Abstract
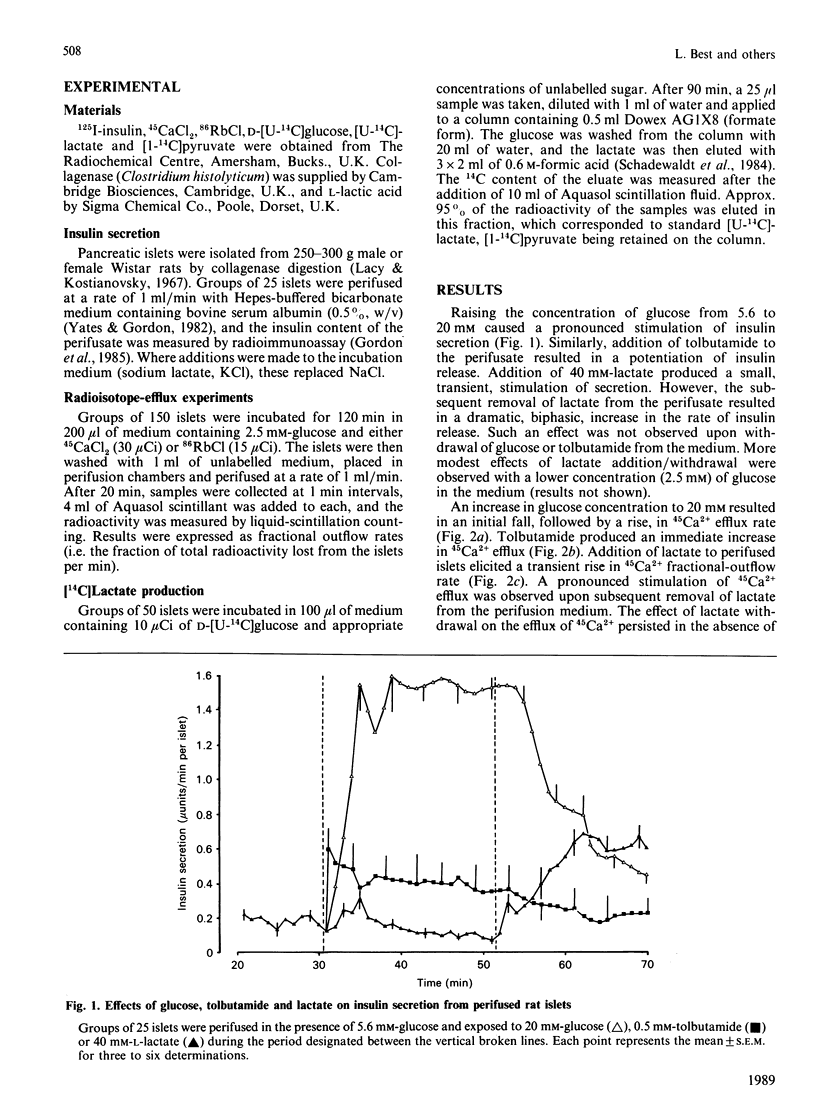
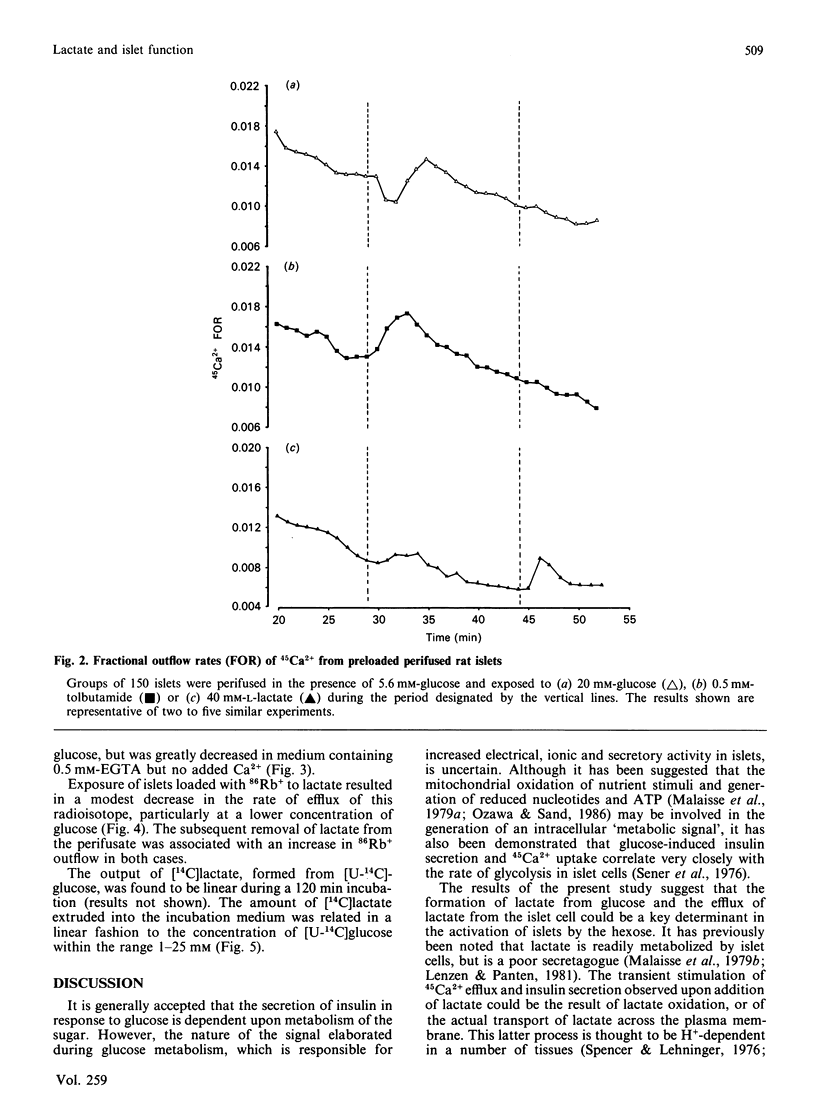
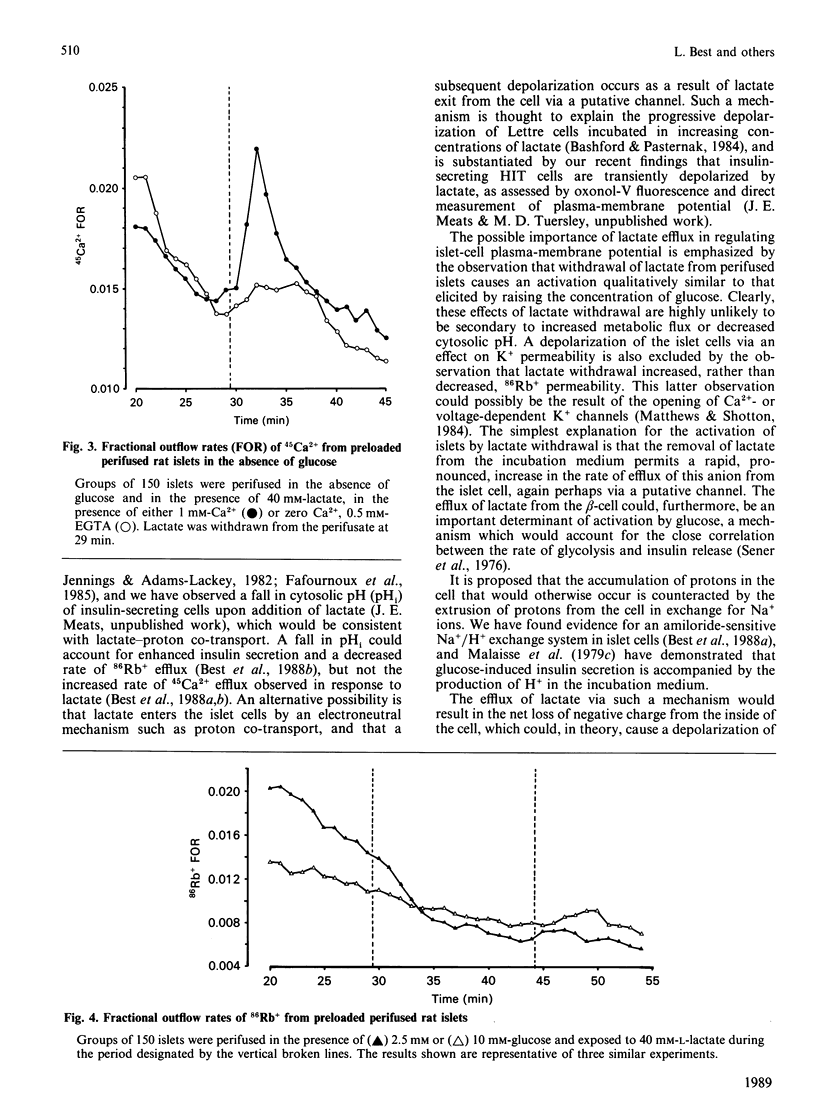
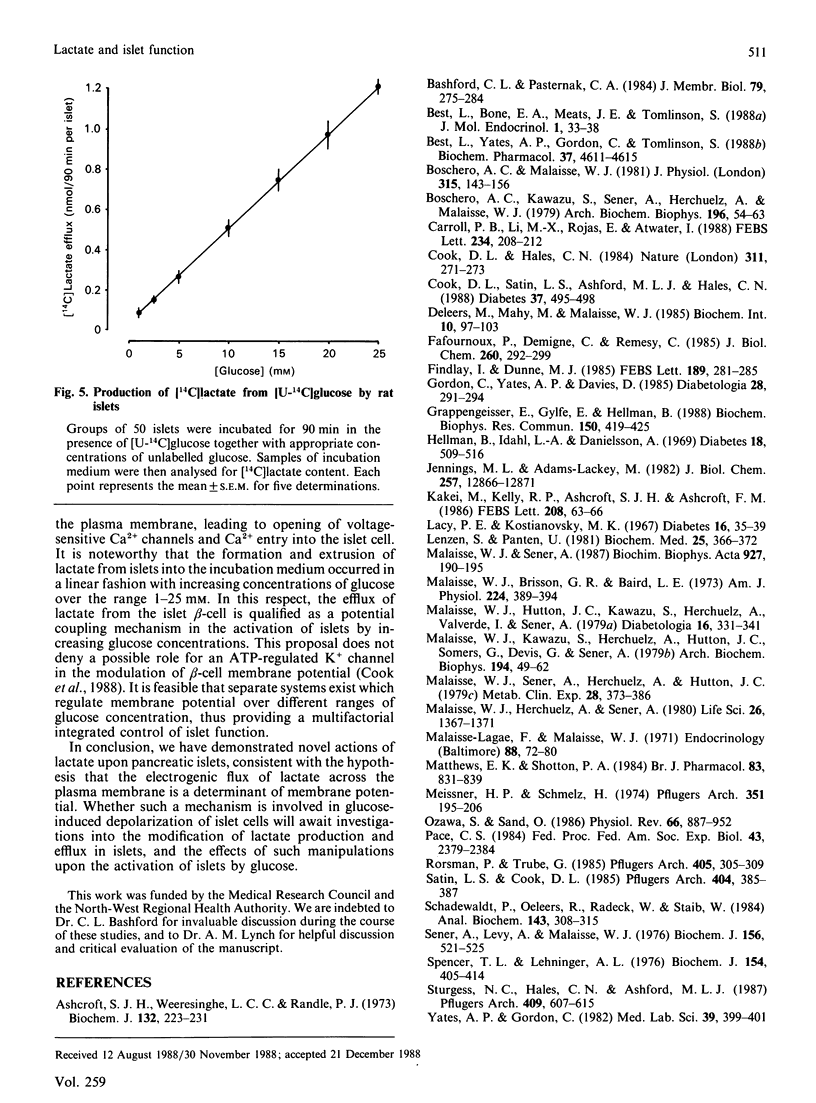
The secretion of insulin from perifused rat pancreatic islets was stimulated by raising the glucose concentration from 5.6 to 20 mM or by exposure to tolbutamide. The addition of sodium lactate (40 mM) to islets perifused in the presence of glucose (5.6 mM) resulted in a small, transient, rise in the rate of secretion. The subsequent removal of lactate, but not glucose or tolbutamide, from the perifusate produced a dramatic potentiation of insulin release. The rate of efflux of 45Ca2+ was also increased when islets were exposed to a high concentration of glucose or lactate or to tolbutamide, and again subsequently upon withdrawal of lactate. Efflux of 86Rb+ was modestly inhibited upon addition of lactate and markedly enhanced by the subsequent withdrawal of lactate from islets. The output of [14C]lactate from islets incubated in the presence of [U-14C]glucose increased linearly with increasing concentrations of glucose (1-25 mM). It is proposed that the activation of islets by the addition or withdrawal of lactate is not due to increased oxidative flux, but occurs as a result of the electrogenic passage of lactate ions across the plasma membrane, resulting in islet-cell depolarization, Ca2+ entry and insulin secretion. The production of lactate via the glycolytic pathway, and the subsequent efflux of lactate from the islet cells with concomitant exchange of H+ for Na+, could be a major determinant of depolarization and hence insulin secretion, in response to glucose.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Weerasinghe L. C., Randle P. J. Interrelationship of islet metabolism, adenosine triphosphate content and insulin release. Biochem J. 1973 Feb;132(2):223–231. doi: 10.1042/bj1320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashford C. L., Pasternak C. A. Plasma membrane potential of Lettré cells does not depend on cation gradients but on pumps. J Membr Biol. 1984;79(3):275–284. doi: 10.1007/BF01871066. [DOI] [PubMed] [Google Scholar]
- Best L., Bone E. A., Meats J. E., Tomlinson S. Is intracellular pH a coupling factor in nutrient-stimulated pancreatic islets? J Mol Endocrinol. 1988 Jul;1(1):33–38. doi: 10.1677/jme.0.0010033. [DOI] [PubMed] [Google Scholar]
- Best L., Yates A. P., Gordon C., Tomlinson S. Modulation by cytosolic pH of calcium and rubidium fluxes in rat pancreatic islets. Biochem Pharmacol. 1988 Dec 15;37(24):4611–4615. doi: 10.1016/0006-2952(88)90328-0. [DOI] [PubMed] [Google Scholar]
- Boschero A. C., Kawazu S., Sener A., Herchuelz A., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release: effects of valinomycin upon pancreatic islet function. Arch Biochem Biophys. 1979 Aug;196(1):54–63. doi: 10.1016/0003-9861(79)90550-2. [DOI] [PubMed] [Google Scholar]
- Carpinelli A. R., Malaisse W. J. Regulation of 86Rb outflow from pancreatic islets: the dual effect of nutrient secretagogues. J Physiol. 1981 Jun;315:143–156. doi: 10.1113/jphysiol.1981.sp013738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll P. B., Li M. X., Rojas E., Atwater I. The ATP-sensitive potassium channel in pancreatic B-cells is inhibited in physiological bicarbonate buffer. FEBS Lett. 1988 Jul 4;234(1):208–212. doi: 10.1016/0014-5793(88)81335-8. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Satin L. S., Ashford M. L., Hales C. N. ATP-sensitive K+ channels in pancreatic beta-cells. Spare-channel hypothesis. Diabetes. 1988 May;37(5):495–498. doi: 10.2337/diab.37.5.495. [DOI] [PubMed] [Google Scholar]
- Deleers M., Mahy M., Malaisse W. J. Glucose increases cytosolic Ca2+ activity in pancreatic islet cells. Biochem Int. 1985 Jan;10(1):97–103. [PubMed] [Google Scholar]
- Fafournoux P., Demigné C., Rémésy C. Carrier-mediated uptake of lactate in rat hepatocytes. Effects of pH and possible mechanisms for L-lactate transport. J Biol Chem. 1985 Jan 10;260(1):292–299. [PubMed] [Google Scholar]
- Findlay I., Dunne M. J. Voltage-activated Ca2+ currents in insulin-secreting cells. FEBS Lett. 1985 Sep 23;189(2):281–285. doi: 10.1016/0014-5793(85)81040-1. [DOI] [PubMed] [Google Scholar]
- Gordon C., Yates A. P., Davies D. Evidence for a direct action of exogenous insulin on the pancreatic islets of diabetic mice: islet response to insulin pre-incubation. Diabetologia. 1985 May;28(5):291–294. doi: 10.1007/BF00271688. [DOI] [PubMed] [Google Scholar]
- Grapengiesser E., Gylfe E., Hellman B. Dual effect of glucose on cytoplasmic Ca2+ in single pancreatic beta-cells. Biochem Biophys Res Commun. 1988 Jan 15;150(1):419–425. doi: 10.1016/0006-291x(88)90537-2. [DOI] [PubMed] [Google Scholar]
- Hellman B., Idahl L. A., Danielsson A. Adenosine triphosphate levels of mammalian pancreatic B cells after stimulation with glucose and hypoglycemic sulfonylureas. Diabetes. 1969 Aug;18(8):509–516. doi: 10.2337/diab.18.8.509. [DOI] [PubMed] [Google Scholar]
- Jennings M. L., Adams-Lackey M. A rabbit erythrocyte membrane protein associated with L-lactate transport. J Biol Chem. 1982 Nov 10;257(21):12866–12871. [PubMed] [Google Scholar]
- Kakei M., Kelly R. P., Ashcroft S. J., Ashcroft F. M. The ATP-sensitivity of K+ channels in rat pancreatic B-cells is modulated by ADP. FEBS Lett. 1986 Nov 10;208(1):63–66. doi: 10.1016/0014-5793(86)81533-2. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Lenzen S., Panten U. Effects of pyruvate, L-lactate, and 3-phenylpyruvate on function of ob/ob mouse pancreatic islets: insulin secretion in relation to 45Ca2+ uptake and metabolism. Biochem Med. 1981 Jun;25(3):366–372. doi: 10.1016/0006-2944(81)90095-8. [DOI] [PubMed] [Google Scholar]
- Malaisse-Lagae F., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. 3. Uptake of 45 calcium by isolated islets of Langerhans. Endocrinology. 1971 Jan;88(1):72–80. doi: 10.1210/endo-88-1-72. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Brisson G. R., Baird L. E. Stimulus-secretion coupling of glucose-induced insulin release. X. Effect of glucose on 45 Ca efflux from perifused islets. Am J Physiol. 1973 Feb;224(2):389–394. doi: 10.1152/ajplegacy.1973.224.2.389. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Herchuelz A., Sener A. The possible significance of intracellular pH in insulin release. Life Sci. 1980 Apr 28;26(17):1367–1371. doi: 10.1016/0024-3205(80)90038-7. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Hutton J. C., Kawazu S., Herchuelz A., Valverde I., Sener A. The stimulus-secretion coupling of glucose-induced insulin release. XXXV. The links between metabolic and cationic events. Diabetologia. 1979 May;16(5):331–341. doi: 10.1007/BF01223623. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Kawazu S., Herchuelz A., Hutton J. C., Somers G., Devis G., Sener A. The stimulus secretion coupling of glucose-induced insulin release. Arch Biochem Biophys. 1979 Apr 15;194(1):49–62. doi: 10.1016/0003-9861(79)90594-0. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Sener A. Glucose-induced changes in cytosolic ATP content in pancreatic islets. Biochim Biophys Acta. 1987 Feb 18;927(2):190–195. doi: 10.1016/0167-4889(87)90134-0. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Sener A., Herchuelz A., Hutton J. C. Insulin release: the fuel hypothesis. Metabolism. 1979 Apr;28(4):373–386. doi: 10.1016/0026-0495(79)90111-2. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Shotton P. A. Efflux of 86Rb from rat and mouse pancreatic islets: the role of membrane depolarization. Br J Pharmacol. 1984 Nov;83(3):831–839. doi: 10.1111/j.1476-5381.1984.tb16239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner H. P., Schmelz H. Membrane potential of beta-cells in pancreatic islets. Pflugers Arch. 1974;351(3):195–206. doi: 10.1007/BF00586918. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Sand O. Electrophysiology of excitable endocrine cells. Physiol Rev. 1986 Oct;66(4):887–952. doi: 10.1152/physrev.1986.66.4.887. [DOI] [PubMed] [Google Scholar]
- Pace C. S. Role of pH as a transduction device in triggering electrical and secretory responses in islet B cells. Fed Proc. 1984 Jun;43(9):2379–2384. [PubMed] [Google Scholar]
- Rorsman P., Trube G. Glucose dependent K+-channels in pancreatic beta-cells are regulated by intracellular ATP. Pflugers Arch. 1985 Dec;405(4):305–309. doi: 10.1007/BF00595682. [DOI] [PubMed] [Google Scholar]
- Satin L. S., Cook D. L. Voltage-gated Ca2+ current in pancreatic B-cells. Pflugers Arch. 1985 Aug;404(4):385–387. doi: 10.1007/BF00585354. [DOI] [PubMed] [Google Scholar]
- Schadewaldt P., Oelers R., Radeck W., Staib W. Oxidative determination of 14C-labeled 2-oxo acids. Anal Biochem. 1984 Dec;143(2):308–315. doi: 10.1016/0003-2697(84)90668-7. [DOI] [PubMed] [Google Scholar]
- Sener A., Levy J., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. Does glycolysis control calcium transport in the B-cell? Biochem J. 1976 Jun 15;156(3):521–525. doi: 10.1042/bj1560521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer T. L., Lehninger A. L. L-lactate transport in Ehrlich ascites-tumour cells. Biochem J. 1976 Feb 15;154(2):405–414. doi: 10.1042/bj1540405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgess N. C., Hales C. N., Ashford M. L. Calcium and ATP regulate the activity of a non-selective cation channel in a rat insulinoma cell line. Pflugers Arch. 1987 Aug;409(6):607–615. doi: 10.1007/BF00584661. [DOI] [PubMed] [Google Scholar]
- Yates A. P., Gordon C. A cell for perifusion studies of isolated tissues. Med Lab Sci. 1982 Oct;39(4):399–401. [PubMed] [Google Scholar]


