Abstract
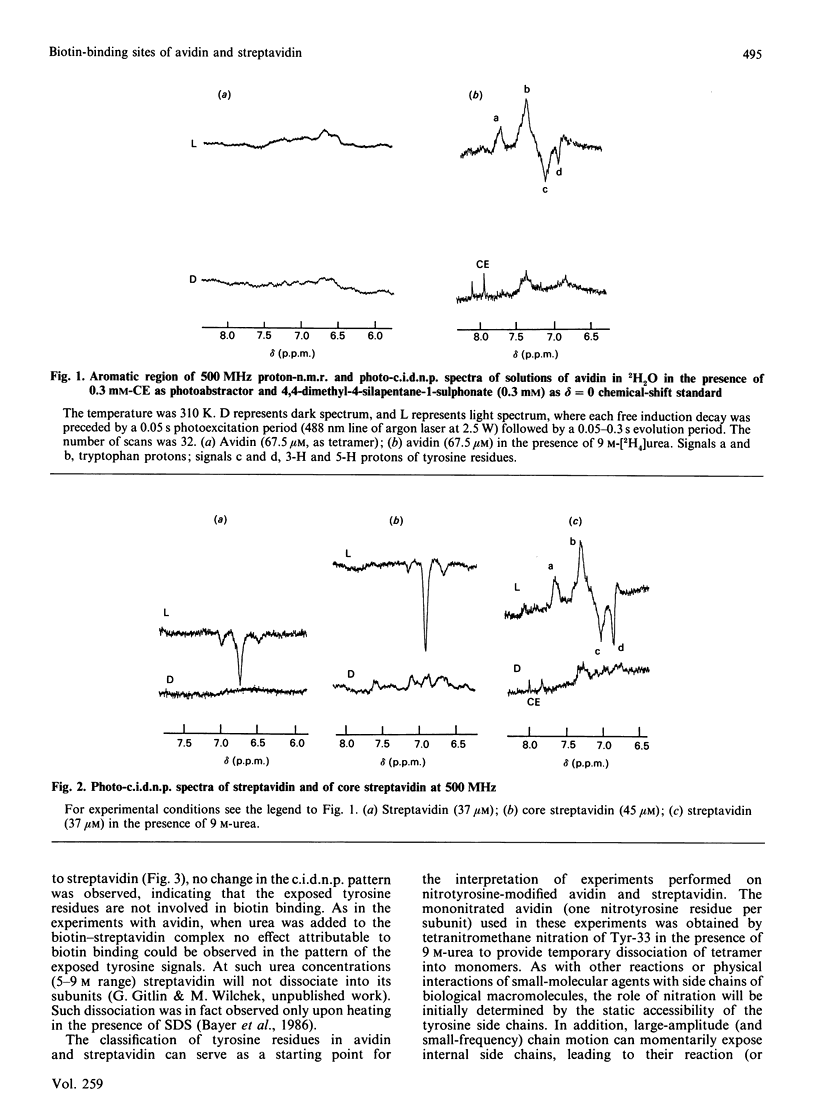
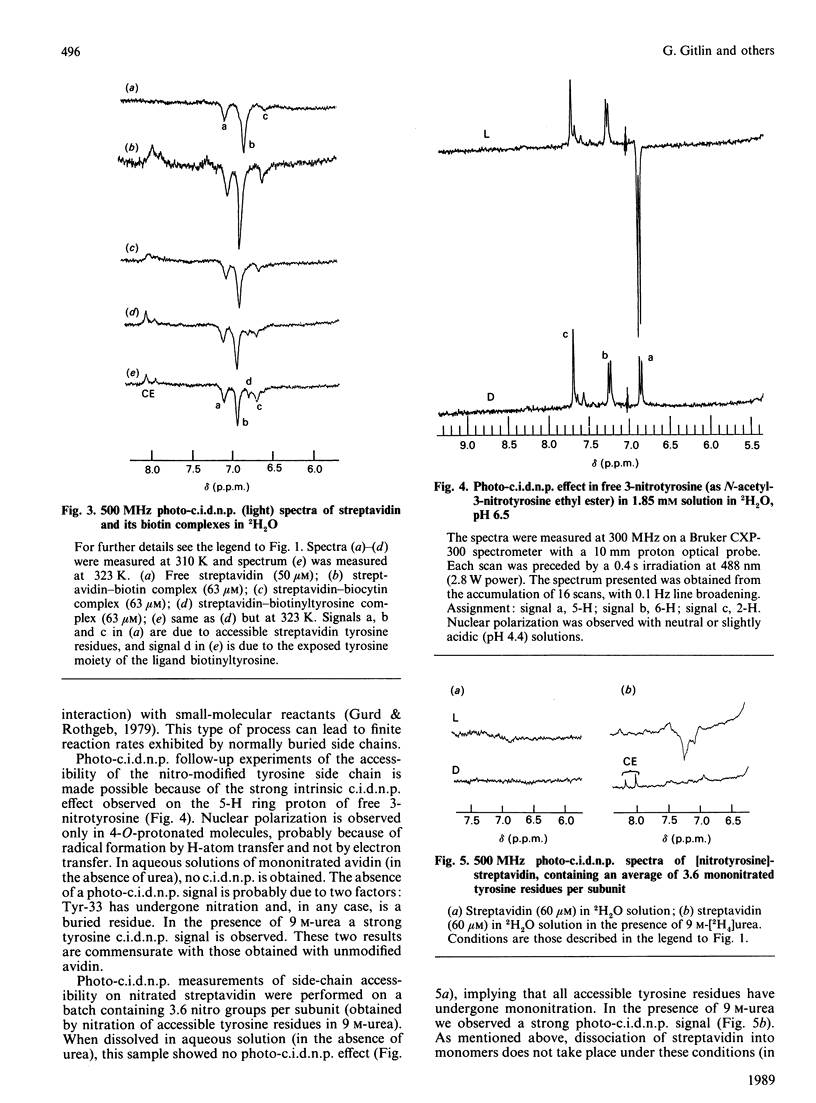
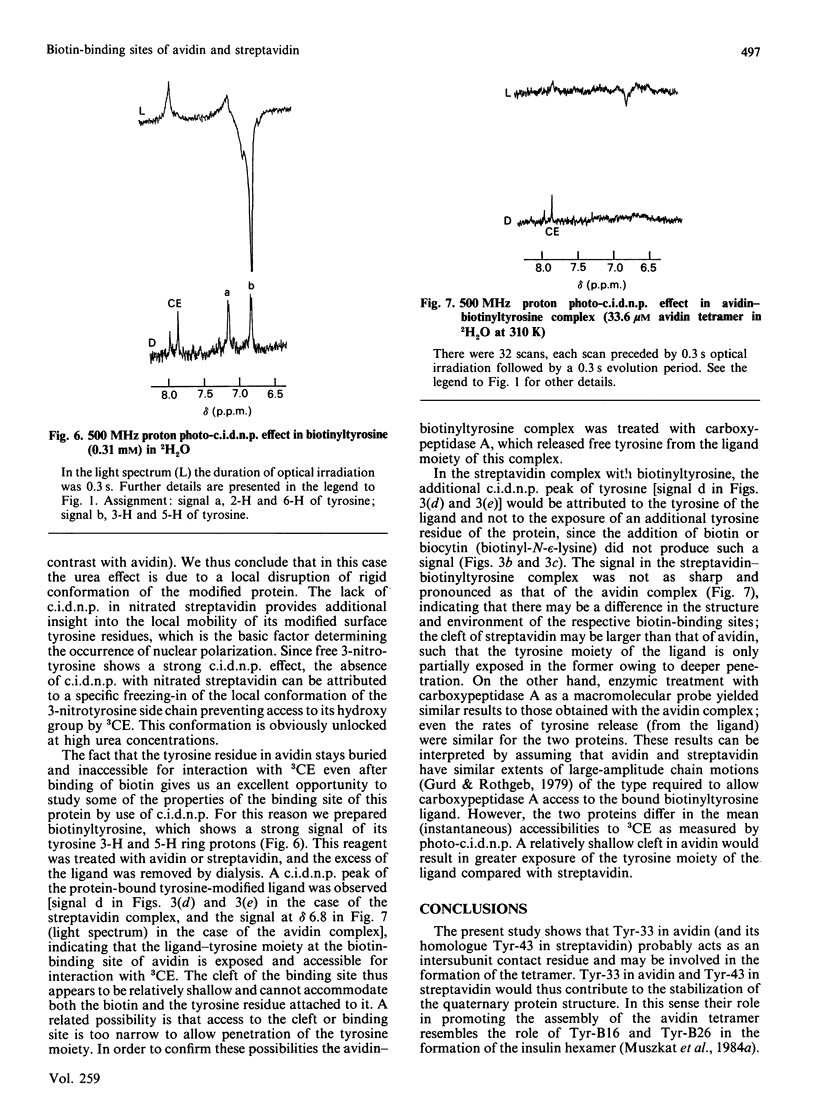
We applied the protein photochemically induced dynamic nuclear polarization (photo-c.i.d.n.p.) method to explore the conformation of the side chains of tyrosine, tryptophan and histidine residues in three biotin-binding proteins. The c.i.d.n.p. spectra of avidin, streptavidin and 'core' streptavidin were compared with those of their complexes with biotin and its derivatives. The data indicate that the single tyrosine residue (Tyr-33) of avidin is clearly inaccessible to the triplet flavin photo-c.i.d.n.p. probe. The same holds for all tryptophan and histidine side chains. Although the analogous Tyr-43 residue of streptavidin is also buried, at least three of the other tyrosine residues of this protein are exposed. The same conclusions apply to the truncated form of the protein, core streptavidin. As judged by the photo-c.i.d.n.p. results, complexing of avidin and streptavidin with biotin, N-epsilon-biotinyl-L-lysine (biocytin) or biotinyltyrosine has little or no effect on tyrosine accessibility in these proteins. Biotinyltyrosine can be used to probe the depth of the corresponding binding site. The accessibility of the tyrosine side chain of biotinyltyrosine in the complex demonstrates the exquisite fit of the biotin-binding cleft of avidin: only the biotin moiety appears to be accommodated, leaving the tyrosine side chain exposed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argaraña C. E., Kuntz I. D., Birken S., Axel R., Cantor C. R. Molecular cloning and nucleotide sequence of the streptavidin gene. Nucleic Acids Res. 1986 Feb 25;14(4):1871–1882. doi: 10.1093/nar/14.4.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer E. A., Ben-Hur H., Gitlin G., Wilchek M. An improved method for the single-step purification of streptavidin. J Biochem Biophys Methods. 1986 Sep;13(2):103–112. doi: 10.1016/0165-022x(86)90022-9. [DOI] [PubMed] [Google Scholar]
- Bayer E., Wilchek M. Insolubilized biotin for the purification of avidin. Methods Enzymol. 1974;34:265–267. doi: 10.1016/s0076-6879(74)34023-2. [DOI] [PubMed] [Google Scholar]
- Gitlin G., Bayer E. A., Wilchek M. Studies on the biotin-binding site of avidin. Lysine residues involved in the active site. Biochem J. 1987 Mar 15;242(3):923–926. doi: 10.1042/bj2420923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin G., Bayer E. A., Wilchek M. Studies on the biotin-binding site of avidin. Tryptophan residues involved in the active site. Biochem J. 1988 Feb 15;250(1):291–294. doi: 10.1042/bj2500291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin G., Bayer E. A., Wilchek M. Studies on the biotin-binding site of streptavidin. Tryptophan residues involved in the active site. Biochem J. 1988 Nov 15;256(1):279–282. doi: 10.1042/bj2560279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N. M. Avidin. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- Green N. M., Konieczny L., Toms E. J., Valentine R. C. The use of bifunctional biotinyl compounds to determine the arrangement of subunits in avidin. Biochem J. 1971 Dec;125(3):781–791. doi: 10.1042/bj1250781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurd F. R., Rothgeb T. M. Motions in proteins. Adv Protein Chem. 1979;33:73–165. doi: 10.1016/s0065-3233(08)60459-3. [DOI] [PubMed] [Google Scholar]
- Lerman C. L., Cohn M. Laser photo-CIDNP NMR of pyridoxal phosphate and pyridoxyllysine residues: an extrinsic probe for non-aromatic amino acid residues in proteins. Biochem Biophys Res Commun. 1980 Nov 17;97(1):121–125. doi: 10.1016/s0006-291x(80)80143-4. [DOI] [PubMed] [Google Scholar]
- Muszkat K. A., Gilon C. CIDNP in tyrosyl protons of luliberin. Nature. 1978 Feb 16;271(5646):685–686. doi: 10.1038/271685a0. [DOI] [PubMed] [Google Scholar]
- Muszkat K. A., Khait I., Hayashi K., Tamiya N. Photochemically induced nuclear polarization study of exposed tyrosines, tryptophans, and histidines in postsynaptic neurotoxins and in membranotoxins of elapid and hydrophid snake venoms. Biochemistry. 1984 Oct 9;23(21):4913–4920. doi: 10.1021/bi00316a014. [DOI] [PubMed] [Google Scholar]
- Muszkat K. A., Khait I., Weinstein S. Photochemically induced nuclear polarization study of the accessibility of tyrosines in insulin. Biochemistry. 1984 Jan 3;23(1):5–10. doi: 10.1021/bi00296a002. [DOI] [PubMed] [Google Scholar]
- Muszkat K. A., Weinstein M., Gilon C. Biochemical applications of chemically induced nuclear polarization in phenols, peptides, catecholamines and related molecules. Biochem J. 1978 Sep 1;173(3):993–996. doi: 10.1042/bj1730993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muszkat K. A., Weinstein S., Khait I., Vered M. Effects of proteinase--inhibitor binding on accessibility of exposed tyrosines. A photochemically induced dynamic nuclear polarization study of bovine pancreatic trypsin inhibitor complexes with trypsin, chymotrypsin, and their zymogens. Biochemistry. 1982 Aug 3;21(16):3775–3779. doi: 10.1021/bi00259a008. [DOI] [PubMed] [Google Scholar]
- Muszkat K. A., Weinstein S., Khait I., Vered M. Photo-CIDNP study of interactions of serine proteinases with their protein inhibitors. Biopolymers. 1983 Jan;22(1):387–390. doi: 10.1002/bip.360220150. [DOI] [PubMed] [Google Scholar]
- Muszkat K. A., Wismontski-Knittel T. Reactivities of tyrosine, histidine, tryptophan, and methionine in radical pair formation in flavin triplet induced protein nuclear magnetic polarization. Biochemistry. 1985 Sep 24;24(20):5416–5421. doi: 10.1021/bi00341a020. [DOI] [PubMed] [Google Scholar]
- Riordan J. F., Sokolovsky M., Vallee B. L. The functional tyrosyl residues of carboxypeptidase A. Nitration with tetranitromethane. Biochemistry. 1967 Nov;6(11):3609–3617. doi: 10.1021/bi00863a036. [DOI] [PubMed] [Google Scholar]
- Wynne D., Wilchek M., Novogrodsky A. A chemical approach for the localization of membrane sites involved in lymphocyte activation. Biochem Biophys Res Commun. 1976 Feb 9;68(3):730–739. doi: 10.1016/0006-291x(76)91206-7. [DOI] [PubMed] [Google Scholar]


