Abstract
Chronic inflammation is associated with diabetes and contributes to the development and progression of micro- and macrovascular complications. Transcutaneous vagus nerve stimulation (tVNS) has been proposed to reduce levels of circulating inflammatory cytokines in non-diabetics by activating the cholinergic anti-inflammatory pathway. We investigated the anti-inflammatory potential of tVNS as a secondary endpoint of a randomized controlled trial in people with diabetes (NCT04143269). 131 people with diabetes (type 1: n = 63; type 2: n = 68), gastrointestinal symptoms and various degrees of autonomic neuropathy were included and randomly assigned to self-administer active (n = 63) or sham (n = 68) tVNS over two successive study periods: (1) Seven days with four daily administrations and, (2) 56 days with two daily administrations. Levels of systemic inflammatory cytokines (IL-6, IL-8, IL-10, TNF-α, IFN-γ) were quantified from blood samples by multiplex technology. Information regarding age, sex, diabetes type, and the presence of cardiac autonomic neuropathy (CAN) was included in the analysis as possible confounders. No differences in either cytokine were seen after study period 1 and 2 between active and sham tVNS (all p-values > 0.08). Age, sex, diabetes type, presence of CAN, and baseline levels of inflammatory cytokines were not associated with changes after treatment (all p-values > 0.07). A tendency towards slight reductions in TNF-α levels after active treatment was observed in those with no CAN compared to those with early or manifest CAN (p = 0.052). In conclusion, tVNS did not influence the level of systemic inflammation in people with diabetes.
Keywords: Diabetes, Inflammation, Vagus nerve stimulation, Autonomic neuropathy
Subject terms: Cytokines, Neuroimmunology, Diabetes
Introduction
Chronic inflammation is considered a central pathophysiological mechanism in the development of diabetes and its complications1. Diabetes activates inflammatory pathways through various mechanisms mainly facilitated by chronic hyperglycemia. This inflammatory response further exacerbates the progression of diabetic complications. Hence, elevated levels of pro-inflammatory cytokines and chemokines contribute to endothelial dysfunction, oxidative stress, and tissue damage, ultimately promoting the development of microvascular and macrovascular complications2.
A few decades ago, an innate anti-inflammatory neuroimmune circuit was discovered by Tracey et al.3. This so-called cholinergic anti-inflammatory pathway is triggered by infection or tissue damage and is mediated through activation of the afferent vagus nerve projecting to the nucleus tractus solitarius within the brainstem. From here the signal is transmitted via the efferent vagus nerve to the splenic nerve causing norepinephrine to be released, which in turn causes the release of post-synaptic acetylcholine from a subpopulation of β2-adrenergic receptor-positive T-cells within the spleen. In the final step of the cholinergic anti-inflammatory pathway, acetylcholine binds to α7 nicotinic acetylcholine receptors (α7nAChRs) on macrophages and causes the release of anti-inflammatory cytokines3,4. This feedback loop between the immune system and the brain is critical in maintaining immune homeostasis and preventing excessive inflammation.
With the discovery of a physiological anti-inflammatory pathway mediated through the vagus nerve, a new research area has emerged in the cross field between immunology and neuromodulation, aiming to exploit this circuit to dampen inflammation in various pathologies with inflammatory aspects5,6. Vagus nerve stimulation (VNS) can be achieved invasively through implantation of electrodes for direct and specific stimulation of the vagus nerve (iVNS). However, also non-invasive approaches have become available in which VNS is achieved transcutaneously (tVNS) either at the cervical level or at the auricular branch of the vagus nerve. Both stimulation sites have been shown to be able to activate common brain areas of vagus nerve projections6,7.
Promising results of iVNS have been shown in animal models of lung injury, colitis, and burn-induced organ dysfunction8–10, and while still in its infancy, clinical studies have also been able to show anti-inflammatory effects of iVNS in Crohn's disease11, and of tVNS in healthy subjects as well as in COVID-19, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis in non-lipopolysaccharide (LPS)-stimulated12–16 and LPS-stimulated blood17. However, these studies have included small sample sizes and/or have been designed as open-label and without sham-control groups. Consequently, more robust evidence is needed regarding the anti-inflammatory effect in humans. Indeed, the therapeutic potential of tVNS in diabetes should be investigated as a novel approach to dampen chronic inflammation and attenuate progression of diabetic complications.
Here, we investigated the hypothesis that tVNS has an anti-inflammatory effect in people with diabetes compared to sham stimulation. This was done as a sub-study of a larger clinical trial18,19. The primary aim of this sub-study was to quantify changes in plasma levels of a palette of cytokines after seven days of high-intensity (four times daily) and 56 days of medium-intensity (twice daily) tVNS treatment. Moreover, the influence of demographic factors (age, sex, diabetes type), presence of autonomic neuropathy, and overall systemic inflammation on the anti-inflammatory effect of tVNS were investigated as secondary aims.
Methods
Study overview
In this investigator-initiated, block-randomized, double-blind, sham-controlled, parallel-group trial, we investigated the effects of tVNS on systemic inflammation in a cohort of people with diabetes19. The primary outcome of the trial was improvements in gastrointestinal symptoms, which has been published elsewhere18. Thus, the findings presented here are based on secondary analyses. The trial was conducted at three Steno Centers in Denmark: Steno Diabetes Center North Jutland, Steno Diabetes Center Aarhus, and Steno Diabetes Center Copenhagen. Participants were recruited from established lists of previous study subjects and through advertisements in patient organizations and social media.
The trial consisted of two successive study periods. In study period 1, lasting seven days, the short-term effects of tVNS were investigated, while the long-term effects were evaluated in study period 2, lasting 56 days. The two study periods were separated by a wash-out period lasting at least 14 days to avoid any carry-over effects. The study was approved by relevant authorities (the Danish Health and Medicines Authority: CIV-19–07-029,105; the North Denmark Region Committee on Health Research Ethics: N-20190020) and was conducted in accordance with the principles of the Helsinki Declaration and Good Clinical Practice.
Study population
The trial included 131 people with diabetes (type 1: n = 63; type 2: n = 68) and gastrointestinal symptoms. For inclusion, upper and lower gastrointestinal symptoms were evaluated by the Gastroparesis Cardinal Symptom Index (GCSI) and the Gastrointestinal Symptom Rating Scale (GSRS), respectively. A combined weighted score of a minimum of 2.3 was required for participation in the trial, as described in19. To evaluate whether the gastrointestinal symptoms were linked to diabetic autonomic neuropathy, participants were only included if they had at least one of the following: (1) abnormal cardiac reflex testing, (2) abnormal sudomotor function, or 3) abnormal score in a patient-reported outcome measure validated to detect autonomic dysfunction (Composite Autonomic Symptoms Score (COMPASS-31)20), for more details see19. As a reference group, 40 healthy participants matched in age and sex were also included in the study for cross-sectional analysis. No tVNS were applied to the healthy cohort. Inclusion in the study was performed by a medical doctor. Written informed consent was collected from all participants before initiation of any study-related activities.
Transcutaneous vagus nerve stimulation
tVNS was self-administered with a handheld battery-powered device (GammaCore Sapphire, ElectroCore LLC, Basking Ridge, New Jersey, USA). The GammaCore Sapphire is a class IIa medical device generating a specialized low-voltage electrical signal through two stainless steel electrodes designed to stimulate the cervical region of the vagal nerve. This signal consists of a burst of 5-kHz sine waves, with each wave lasting 200 microseconds and the entire burst lasting 1 ms. The bursts are repeated at a frequency of 25 Hz (once every 40 ms), resulting in a peak voltage of 24 V and a peak output current of 60 mA21. A single tVNS dose lasts for 120 s. The device allows for manual modulation of the intensity of the stimulation through a digital user interface within a range of 1 to 40 arbitrary units. Participants were randomized to receive either active or sham tVNS in a parallel manner, and thus, the randomization was maintained during the entire study. Sham stimulation was applied by a device identical in appearance to the active device but with no electrical functionality. In this study, the stimulation protocol was as follows: In study period 1, two doses of tVNS (one on each side) were applied to the cervical part of the vagal nerve four times a day (eight doses in total per day for seven days, i.e., short-term, high-dose treatment). During the wash-out period of at least 14 days, no stimulations were applied. In study period 2, tVNS was performed bilaterally two times a day (four doses in total per day for 56 days, i.e., long-term, moderate-dose treatment). Participants received thorough instructions on how to operate the GammaCore device. Correct positioning of the device was achieved by locating the pulse in the triangular area between the sternocleidomastoid muscle and the trachea and placing the electrodes of the device here. The participants were instructed to use the maximum tolerated intensity for each stimulation, defined as the intensity just prior to experiencing pain in the area. Instructions regarding GammaCore use were provided by unblinded study personnel due to the visual feedback from active stimulation such as mild pulling of the oral commissure. Unblinded study personnel were only involved in the instruction sessions, while blinded study personnel collected data.
Plasma concentrations of inflammatory cytokines
Venous blood was collected from participants in the morning after a fasting period of at least six hours before initiation of tVNS and after seven (study period 1) or 56 days (study period 2) of tVNS (four blood samples in total). A single blood sample was likewise collected from healthy participants as reference. Blood was collected in EDTA tubes and centrifuged at 1.000 g at 4 °C for 10 min. Isolated plasma was aliquoted in adequate volumes and stored in a freezer at − 80 °C. Samples were thawed and analyzed together when the entire data set was complete. Plasma concentrations of interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ) were quantified using the V-PLEX Proinflammatory Panel 1 Human Kit (Meso Scale Diagnostics (MSD), Gaithersburg, Maryland, USA) as previously done22,23.
For sample values below the detection limit of the assay, a value equal to the detection limit divided by the square root of 2 was assigned24. A threshold of 20% was set for the coefficient of variance between duplicate measurements. Likewise, a threshold of at least 70% detectable samples per analyte was applied.
Cardiac autonomic neuropathy
The presence of cardiac autonomic neuropathy (CAN) was evaluated by cardiac autonomic reflex testing (CARTs) by a handheld device (VAGUS, Medicus Engineering Aps, Aarhus, Denmark)25. Recordings were conducted during various conditions, including rest, transition from lying to standing, deep breathing, and the Valsalva maneuver. To ensure accurate interpretation, age-specific cut-off values were implemented for classification of no CAN (no abnormal CART), early CAN (one abnormal CART), and manifest CAN (more than one abnormal CART)26. Abnormal values in one or more recordings were considered indication of CAN and were used to stratify participants in a binormal manner (+ CAN/-CAN).
Statistics
Data were collected and managed in the electronic data management tool REDCap, provided by the Region of North Jutland27, and analyzed according to per-protocol norms. The Shapiro–Wilk test of normality was used for evaluation of data distribution. Baseline-corrected concentrations of inflammatory cytokines in the diabetes cohort (after-treatment levels subtracted from pre-treatment levels) were used for all analyses.
The effect of tVNS was analyzed using linear regression with plasma concentrations as the dependent variable and treatment (active tVNS/sham tVNS) as the independent variable (model 1). Results were adjusted for age, sex, diabetes type (type 1/type 2) (model 2), and presence of CAN (+ CAN/-CAN) (model 3). Lastly, results were adjusted for baseline levels of plasma concentrations (model 4).
The power calculation for the study was determined based on the primary endpoint18,19, and the analyses presented here are thus exploratory. Statistical analyses were performed using Stata software (StataCorp LLC, v17.0). Figures were made in GraphPad Prism (version 10.1.0 for Windows).
Results
Study population
In total, 145 participants were included in the study. Of these, 14 dropped out prior to study period 1 (withdrawal of consent: 6, exclusion by study personnel: 8). Thus, 131 participants entered study period 1, of which 68 participants were randomized to sham tVNS, while 63 participants were randomized to active tVNS. Study period 1 was completed by 127 participants (sham: 65, active: 62), and study period 2 was completed by 116 participants (sham: 57, active: 59). The total number of drop-out was thus 15 (sham: 11, active: 4). No differences in baseline characteristics at inclusion in the study were found between treatment groups (Table 1). The primary endpoint of the study was alleviation of gastrointestinal symptoms; however, no such effect was found18.
Table 1.
Baseline characteristics of participants included in the study.
| Sham tVNS (n = 68) | Active tVNS (n = 63) | |
|---|---|---|
| Age, years | 58 (20–65) | 55 (45–67) |
| Sex | ||
| Male, n (%) | 24 (35) | 29 (46) |
| Female, n (%) | 44 (65) | 34 (54) |
| BMI, kg/m2 | 29 (25–35) | 28 (25–32) |
| Diabetes type | ||
| Type 1, n (%) | 32 (47) | 31 (49) |
| Type 2, n (%) | 36 (53) | 32 (51) |
| Diabetes duration, years | 18 (11–33) | 20 (10–29) |
| HbA1c, mmol/mol | 60 (54–68) | 58 (52–67) |
| CAN | ||
| No CAN, n (%) | 31 (48) | 22 (38) |
| Early CAN, n (%) | 19 (30) | 14 (24) |
| Manifest CAN, n (%) | 15 (23) | 22 (38) |
| IL-6, pg/mL | 1.2 (1.0–2.2) | 1.2 (0.8–1.6) |
| IL-8, pg/mL | 6.1 (4.6–8.5) | 7.5 (5.7–10.4) |
| IL-10, pg/mL | 0.3 (0.2–0.4) | 0.4 (0.2–0.5) |
| TNF-α, pg/mL | 1.1 (1.0–1.5) | 1.3 (1.0–1.6) |
| IFN-γ, pg/mL | 6.7 (4.5–12.5) | 6.6 (4.3–10.4) |
| Medication | ||
| Statins, n (%) | 42 (63) | 37 (59) |
| Glucocorticoids, n (%) | 2 (3) | 2 (3) |
| NSAIDs, n (%) | 10 (15) | 12 (19) |
| Acetylsalicylic acid, n (%) | 10 (15) | 14 (22) |
| GLP-1 receptor agonists, n (%) | 20 (30) | 14 (22) |
| SGLT-2 inhibitors, n (%) | 18 (27) | 17 (27) |
| DPP-4 inhibitors, n (%) | 2 (3) | 2 (3) |
Data are displayed as median (1st; 3rd quartiles) or number and percentages.
BMI Body mass index, CAN Cardiac autonomic neuropathy, DPP-4 dipeptidyl peptidase-4, GLP-1, glucagon-like peptide-1, IFN Interferon, IL Interleukin, NSAIDs nonsteroidal anti-inflammatory drugs; SGLT-2 sodium-glucose transport protein-2, TNF Tumor necrosis factor.
Plasma concentrations of inflammatory cytokines
Of the 10 analytes investigated, five (IL-1β, IL-2, IL-4, IL-12p70, and IL-13) were excluded from the analysis due to poor data quality as defined in the methods section. The remaining five analytes (IL-6, IL-8, IL-10, TNF-α, and IFN-γ) were successfully detected in > 99% of samples.
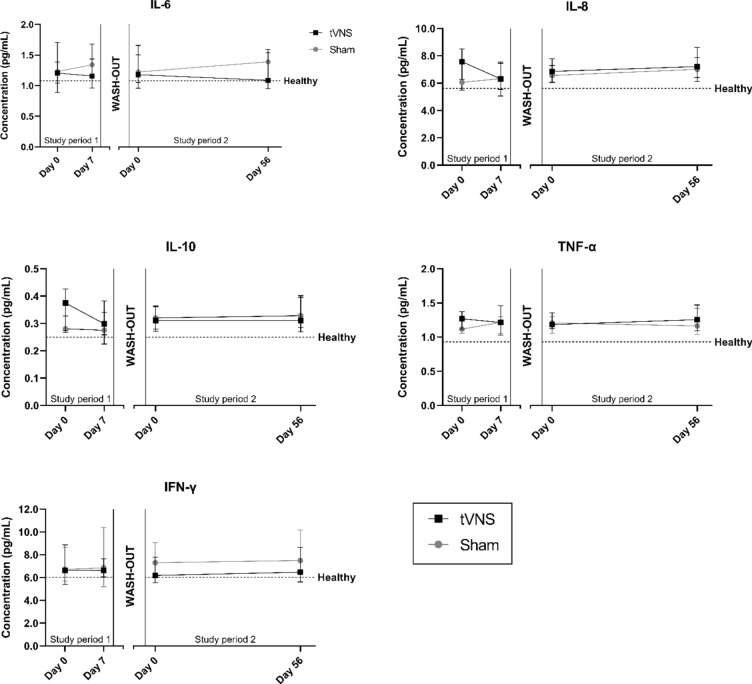
No differences in baseline levels of either analyte were seen between treatment groups (Table 1). For all analytes, median baseline levels of people with diabetes were above median levels of the healthy cohort (Fig. 1), with statistically significant differences in IL-8, IL-10 and TNF-α (all p < 0.02). Baseline-corrected levels of either analyte were independent of the treatment group in both study period 1 and 2 (all p > 0.08) (Table 2 – model 1).
Fig. 1.
Plasma concentrations of inflammatory cytokines during short-term, high-dose treatment (study period 1) and long-term, moderate-dose treatment (study period 2). The wash-out period was at least 14 days. Data is displayed as median with 95% confidence intervals. Horizontal dashed lines indicate median concentration of the healthy control cohort.
Table 2.
Results from linear regression analysis of the relationship between levels of inflammatory cytokines and active/sham stimulation.
| Model 1: No adjustments | Model 2: Demographic adjustments | Model 3: Adjustment for autonomic neuropathy | Model 4: Adjustment for inflammatory level | |||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| Study period 1 | ||||||||
| IL-6 | 0.19 (− 0.81–1.19) | 0.71 | 0.27 (− 0.78–1.32) | 0.61 | 0.49 (− 0.54–1.52) | 0.35 | − 0.03 (− 0.90–0.83) | 0.94 |
| IL-8 | − 0.97 (− 2.05–0.11) | 0.08 | − 1.02 (− 2.13–0.09) | 0.07 | − 0.77 (− 1.20–0.45) | 0.21 | − 0.21 (− 1.12–0.70) | 0.65 |
| IL-10 | − 0.01 (− 0.06–0.05) | 0.76 | − 0.01 (− 0.06–0.05) | 0.76 | 0.01 (− 0.05–0.06) | 0.84 | 0.00 − 0.05–0.06) | 0.87 |
| TNF-α | − 0.07 (− 0.18–0.04) | 0.21 | − 0.07 (− 0.19–0.04) | 0.21 | − 0.09 (− 0.22–0.03) | 0.14 | − 0.09 (− 0.22–0.03) | 0.15 |
| IFN-γ | − 0.32 (− 8.26–7.61) | 0.94 | − 1.73 (− 10.06–6.59) | 0.68 | − 0.91 (− 10.01–8.19) | 0.84 | 0.53 (− 8.31–9.37) | 0.91 |
| Study period 2 | ||||||||
| IL-6 | − 0.23 (− 0.70–0.25) | 0.34 | − 0.21 (− 0.71–0.29) | 0.40 | − 0.22 (− 0.75–0.31) | 0.42 | − 0.31 (− 0.83–0.21) | 0.24 |
| IL-8 | − 0.28 (− 1.62–1.06) | 0.68 | − 0.43 (− 1.82–0.97) | 0.54 | − 0.07 (− 1.32–1.19) | 0.92 | 0.04 (− 1.10–1.18) | 0.94 |
| IL-10 | 0.04 (− 0.04–0.12) | 0.35 | 0.04 (− 0.05–0.13) | 0.36 | 0.02 (− 0.06–0.11) | 0.58 | 0.02 (− 0.07–0.10) | 0.70 |
| TNF-α | 0.04 (− 0.20–0.28) | 0.74 | 0.04 (− 0.20–0.29) | 0.72 | 0.12 (− 0.15–0.38) | 0.39 | 0.02 (− 0.20–0.25) | 0.83 |
| IFN-γ | 13.53 (− 18.71–45.76) | 0.41 | 17.93 (− 15.62–51.47) | 0.29 | 21.66 (− 14.93–28.25) | 0.24 | − 3.42 (− 14.40–7.56) | 0.54 |
Effect of tVNS based on age, sex, and diabetes type
To explore underlying mechanisms, changes in concentrations of inflammatory cytokines were adjusted for age, sex, and diabetes type. Based on this analysis, no differences between active and sham stimulation were seen (all p > 0.07) (Table 2 – model 2).
Effect of tVNS based on the presence of autonomic neuropathy
When including information regarding the integrity of autonomic function to the analysis, no differences in delta concentrations of inflammatory cytokines between active and sham tVNS stimulation were observed in either study period (all p > 0.14) (Table 2 – model 3). However, the change in TNF-α levels between CAN-status (no CAN versus early/manifest CAN) during active treatment approached statistical significance (-0.11 (-0.28–0.05) vs. 0.06 (-0.04–0.16), p = 0.052).
Effect of tVNS based on baseline levels of inflammatory cytokines
No differences in delta concentrations of inflammatory cytokines between active and sham tVNS in either study period were seen when including information regarding baseline level of inflammatory in the statistical model (all p > 0.15) (Table 2 – model 4).
A negative coefficient indicates that the levels of inflammatory cytokines were decreased during active stimulation compared to sham stimulation. Model 1 represents the unadjusted results. In model 2, adjustments for age, sex and diabetes type were included. In model 3, adjustments for the presence of autonomic neuropathy were included along with factors from model 2. In model 4, baseline inflammatory cytokine levels were included in the adjustments along with factors from models 2 and 3.
Discussion
In this exploratory sub-study of a randomized, double-blind, sham-controlled trial we found no clinically relevant reductions in plasma concentrations of inflammatory cytokines after short term (seven days) or long-term (56 days) tVNS treatment in people with diabetes in spite of elevated baseline concentrations.
Transcutaneous vagus nerve stimulation as anti-inflammatory therapy
Since the discovery of the cholinergic anti-inflammatory pathway by Tracey et al. in the beginning of the new millennium, much research has been focused on utilizing this pathway for therapeutic purposes5. Several animal studies have shown promising results in lowering levels of pro-inflammatory cytokines with iVNS both locally8,28 and systemically29,30.
In humans, non-invasive tVNS has several advantages over iVNS, which require surgical implantation thereby limiting the therapeutic potential. However, the number of clinical trials investigating cervical tVNS as an anti-inflammatory therapy is limited, and prior to our study only a single study was designed as a blinded randomized-controlled trial5. Therefore, the evidence provided in most clinical studies is of limited importance, given that the degree of sham response is unknown. In the previous randomized-controlled trial, 20 healthy participants with intact autonomic regulation (10 receiving active tVNS, 10 receiving sham tVNS) were exposed to three bilateral tVNS stimulations delivered by the GammaCore device over the course of 10 h. Results showed that several cytokine levels (including TNF-α) in non-LPS-stimulated whole blood cultures were significantly decreased in the active tVNS group compared to the sham tVNS group17. This result is in accordance with findings from an open-label study in healthy participants conducted previously in our group14. The fact that we were unable to reproduce such an anti-inflammatory effect of tVNS in a cohort of people with diabetes raises the question of whether the diabetes pathology per se, and particularly autonomic dysfunction, may complicate the application of tVNS in this patient group. An intact vagal nerve must be considered crucial for achieving the maximum effect of tVNS therapy. In diabetes, neuropathy may be present in both large and small nerve fibers, and indeed, demyelination and degeneration of the vagal nerve have been reported in connection with diabetes31. We therefore deliberately included people with various degrees of diabetic autonomic neuropathy. As such, approximately half of our cohort had verified CAN, while the other half of our cohort were included based on abnormal sudomotor function or the presence of symptoms indicative of autonomic dysfunction. The response to tVNS could potentially differ between participants with and without verified CAN in the sense that disrupted integrity of the vagal control of cardiac function may coincide with a vagal nerve not able to activate the inflammatory reflex despite transcutaneous electrical stimulation. We therefore hypothesized that tVNS could cause a reduction in inflammatory cytokines in the subgroup of participants with intact cardiac vagal function (-CAN). However, no differences were found when including this information in the analysis. Nonetheless, there was tendency towards reductions in TNF-α levels in people with no CAN compared to those with early/manifest CAN (p = 0.052). Of note, the intensity of stimulations used by participants also did not influence the cytokine levels (results not shown). However, the applied intensities in most participants approached the maximum value of the GammaCore device18.
Finally, despite a notable degree of plasticity within vagal neurocircuits, disrupted central signaling within the brainstem, midbrain, and other central projections towards the efferent vagus nerve, may also have disturbed the afferent vagus nerve from mediating an anti-inflammatory response, thus, preventing the activation of the efferent cholinergic anti-inflammatory reflex. Alterations in brain signaling have previously been demonstrated in people with diabetes probably caused by central neuropathic processes32. It can be speculated if circumvention of central processing by specifically stimulating the left efferent vagus nerve could be more suitable for activation of the cholinergic anti-inflammatory pathway in diabetes. However, this approach may pose the risk of cardiovascular side effects. Direct stimulation of the splenic nerve as the final neural element of the cholinergic anti-inflammatory pathway has been shown to induce anti-inflammatory responses in a rodent model of rheumatoid arthritis33. Translation of this finding to a clinical setting is currently being investigated in a feasibility study in patients with rheumatoid arthritis (NCT05003310). The implications for this approach in people with diabetes is also intriguing, but the application is limited by the need for invasive surgery. Moreover, while rheumatoid arthritis is considered a high-grade systemic inflammatory disease34, the low-grade systemic inflammation seen in diabetes could potentially complicate the detection of anti-inflammatory responses of VNS simply because of more subtle absolute decreases in cytokine levels.
Strengths and limitations
The major strength of this study is that it is the first to investigate the anti-inflammatory effect of tVNS in a large-scale, randomized, sham-controlled trial, and as such, the results have higher credibility than previous smaller, non-randomized, and open-label studies. Moreover, our cohort of people with diabetes was characterized in detail regarding autonomic and peripheral neuropathy. However, several limitations are also present.
Firstly, we included both people with type 1 and type 2 diabetes. Though chronic inflammation is implicated in both pathologies, differences regarding levels of inflammatory biomarkers in people with type 1 and type 2 diabetes may exist35. This indicates that the underlying mechanisms of the diabetic state influence the level of chronic inflammation. It is plausible that the potential anti-inflammatory effects of tVNS may also differ between type 1 and type 2 diabetes. However, our study showed no effect of tVNS in either type of diabetes. Moreover, we included people with both short- and long-term diabetes duration. The degree of low-grade inflammation may also differ between these two subgroups. Indeed, we have shown previously that levels of anti-inflammatory IL-10 were increased in people with long-term type 2 diabetes compared to people with short-term disease duration, possibly due to a compensatory mechanism22.
Secondly, individual compliance with the stimulation protocol may have been a challenge in the current trial, especially during the second study period lasting for 56 days. Commitment to the study could potentially decrease during this relatively long period of time – perhaps particularly in the sham group in which no sensory feedback was obtained from the stimulation. To monitor compliance, participants were asked to note the time and day of each stimulation in a study diary, and from these entries, compliance rates of 89% and 86% in the active group and 86% and 85% in the placebo group were observed for study period 1 and study period 2, respectively18. However, there was no objective way of verifying these diary entries. While compliance issue can be entirely avoided by using implantable automated VNS devices, this approach includes invasive surgery and for this patient group, the intention was to test an easy-to-use over-the-counter treatment option.
Thirdly, while a cross-over design could have raised the validity of the study, a parallel-group design was chosen due to challenges of maintaining blinding if the participants were to experience both active and sham stimulation. The sham device mirroring the active GammaCore Sapphire emits a similar sound as the active device, but without generating any electrical current, as it cannot be imitated without risking actual activation of the vagus nerve. This contrasts previous versions of the S-300 GammaCore sham device, which produced a slight vibration, posing a theoretical risk of physical activation of the vagus nerve through carotid massage36.
Fourthly, we measured actual cytokine levels in this study, but LPS-stimulation of collected blood samples could have been applied. This approach may have increased the relative difference in cytokine response between treatment groups given that tVNS treatment induced a phenotypic switch of immune cells. However, the non-LPS-stimulated approach reflects actual levels of systemic cytokines more accurately, which may also be considered an advantage. Moreover, by measuring cytokine levels systemically, information regarding local changes in inflammatory factors after tVNS is unknown.
Lastly, the aims investigated in the present study were secondary aims of a larger randomized controlled trial, and accordingly the power calculation was not intended for detecting subtle changes in systemic inflammatory cytokines. Thus, the lack of significant findings may be due to type 2 errors.
A general limitation in the existing VNS studies is the limited generalizability of results, which is complicated by the different stimulation paradigms employed in studies. To advance the field systematically, sufficient reporting of methodological details such as stimulation modality (iVNS, cervical tVNS, auricular tVNS), device manufacturer, electrode design, stimulation intensity (frequency, amplitude, etc.), anatomical location (unilateral, bilateral), and dosing (how many times a day) should be reported as a minimum37. Going forward, a general consensus regarding optimal stimulation protocols and identification of the optimal clinical endpoint would increase the comparability and robustness of results. Studies solely dedicated to this goal are highly warranted. Moreover, it is likely that the optimal stimulation approach depend on pathologies and patient groups, and thus disease-specific dose–response studies are highly warranted. The stimulation protocol applied in this study was chosen based on the literature and with the aim of targeting a clinically applicable treatment, but the possibility of insufficient stimulation as the cause of negative findings cannot be discarded. However, in study period 1, participants used the device four times a day bilaterally, and it seems unlikely to maintain a high compliance if an anti-inflammatory response requires additional doses. In such case, automated iVNS devices seem more suited for this purpose.
Conclusion
In conclusion, this randomized, double-blind, sham-controlled trial showed that both short-term and long-term cervical tVNS were ineffective in reducing plasma levels of inflammatory cytokines in a cohort of people with diabetes and autonomic neuropathy. This could be associated with neurodegeneration and altered brain signaling in diabetes. Optimization of stimulation protocols and possibly early intervention prior to the development of autonomic neuropathy could be investigated in future studies.
Author contributions
Study design and conceptualization by AMD, CB, KK, FKK, and BB. HK, DK, DB included all participants. TO, HK, DK, and DB collected data. TO, JS, BB, CSH, PR, and CB analysed and interpreted the data. TO wrote the first manuscript draft. All authors reviewed and contributed to the final manuscript.
Funding
Novo Nordic Foundation, NNF180C0052045.
Data availability
Data of this study are available from the corresponding author upon reasonable request.
Competing interests
All other authors have no competing interests to declare.
Ethical approval
The study was approved by relevant authorities (the Danish Health and Medicines Authority: CIV-19–07-029,105; the North Denmark Region Committee on Health Research Ethics: N-20190020) and was conducted in accordance with the principles of the Helsinki Declaration and Good Clinical Practice.
Consent to participate
Written informed consent was collected from all participants before initiation of any study-related activities.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tsalamandris, S. et al. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. cardiol.10.15420/ecr.2018.33.1 (2019). 10.15420/ecr.2018.33.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nedosugova, L. V. et al. Inflammatory mechanisms of diabetes and its vascular complications. Biomedicines10.3390/biomedicines10051168 (2022). 10.3390/biomedicines10051168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tracey, K. J. The inflammatory reflex. Nature10.1038/NATURE01321 (2002). 10.1038/NATURE01321 [DOI] [PubMed] [Google Scholar]
- 4.Kuwabara, S., Goggins, E. & Tanaka, S. Neuroimmune circuits activated by Vagus nerve stimulation. Nephron10.1159/000518176 (2022). 10.1159/000518176 [DOI] [PubMed] [Google Scholar]
- 5.Kelly, M. J., Breathnach, C., Tracey, K. J. & Donnelly, S. C. Manipulation of the inflammatory reflex as a therapeutic strategy. Cell Rep. Med.10.1016/j.xcrm.2022.100696 (2022). 10.1016/j.xcrm.2022.100696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tynan, A., Brines, M. & Chavan, S. S. Control of inflammation using non-invasive neuromodulation: past, present and promise. Int. Immunol.10.1093/intimm/dxab073 (2022). 10.1093/intimm/dxab073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nonis, R., D’Ostilio, K., Schoenen, J. & Magis, D. Evidence of activation of vagal afferents by non-invasive vagus nerve stimulation: An electrophysiological study in healthy volunteers. Cephalalgia37, 1285–1293. 10.1177/0333102417717470 (2017). 10.1177/0333102417717470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu, W. et al. Protective effect of electrical stimulation of the vagus nerve in lipopolysaccharide-induced acute lung injury in rats. Mol. med. rep.10.3892/mmr.2021.12004 (2021). 10.3892/mmr.2021.12004 [DOI] [PubMed] [Google Scholar]
- 9.Jin, H. et al. Anti-inflammatory effects and mechanisms of vagal nerve stimulation combined with electroacupuncture in a rodent model of TNBS-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol.10.1152/ajpgi.00254.2016 (2017). 10.1152/ajpgi.00254.2016 [DOI] [PubMed] [Google Scholar]
- 10.Niederbichler, Andreas D, Stephan Papst, Leif Claassen, Andreas Jokuszies, Lars Steinstraesser, Tobias Hirsch, Mehmet A Altintas, et al. 2009. Burn-induced organ dysfunction: vagus nerve stimulation attenuates organ and serum cytokine levels. Burns : J. Int. Soc. Burn Injuries 35. Netherlands: 783–789. 10.1016/j.burns.2008.08.023. (2009). [DOI] [PubMed]
- 11.Bonaz, B. et al. Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterol. Motil.10.1111/nmo.12792 (2016). 10.1111/nmo.12792 [DOI] [PubMed] [Google Scholar]
- 12.Seitz, T. et al. Percutaneous auricular Vagus nerve stimulation reduces inflammation in critical Covid-19 patients. Front. physiol.10.3389/fphys.2022.897257 (2022). 10.3389/fphys.2022.897257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drewes, A. M. et al. Short-term transcutaneous non-invasive vagus nerve stimulation may reduce disease activity and pro-inflammatory cytokines in rheumatoid arthritis: results of a pilot study. Scand. J. Rheumatol.10.1080/03009742.2020.1764617 (2021). 10.1080/03009742.2020.1764617 [DOI] [PubMed] [Google Scholar]
- 14.Brock, C. et al. Transcutaneous cervical vagal nerve stimulation modulates cardiac vagal tone and tumor necrosis factor-alpha. Neurogastroenterol. Motil.10.1111/nmo.12999 (2017). 10.1111/nmo.12999 [DOI] [PubMed] [Google Scholar]
- 15.Staats, P., Giannakopoulos, G., Blake, J., Liebler, E. & Levy, R. M. The use of non-invasive vagus nerve stimulation to treat respiratory symptoms associated with COVID-19: A theoretical hypothesis and early clinical experience. Neuromod. J. Int. Neurom. Soc.10.1111/ner.13172 (2020). 10.1111/ner.13172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brock, C. et al. Vagal nerve stimulation-modulation of the anti-inflammatory response and clinical outcome in psoriatic arthritis or ankylosing spondylitis. Mediators Inflamm.2021, 9933532. 10.1155/2021/9933532 (2021). 10.1155/2021/9933532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lerman, Imanuel, Richard Hauger, Linda Sorkin, James Proudfoot, Bryan Davis, Andy Huang, Katie Lam, Bruce Simon, and Dewleen G. Baker. Noninvasive Transcutaneous Vagus Nerve Stimulation Decreases Whole Blood Culture-Derived Cytokines and Chemokines: A Randomized, Blinded, Healthy Control Pilot Trial. Neuromodulation : journal of the International Neuromodulation Society 19. United States: Blackwell Publishing Inc.: 283–290. 10.1111/ner.12398. (2016). [DOI] [PubMed]
- 18.Kornum, D. S. et al. Transcutaneous vagal nerve stimulation for treating gastrointestinal symptoms in individuals with diabetes: a randomised, double-blind, sham-controlled, multicentre trial. Diabetologia10.1007/s00125-024-06129-0 (2024). 10.1007/s00125-024-06129-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okdahl, T. et al. Study protocol for a multicentre, randomised, parallel group, sham-controlled clinical trial investigating the effect of transcutaneous vagal nerve stimulation on gastrointestinal symptoms in people with diabetes complicated with diabetic autonomic neurop. BMJ Open11, 1–13. 10.1136/bmjopen-2020-038677 (2021). 10.1136/bmjopen-2020-038677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greco, C. et al. Validation of the Composite Autonomic Symptom Score 31 (COMPASS 31) for the assessment of symptoms of autonomic neuropathy in people with diabetes. Diabetic Med.34, 834–838. 10.1111/dme.13310 (2017). 10.1111/dme.13310 [DOI] [PubMed] [Google Scholar]
- 21.Electrocore. 2018. Instructions for Use for gammaCore Sapphire TM.
- 22.Okdahl, T. et al. Low-grade inflammation in type 2 diabetes: a cross-sectional study from a Danish diabetes outpatient clinic. BMJ Open10.1136/BMJOPEN-2022-062188 (2022). 10.1136/BMJOPEN-2022-062188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wegeberg, A. M. et al. Elevated levels of interleukin-12/23p40 may serve as a potential indicator of dysfunctional heart rate variability in type 2 diabetes. Cardiovasc. Diabetol.10.1186/s12933-021-01437-w (2022). 10.1186/s12933-021-01437-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Croghan, Carry W., and Peter P. Egeghy. Methods of Dealing With Values Below the Limit of Detection Using Sas. Southern SAS User Group: 5. (2003).
- 25.Wegeberg, Anne Marie, Elin D. Lunde, Sam Riahi, Niels Ejskjaer, Asbjørn M. Drewes, Birgitte Brock, Rodica Pop-Busui, and Christina Brock. Cardiac vagal tone as a novel screening tool to recognize asymptomatic cardiovascular autonomic neuropathy: Aspects of utility in type 1 diabetes. Diabetes research and clinical practice 170. Diabetes Res Clin Pract. 10.1016/J.DIABRES.2020.108517. (2020). [DOI] [PubMed]
- 26.Spallone, V., F. Bellavere, L. Scionti, S. Maule, R. Quadri, G. Bax, P. Melga, et al. Recommendations for the use of cardiovascular tests in diagnosing diabetic autonomic neuropathy. Nutrition, metabolism, and cardiovascular diseases : NMCD 21. Nutr Metab Cardiovasc Dis: 69–78. 10.1016/J.NUMECD.2010.07.005. (2011). [DOI] [PubMed]
- 27.Harris, Paul A., Robert Taylor, Robert Thielke, Jonathon Payne, Nathaniel Gonzalez, and Jose G. Conde. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics 42. Elsevier Inc.: 377–381. 10.1016/j.jbi.2008.08.010. (2009). [DOI] [PMC free article] [PubMed]
- 28.Namgung, Uk, Ki-Joong Joong Kim, Byung-Gon Gon Jo, and Jong-Min Min Park. Vagus nerve stimulation modulates hippocampal inflammation caused by continuous stress in rats. Journal of neuroinflammation 19. England: BioMed Central Ltd: 33. 10.1186/s12974-022-02396-z. (2022). [DOI] [PMC free article] [PubMed]
- 29.Komegae, Evilin Naname, David George Stephen Farmer, Virginia Leah Brooks, Michael Joseph McKinley, Robin Michael McAllen, and Davide Martelli. Vagal afferent activation suppresses systemic inflammation via the splanchnic anti-inflammatory pathway. Brain, behavior, and immunity 73. Netherlands: Academic Press Inc.: 441–449. 10.1016/j.bbi.2018.06.005. (2018). [DOI] [PMC free article] [PubMed]
- 30.Deng, Jielin, Yunqiu Jiang, Meng Wang, Ling Shao, and Changjin Deng. 2021. Activation of vagovagal reflex prevents hepatic ischaemia-reperfusion-induced lung injury via anti-inflammatory and antioxidant effects. Exp. physiol. 10.1113/EP089865. (2021). [DOI] [PubMed]
- 31.Guo, Y.-P., Mcleod, J. G. & Baverstock, J. Pathological changes in the vagus nerve in diabetes and chronic alcoholism. Neurosurg. Psychiatry50, 1449–1453. 10.1136/jnnp.50.11.1449 (1987). 10.1136/jnnp.50.11.1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Croosu, S. S. et al. Altered functional connectivity between brain structures in adults with type 1 diabetes and polyneuropathy. Brain Res.10.1016/j.brainres.2022.147882 (2022). 10.1016/j.brainres.2022.147882 [DOI] [PubMed] [Google Scholar]
- 33.Guyot, Mélanie, Thomas Simon, Clara Panzolini, Franck Ceppo, Douglas Daoudlarian, Emilie Murris, Eric Macia, et al. Apical splenic nerve electrical stimulation discloses an anti-inflammatory pathway relying on adrenergic and nicotinic receptors in myeloid cells. Brain, behave. Immune. 10.1016/j.bbi.2019.03.015. (2019). [DOI] [PubMed]
- 34.Sattar, N., McCarey, D. W., Capell, H. & McInnes, I. B. Explaining How “High-Grade” Systemic Inflammation Accelerates Vascular Risk in Rheumatoid Arthritis. Circulation108, 2957–2963. 10.1161/01.CIR.0000099844.31524.05 (2003). 10.1161/01.CIR.0000099844.31524.05 [DOI] [PubMed] [Google Scholar]
- 35.Donath, Marc Y., Charles A. Dinarello, and Thomas Mandrup-Poulsen. 2019. Targeting innate immune mediators in type 1 and type 2 diabetes. Nature Reviews Immunology 19. Springer US: 734–746. 10.1038/s41577-019-0213-9. [DOI] [PubMed]
- 36.Schweitzer, P. & Teichholz, L. E. Carotid sinus massage. Its diagnostic and therapeutic value in arrhythmias. Am. J. Med.78, 645–654. 10.1016/0002-9343(85)90408-5 (1985). 10.1016/0002-9343(85)90408-5 [DOI] [PubMed] [Google Scholar]
- 37.Farmer, A. D. et al. International consensus based review and recommendations for minimum reporting standards in research on transcutaneous Vagus nerve stimulation (Version 2020). Front. Hum. Neurosci.10.3389/fnhum.2020.568051 (2021). 10.3389/fnhum.2020.568051 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data of this study are available from the corresponding author upon reasonable request.