Abstract
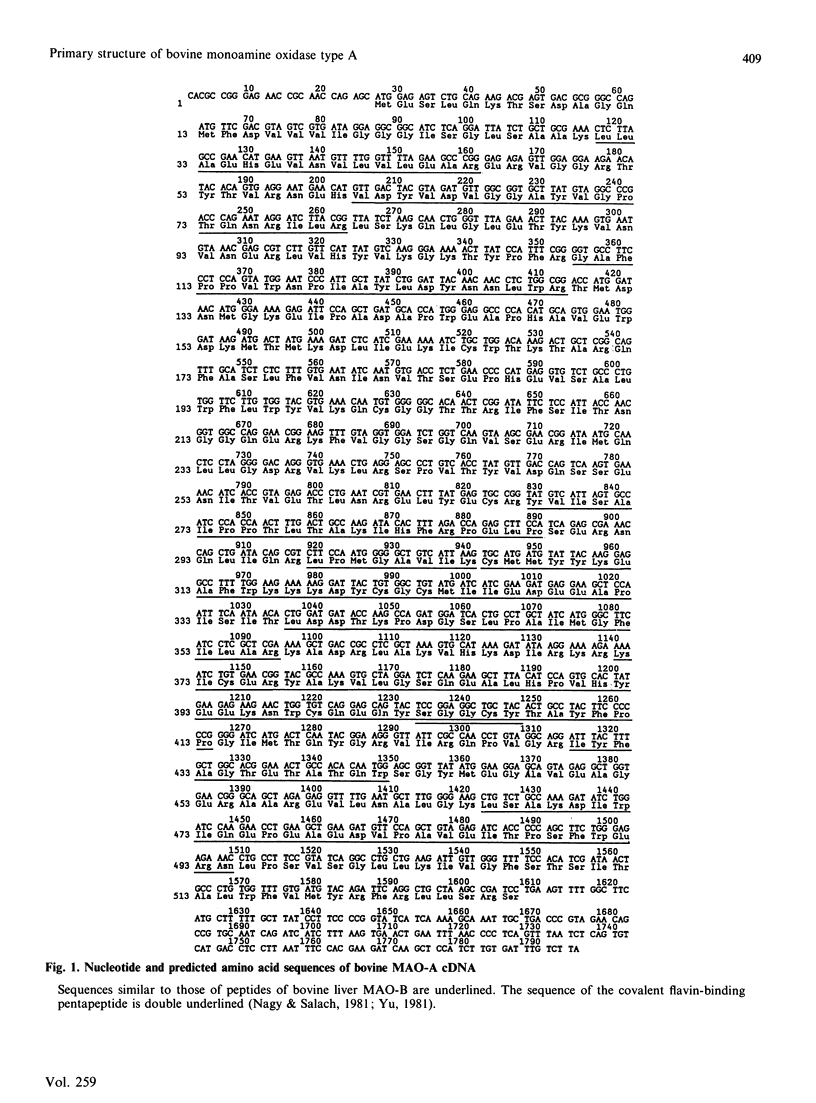
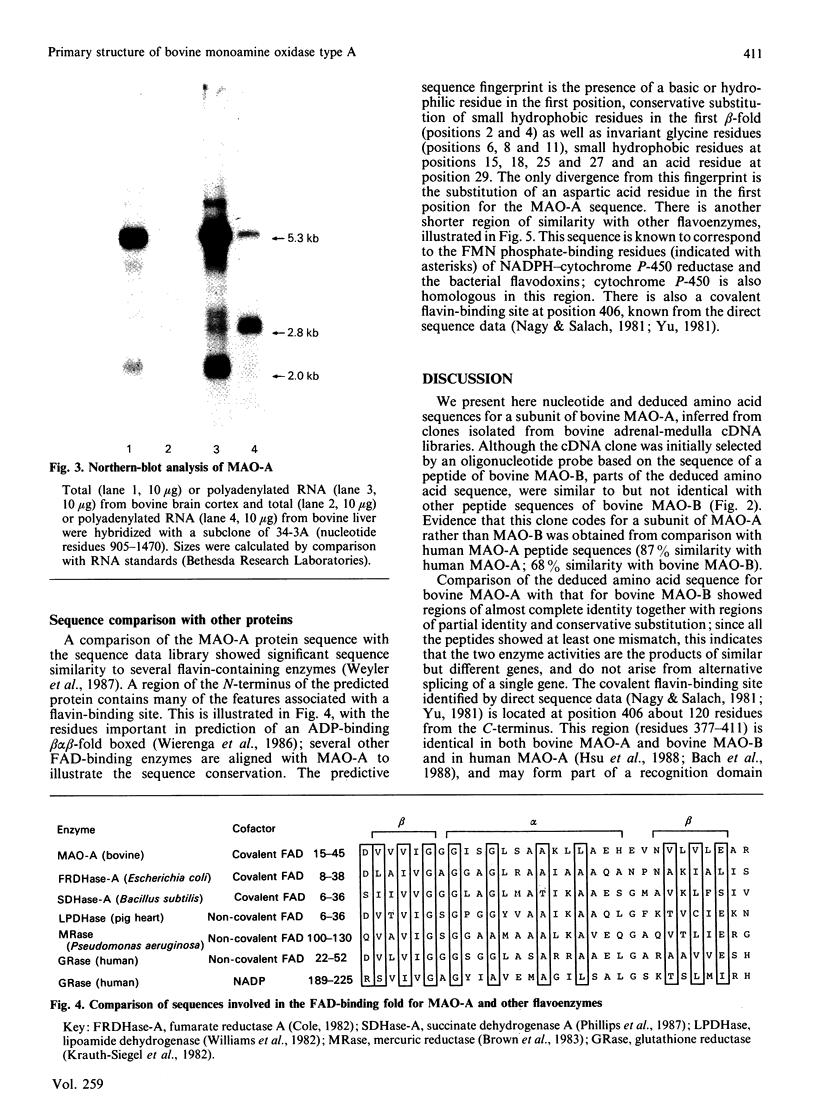
We have isolated cDNA clones believed to encompass the full-length coding sequences for a subunit of bovine monoamine oxidase type A (MAO-A). The clones code for an apoprotein of 527 amino acid residues corresponding to a molecular mass of 59,806 Da. The inferred protein sequences show an overall similarity of 68% with partial amino acid sequences of bovine type B MAO (about 41% of the total sequence), as well as a greater similarity (greater than 90%) with some regions including that for the published sequence of the flavin-binding region. Sequence comparisons indicate that these two forms of MAO are encoded by distinct genes. Comparison of this sequence with other flavoenzymes showed similarity with regions associated with non-covalent flavin-binding sites. Analysis of mRNAs coding for MAO enzymes showed a heterogeneity of transcripts consistent with several different forms of monoamine oxidase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach A. W., Lan N. C., Johnson D. L., Abell C. W., Bembenek M. E., Kwan S. W., Seeburg P. H., Shih J. C. cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4934–4938. doi: 10.1073/pnas.85.13.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. P., Hemsworth B. A. Effect of phospholipid depletion by phospholipases on the properties and formation of the multiple monoamine oxidase forms in the rat liver. Eur J Biochem. 1978 Dec 1;92(1):165–174. doi: 10.1111/j.1432-1033.1978.tb12734.x. [DOI] [PubMed] [Google Scholar]
- Brown G. K., Powell J. F., Craig I. W. Molecular weight differences between human platelet and placental monoamine oxidase. Biochem Pharmacol. 1980 Oct 1;29(19):2595–2603. doi: 10.1016/0006-2952(80)90073-8. [DOI] [PubMed] [Google Scholar]
- Brown N. L., Ford S. J., Pridmore R. D., Fritzinger D. C. Nucleotide sequence of a gene from the Pseudomonas transposon Tn501 encoding mercuric reductase. Biochemistry. 1983 Aug 16;22(17):4089–4095. doi: 10.1021/bi00286a015. [DOI] [PubMed] [Google Scholar]
- Cawthon R. M., Breakefield X. O. Differences in A and B forms of monoamine oxidase revealed by limited proteolysis and peptide mapping. Nature. 1979 Oct 25;281(5733):692–694. doi: 10.1038/281692a0. [DOI] [PubMed] [Google Scholar]
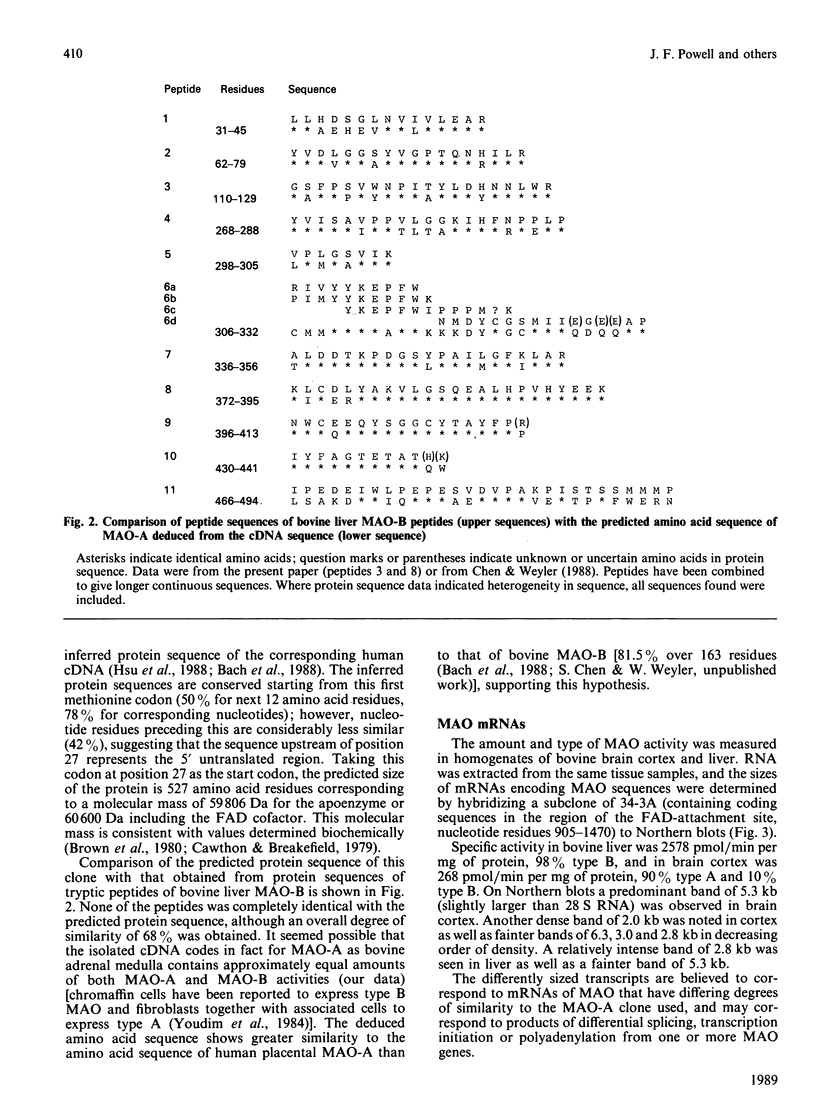
- Chen S. A., Weyler W. Partial amino acid sequence analysis of human placenta monoamine oxidase A and bovine liver monoamine oxidase B. Biochem Biophys Res Commun. 1988 Oct 14;156(1):445–450. doi: 10.1016/s0006-291x(88)80861-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cole S. T. Nucleotide sequence coding for the flavoprotein subunit of the fumarate reductase of Escherichia coli. Eur J Biochem. 1982 Mar 1;122(3):479–484. doi: 10.1111/j.1432-1033.1982.tb06462.x. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Denney R. M., Fritz R. R., Patel N. T., Abell C. W. Human liver MAO-A and MAO-B separated by immunoaffinity chromatography with MAO-B-specific monoclonal antibody. Science. 1982 Mar 12;215(4538):1400–1403. doi: 10.1126/science.7063850. [DOI] [PubMed] [Google Scholar]
- Edelstein S. B., Breakefield X. O. Monoamine oxidases A and B are differentially regulated by glucocorticoids and "aging" in human skin fibroblasts. Cell Mol Neurobiol. 1986 Jun;6(2):121–150. doi: 10.1007/BF00711066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Fujii-Kuriyama Y., Mizukami Y., Kawajiri K., Sogawa K., Muramatsu M. Primary structure of a cytochrome P-450: coding nucleotide sequence of phenobarbital-inducible cytochrome P-450 cDNA from rat liver. Proc Natl Acad Sci U S A. 1982 May;79(9):2793–2797. doi: 10.1073/pnas.79.9.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V., Sandler M. Clinical chemistry of monoamine oxidase. Cell Biochem Funct. 1986 Apr;4(2):89–97. doi: 10.1002/cbf.290040203. [DOI] [PubMed] [Google Scholar]
- Gomes B., Naguwa G., Kloepfer H. G., Yasunobu K. T. Amine oxidase. XV. The sulfhydryl groups of beef liver mitochondrial amine oxidase. Arch Biochem Biophys. 1969 Jun;132(1):28–33. doi: 10.1016/0003-9861(69)90335-x. [DOI] [PubMed] [Google Scholar]
- Greenawalt J. W. Localization of monoamine oxidase in rat liver mitochondria. Adv Biochem Psychopharmacol. 1972;5:207–226. [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase T., Müller U., Riezman H., Schatz G. A 70-kd protein of the yeast mitochondrial outer membrane is targeted and anchored via its extreme amino terminus. EMBO J. 1984 Dec 20;3(13):3157–3164. doi: 10.1002/j.1460-2075.1984.tb02274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Williams K. R., Konigsberg W. H., Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated neuronal phosphoprotein. I. Amino acid sequence around the phosphorylated threonine. J Biol Chem. 1984 Dec 10;259(23):14486–14490. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Tipton K. F. A kinetic evaluation of monoamine oxidase activity in rat liver mitochondrial outer membranes. Biochem J. 1974 Jun;139(3):645–652. doi: 10.1042/bj1390645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y. P., Weyler W., Chen S., Sims K. B., Rinehart W. B., Utterback M. C., Powell J. F., Breakefield X. O. Structural features of human monoamine oxidase A elucidated from cDNA and peptide sequences. J Neurochem. 1988 Oct;51(4):1321–1324. doi: 10.1111/j.1471-4159.1988.tb03105.x. [DOI] [PubMed] [Google Scholar]
- Iacangelo A., Affolter H. U., Eiden L. E., Herbert E., Grimes M. Bovine chromogranin A sequence and distribution of its messenger RNA in endocrine tissues. Nature. 1986 Sep 4;323(6083):82–86. doi: 10.1038/323082a0. [DOI] [PubMed] [Google Scholar]
- Johnston J. P. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968 Jul;17(7):1285–1297. doi: 10.1016/0006-2952(68)90066-x. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauth-Siegel R. L., Blatterspiel R., Saleh M., Schiltz E., Schirmer R. H., Untucht-Grau R. Glutathione reductase from human erythrocytes. The sequences of the NADPH domain and of the interface domain. Eur J Biochem. 1982 Jan;121(2):259–267. doi: 10.1111/j.1432-1033.1982.tb05780.x. [DOI] [PubMed] [Google Scholar]
- Levitt P., Pintar J. E., Breakefield X. O. Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6385–6389. doi: 10.1073/pnas.79.20.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomedico P. T., Saunders G. F. Preparation of pancreatic mRNA: cell-free translation of an insulin-immunoreactive polypeptide. Nucleic Acids Res. 1976 Feb;3(2):381–391. doi: 10.1093/nar/3.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey S. P., Johannessen J. N., Chiueh C. C., Burns R. S., Herkenham M. A. Intraneuronal generation of a pyridinium metabolite may cause drug-induced parkinsonism. Nature. 1984 Oct 4;311(5985):464–467. doi: 10.1038/311464a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamiura N., Yasunobu K. T. Bovine liver monoamine oxidase. A modified purification procedure and preliminary evidence for two subunits and one FAD. Arch Biochem Biophys. 1978 Aug;189(2):481–489. doi: 10.1016/0003-9861(78)90237-0. [DOI] [PubMed] [Google Scholar]
- Nagy J., Salach J. I. Identity of the active site flavin-peptide fragments from the human "A"-form and the bovine "B"-form of monoamine oxidase. Arch Biochem Biophys. 1981 May;208(2):388–394. doi: 10.1016/0003-9861(81)90523-3. [DOI] [PubMed] [Google Scholar]
- Phillips M. K., Hederstedt L., Hasnain S., Rutberg L., Guest J. R. Nucleotide sequence encoding the flavoprotein and iron-sulfur protein subunits of the Bacillus subtilis PY79 succinate dehydrogenase complex. J Bacteriol. 1987 Feb;169(2):864–873. doi: 10.1128/jb.169.2.864-873.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintar J. E., Levitt P., Salach J. I., Weyler W., Rosenberg M. B., Breakefield X. O. Specificity of antisera prepared against pure bovine MAO-B. Brain Res. 1983 Oct 3;276(1):127–139. doi: 10.1016/0006-8993(83)90554-1. [DOI] [PubMed] [Google Scholar]
- Porter T. D., Kasper C. B. NADPH-cytochrome P-450 oxidoreductase: flavin mononucleotide and flavin adenine dinucleotide domains evolved from different flavoproteins. Biochemistry. 1986 Apr 8;25(7):1682–1687. doi: 10.1021/bi00355a036. [DOI] [PubMed] [Google Scholar]
- Rice D. W., Schulz G. E., Guest J. R. Structural relationship between glutathione reductase and lipoamide dehydrogenase. J Mol Biol. 1984 Apr 15;174(3):483–496. doi: 10.1016/0022-2836(84)90332-2. [DOI] [PubMed] [Google Scholar]
- Saadat S., Stehle A. D., Lamouroux A., Mallet J., Thoenen H. Influence of cell-cell contact on levels of tyrosine hydroxylase in cultured bovine adrenal chromaffin cells. J Biol Chem. 1987 Sep 25;262(27):13007–13014. [PubMed] [Google Scholar]
- Salach J. I. Monoamine oxidase from beef liver mitochondria: simplified isolation procedure, properties, and determination of its cysteinyl flavin content. Arch Biochem Biophys. 1979 Jan;192(1):128–137. doi: 10.1016/0003-9861(79)90078-x. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Buss J. E. The covalent modification of eukaryotic proteins with lipid. J Cell Biol. 1987 Jun;104(6):1449–1453. doi: 10.1083/jcb.104.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Yasunobu K. T., Yoch D. C. Complete amino acid sequence of azotoflavin, a flavodoxin from Azotobacter vinelandii. Biochemistry. 1977 Aug 9;16(16):3525–3537. doi: 10.1021/bi00635a005. [DOI] [PubMed] [Google Scholar]
- Tanka M., Haniu M., Yasunobu F., Mayhew S. G. The amino acid sequence of the Clostridium MP flavodoxin. J Biol Chem. 1974 Jul 25;249(14):4393–4396. [PubMed] [Google Scholar]
- Westlund K. N., Denney R. M., Kochersperger L. M., Rose R. M., Abell C. W. Distinct monoamine oxidase A and B populations in primate brain. Science. 1985 Oct 11;230(4722):181–183. doi: 10.1126/science.3875898. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., Terpstra P., Hol W. G. Prediction of the occurrence of the ADP-binding beta alpha beta-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986 Jan 5;187(1):101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- Williams C. H., Jr, Arscott L. D., Schulz G. E. Amino acid sequence homology between pig heart lipoamide dehydrogenase and human erythrocyte glutathione reductase. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2199–2201. doi: 10.1073/pnas.79.7.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. R., Stone K. L., LoPresti M. B., Merrill B. M., Planck S. R. Amino acid sequence of the UP1 calf thymus helix-destabilizing protein and its homology to an analogous protein from mouse myeloma. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5666–5670. doi: 10.1073/pnas.82.17.5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim M. B., Banerjee D. K., Pollard H. B. Isolated chromaffin cells from adrenal medulla contain primarily monoamine oxidase B. Science. 1984 May 11;224(4649):619–621. doi: 10.1126/science.6424235. [DOI] [PubMed] [Google Scholar]
- Yu P. H. Studies on the pargyline-binding site of different types of monoamine oxidase. Can J Biochem. 1981 Jan;59(1):30–37. doi: 10.1139/o81-005. [DOI] [PubMed] [Google Scholar]