekema DJ, Hsueh PR, Mendes RE, et al. The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother 2019; 63:e00355-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Demirdal T, Sen P, Emir B. Predictors of mortality in invasive pneumococcal disease: a meta-analysis. Expert Rev Anti Infect Ther 2021; 19:927–44. [DOI] [PubMed] [Google Scholar]
- 3. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the outcomes of adults with Enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis 2018; 66:172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. CLSI . Performance standards for antimicrobial susceptibility testing. CLSI supplemental M100. Wayne, PA: Clinical and Laboratory Standards Institute, 2022. [Google Scholar]
- 6. Yahav D, Franceschini E, Koppel F, et al. Seven versus 14 days of antibiotic therapy for uncomplicated gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis 2019; 69:1091–8. [DOI] [PubMed] [Google Scholar]
- 7. Wald-Dickler N, Spellberg B. Short-course antibiotic therapy-replacing constantine units with “shorter is better”. Clin Infect Dis 2019; 69:1476–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heil EL, Bork JT, Abbo LM, et al. Optimizing the management of uncomplicated gram-negative bloodstream infections: consensus guidance using a modified Delphi process. Open Forum Infect Dis 2021; 8:ofab434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hojat LS, Bessesen MT, Huang M, et al. Effectiveness of shorter versus longer durations of therapy for common inpatient infections associated with bacteremia: a multicenter, propensity-weighted cohort study. Clin Infect Dis 2020; 71:3071–8. [DOI] [PubMed] [Google Scholar]
- 10. Boulos JM, Fabre V, Dzintars K, et al. 195. Duration of therapy for streptococcal bacteremia. Open Forum Infect Dis 2021; 8(Suppl 1):S205. [Google Scholar]
- 11. Ramos-Otero GP, Sarangarm P, Walraven C. A retrospective analysis of intravenous vs oral antibiotic step-down therapy for the treatment of uncomplicated streptococcal bloodstream infections. J Clin Pharmacol 2022; 62:1372–8. [DOI] [PubMed] [Google Scholar]
- 12. Waked R, Craig WY, Mercuro NJ, Wungwattana M, Wood E, Rokas KE. Uncomplicated streptococcal bacteremia: the era of oral antibiotic step-down therapy? Int J Antimicrob Agents 2023; 61:106736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.