Abstract
Background
The benefit of pulmonary vein isolation (PVI) in patients with atrial fibrillation (AF) and heart failure with reduced ejection fraction (HFrEF) is well established; its efficacy in patients with heart failure preserved ejection fraction (HFpEF) is less clear.
Objective
The objective of the study was to compare AF and heart failure (HF) rehospitalizations after PVI in patients with HFpEF vs HFrEF.
Methods
The IBM MarketScan Database was used to identify patients undergoing PVI for AF. Patients were categorized by HF status: absence of HF, presence of HFrEF, or presence of HFpEF. Primary outcomes were HF and arrhythmia hospitalizations after PVI.
Results
A total of 32,524 patients were analyzed: 27,900 with no HF (86%), 2948 with HFrEF (9%), and 1676 with HFpEF (5%). Compared with those with no HF, both patients with HFrEF and HFpEF were more likely to be hospitalized for HF (hazard ratio [HR] 7.27; P < .01 for HFrEF and HR 9.46; P < .01 for HFpEF) and for AF (HR 1.17; P < .01 for HFrEF and HR 1.74; P < .01 for HFpEF) after PVI. In matched analysis, 23% of patients with HFrEF and 24% patients with HFpEF demonstrated a reduction in HF hospitalizations (P = .31) and approximately one-third demonstrated decreased arrhythmia rehospitalizations (P = .57) in the 6 months after PVI. Compared with those with HFrEF in longer-term follow-up (>1 year), patients with HFpEF were more likely to have HF (HR 1.30; P < .01) and arrhythmia (HR 1.19; P < .01) rehospitalizations.
Conclusion
Reductions in HF and arrhythmia hospitalizations are observed early after PVI across all patients with HF, but patients with HFpEF demonstrate higher HF rehospitalization and arrhythmia recurrence in longer-term follow-up than do patients with HFrEF.
Keywords: Atrial fibrillation, Pulmonary vein isolation, Heart failure with preserved ejection fraction, Heart failure with reduced ejection fraction, Diastolic dysfunction
Key Findings.
-
▪
After pulmonary vein isolation (PVI), patients with heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF) both demonstrate improvements in rehospitalizations for heart failure (HF) and arrhythmia early after PVI.
-
▪
Although reductions in HF and arrhythmia hospitalizations are observed early after PVI across all patients with HF, patients with HFpEF demonstrate higher HF rehospitalization and arrhythmia recurrence in longer-term follow-up than patients with HFrEF.
-
▪
While patients with HFpEF may benefit from PVI for the treatment of AF, increased vigilance and closer follow-up are required in the management of patients with HFpEF after PVI.
Introduction
Catheter ablation for atrial fibrillation (AF) to achieve pulmonary vein isolation (PVI) is an established treatment modality for symptomatic patients with either paroxysmal or persistent AF, reflected in the class I and IIA indications for AF management in both the European Society of Cardiology and the American Heart Association, American College of Cardiology, and Heart Rhythm Society guidelines.1,2 Most data on outcomes after PVI have focused on the treatment of patients with normal cardiac function without a history of symptomatic heart failure (HF). With that noted, there have been a growing number of prospective cohort and small randomized trials, as well as post hoc analyses, exploring the outcomes of AF ablation in patients with HF with reduced ejection fraction (HFrEF).3, 4, 5, 6, 7, 8 These trials indicate that PVI is associated with improved ventricular remodeling as well as beneficial clinical outcomes after ablation, including improved quality of life, reduced HF or arrhythmia hospitalization, and a trend toward improved survival when compared to patients with AF who are medically managed. However, there is a relative paucity of data about the potential benefits of PVI for patients with AF and HF with preserved ejection fraction (HFpEF).
In this study, we sought to analyze the real-world outcomes after PVI in patients with HFpEF as compared with patients with HFrEF and those with no HF. Specifically, we explored the impact of PVI on HF and arrhythmia rehospitalizations in the 6 months before vs the 6 months after therapy.
Methods
Data source
The present study was a retrospective cohort analysis of the 2003–2020 IBM MarketScan Commercial Claims and Encounters and Medicare Supplemental databases, both of which compile adjudicated claims for inpatient and outpatient services. The MarketScan Commercial Claims and Encounters Database contains data gathered from 28 million inpatient records, representing ∼50% of US hospital discharges annually. The Medicare Supplemental Database includes Medicare-eligible retirees with employer-sponsored Medicare Supplemental plans.9 The present study used existing large de-identified databases for which institutional review board exemption was obtained (IRB22-1859) and for which ethical guidelines have been followed according to the Helsinki Declaration on human research. Diagnosis and procedural codes used to identify diagnoses, procedures, baseline characteristics, and outcomes are listed in Online Supplemental Table 1.
Study population
We selected patients who underwent first time AF ablation between January 1, 2013, and December 31, 2020, by selecting patients with both a diagnosis of AF and procedural code 93656. Notably, procedural code 93656, introduced in 2013, was the first to explicitly mention PVI. Our cohort therefore does not include any patients undergoing PVI for AF before 2013. Patients with a history of open surgical ablation (“Maze” procedure), atrioventricular node ablation, or heart transplantation were excluded. Patients were also required to have 6 months of continuous enrollment before and after (unless a patient died, in which case they were included in the data set) the month of PVI to ensure appropriate recording of baseline comorbidities and to assess impact on clinical outcomes.
Patients were grouped on the basis of the type of HF. Patients were categorized as having HFrEF if they had a diagnostic code for systolic dysfunction without a diagnostic code for either diastolic dysfunction or a combination of systolic and diastolic dysfunction. Patients were categorized as having HFpEF if they had a diagnostic code of diastolic dysfunction without a diagnostic code of systolic dysfunction or a combination of systolic and diastolic dysfunction. Patients with codes indicating unspecified HF, with codes indicating combined systolic and diastolic dysfunction, as well as patients who have had individual diagnoses of both systolic and diastolic dysfunction were excluded from the study. In addition, type of AF was identified: patients were categorized as having paroxysmal AF if they had a diagnostic code for paroxysmal AF and without International Classification of Diseases (ICD) codes indicating persistent or permanent AF. Patients were categorized as having persistent AF if they had a diagnostic code for persistent AF and without ICD codes indicating permanent AF. Patients were identified as having permanent AF if they had any ICD code indicating permanent AF. All other patients with AF were categorized as unspecified AF.
Patient demographic characteristics, including age, sex, and US region, were collected from records on the day of first PVI. Comorbidities selected for this study are depicted in Table 1. Comorbidities were collected across all available fields (≤15) from all inpatient and outpatient encounter records before and including the day of the index procedure. Medication use was identified using the National Drug Code.
Table 1.
Baseline characteristics of the unmatched cohorts
| Characteristic | No HF (n = 27,900) | HFrEF (n = 2948) | HFpEF (n = 1676) | P |
|---|---|---|---|---|
| Sex | ||||
| Male | 19,449 (70) | 2,379 (81) | 943 (56) | <.01 |
| Age | ||||
| Median (IQR) (y) | 59 (54–64) | 60 (55–64) | 63 (58–72) | <.01 |
| Pre- and post-PVI enrollment (median [IQR]) | ||||
| Pre-PVI enrollment (y) | 3.84 (1.83–7.14) | 4.27 (2.23–8.08) | 4.51 (2.38–8.66) | <.01 |
| Post-PVI follow-up (y) | 1.96 (1.16–3.34) | 1.84 (1.10–3.06) | 1.76 (1.08–2.93) | <.01 |
| US region | ||||
| Northeast | 5,533 (20) | 673 (23) | 322 (19) | <.01 |
| North Central | 6,311 (23) | 734 (25) | 473 (28) | |
| South | 11,576 (41) | 1,132 (38) | 674 (40) | |
| West | 4,274 (15) | 383 (13) | 200 (12) | |
| Unknown | 206 (1) | 26 (1) | 7 (0) | |
| Atrial fibrillation type | ||||
| Paroxysmal atrial fibrillation | 8,532 (31) | 493 (17) | 318 (19) | <.01 |
| Persistent atrial fibrillation | 7,673 (28) | 1,433 (49) | 733 (44) | <.01 |
| Permanent atrial fibrillation | 20 (0.1) | 2 (0.1) | 2 (0.1) | .78 |
| Unspecified Atrial fibrillation type | 11,675 (42) | 1,020 (35) | 623 (37) | <.01 |
| Procedural characteristics | ||||
| Addition of intracardiac catheter ablation of a mechanism distinct from the primary ablation mechanism (CPT code 93655) | 9,782 (35) | 1,139 (39) | 626 (37) | <.01 |
| Additional linear/focal ablation of the left and right atrium for the treatment of remaining atrial fibrillation after completion of PVI (CPT code 93657) | 9,907 (36) | 1,261 (43) | 703 (42) | <.01 |
| Comorbidities | ||||
| Chronic kidney disease | 1,414 (5) | 331 (11) | 293 (17) | <.01 |
| Chronic pulmonary disease | 4,034 (14) | 576 (20) | 452 (27) | <.01 |
| Coronary artery disease | 4,219 (15) | 953 (32) | 526 (31) | <.01 |
| Diabetes mellitus | 6,391 (23) | 986 (33) | 720 (43) | <.01 |
| End-stage renal disease | 429 (1) | 115 (4) | 103 (6) | <.01 |
| Hyperlipidemia | 20,501 (73) | 2,329 (79) | 1,441 (86) | <.01 |
| Hypertension | 21,203 (76) | 2,530 (86) | 1,579 (94) | <.01 |
| Mitral valve disease | 10,850 (39) | 1,735 (59) | 963 (57) | <.01 |
| Myocardial infarction | 2,057 (7) | 658 (22) | 308 (18) | <.01 |
| Obesity | 9,981 (36) | 1,239 (42) | 953 (57) | <.01 |
| Obstructive sleep apnea | 10,392 (37) | 1,279 (43) | 853 (51) | <.01 |
| Peripheral vascular disease | 3,381 (12) | 528 (18) | 468 (28) | <.01 |
| Pulmonary hypertension | 253 (1) | 95 (3) | 86 (5) | <.01 |
| Stroke/TIA | 2,328 (8) | 371 (13) | 226 (13) | <.01 |
| Thyrotoxicosis | 1,008 (4) | 150 (5) | 84 (5) | <.01 |
| Tobacco use | 3,938 (14) | 546 (19) | 340 (20) | <.01 |
| Valvular (nonmitral) heart disease | 9,059 (32) | 1,430 (49) | 886 (53) | <.01 |
| 6-month pre-PVI hospitalization rates | ||||
| All-cause hospitalization | 12,073 (43) | 1,663 (56) | 1,075 (64) | <.01 |
| Noncardiovascular disease hospitalization | 6,527 (20) | 591 (20) | 432 (26) | <.01 |
| Arrhythmia hospitalization | 8,174 (29) | 1,140 (39) | 780 (47) | <.01 |
| Heart failure hospitalization | 0 (0) | 953 (32) | 527 (31) | <.01 |
| Cerebrovascular disease hospitalization | 135 (0) | 37 (1) | 8 (0) | <.01 |
| Other cardiovascular disease hospitalization | 619 (2) | 68 (2) | 63 (4) | .03 |
| CHA2DS2-VASc score | ||||
| 0 | 3,489 (13) | 0 (0) | 0 (0) | <.01 |
| 1 | 8,932 (32) | 214 (7) | 33 (2) | <.01 |
| 2 | 7,469 (27) | 835 (28) | 240 (14) | <.01 |
| 3 | 4,333 (16) | 793 (27) | 401 (24) | <.01 |
| 4 | 2,215 (8) | 499 (17) | 391 (23) | <.01 |
| 5 | 955 (3) | 304 (10) | 287 (17) | <.01 |
| ≥6 | 507 (2) | 303 (10) | 324 (19) | <.01 |
| Average | 1.92 (1.39) | 3.30 (1.52) | 4.01 (1.59) | <.01 |
| Medications | ||||
| Class I or III antiarrhythmic medication | 16,917 (61) | 1,896 (64) | 1,085 (65) | .56 |
| DOAC | 17,330 (62) | 1,977 (67) | 1,094 (65) | <.01 |
| Warfarin | 3,680 (13) | 573 (19) | 341 (20) | <.01 |
| β-Blocker | 13,383 (48) | 2,146 (73) | 1,019 (61) | <.01 |
| ACE inhibitor or angiotensin II receptor blocker | 8,121 (29) | 1,754 (60) | 720 (43) | <.01 |
| Mineralocorticoid receptor antagonist | 445 (2) | 467 (16) | 123 (7) | <.01 |
| Sacubitril-valsartan | 11 (0) | 129 (4) | 3 (0) | <.01 |
| SGLT2 inhibitor | 344 (1) | 54 (2) | 28 (2) | .01 |
| Hydralazine/isosorbide dinitrate | 0 (0) | 6 (0) | 1 (0) | <.01 |
| Digoxin | 1,494 (5) | 488 (17) | 183 (11) | <.01 |
Values are presented count (percentage of the cohort) unless stated otherwise.
ACE = angiotensin-converting enzyme; CPT = Current Procedural Terminology; DOAC = direct oral anticoagulant; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; PVI = pulmonary vein isolation; SGLT2 = sodium-glucose cotransporter 2; TIA = transient ischemic attack.
Outcomes
Two primary outcomes were assessed: (1) HF hospitalization (HFH) and (2) arrhythmia hospitalization as a result of AF or atrial flutter. Outcomes were identified as any inpatient encounter at any time after PVI with either an HF or an AF/atrial flutter diagnosis queried across all available diagnostic fields. Notably, all diagnostic fields, as opposed to only the principal diagnosis, were queried because of the often coincident diagnosis of both HF and AF. If only the principal diagnostic field were to be queried, outcomes would likely underrepresent AF and HF events. Importantly, all patients in the data set have a history of HF and AF by necessity. Therefore, querying all diagnostic fields are not expected to bias the results in one group more than the other.
In addition, patients were assessed for an absolute decrease, no change, or increase in the number of both HF and arrhythmia hospitalizations in the 6 months after vs 6 months before catheter ablation for AF. A 6-month periprocedural period was chosen with the rationale that patients are more likely to be symptomatic from AF in the lead up to invasive therapeutic intervention; therefore, assessing this periprocedural period would provide clinically relevant outcomes for patients to assess the possible impact of ablation on immediate relief and hospitalization burden. In contrast to other studies assessing PVI, which have included a 3-month blanking period after PVI, we did not use a blanking period in our study so that these periprocedural differences could be assessed.
Statistics
Baseline characteristics between patients with no HF, patients with HFpEF, and patients with HFrEF were compared. Continuous variables were assessed for normality using the Kolmogorov-Smirnov test. Non-normally distributed continuous variables were reported as median (interquartile range [IQR]) and were compared using the Kruskal-Wallis test. Categorical characteristics were reported as count (percentage) and were compared using the χ2 test of association.
Kaplan-Meier curves were constructed to measure event-free survival. Differences in primary outcomes during follow-up were assessed using univariable Cox proportional hazards models.
Statistical significance was determined using α < .05. All data analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Baseline comorbidities, a binary age variable (<65 years vs ≥65 years), sex, and pre-PVI medication prescription were separately assessed as predictors of time to first post-PVI HF or arrhythmia rehospitalization in patients with HF by creating a Cox proportional hazards model as follows: (1) a univariable model was fit for each predictor, and only predictors significant at P ≤ .05 were selected; and (2) those predictors were subsequently used for a multivariable model, and backward elimination was used to eliminate nonsignificant predictors for which P > .05. Collinearity of the predictors included in the model was assessed using a Spearman correlation—0.7 was used as the correlation coefficient cutoff, whereby no predictors were collinear.
To mitigate the effects of unmeasured confounders that could account for differences in outcomes between patients with HFpEF and patients with HFrEF, a secondary analysis was performed using a matched cohort with a match ratio of 1:1. Propensity scores were calculated for every patient according to a multivariable Cox proportional hazards model that included all predictors listed in Online Supplemental Table 2. Patients were propensity score matched using the SAS Greedy 5→1 Digit Match Macro.10
Results
Study population
The study cohort included 32,524 patients: 27,900 patients with no history of HF before PVI (86%), 2948 with a history of HFrEF (9%), and 1676 with a history of HFpEF (5%) (Figure 1). Significant differences were noted among multiple baseline characteristics (Table 1). Compared with those with no history of HF or with a history of HFrEF, patients with HFpEF were significantly more likely to have chronic kidney disease, chronic pulmonary disease, diabetes mellitus, end-stage renal disease, hyperlipidemia, hypertension, pulmonary hypertension, obesity, obstructive sleep apnea, peripheral vascular disease, and nonmitral valvular disease (P < .01). Patients with HFpEF were also significantly more likely to have had noncardiovascular disease, arrhythmia, and all-cause hospitalizations in the 6 months before PVI than those with HFrEF or no HF (P < .01).
Figure 1.
Study cohort. A total of 55,176 patients were identified as having undergone pulmonary vein isolation (PVI) for atrial fibrillation from 2013 to 2020. Patients with fewer than 6 months of continuous enrollment before and after PVI (unless patients died) were excluded. Patients having undergone the Maze procedure, atrioventricular (AV) node ablation, or heart transplantation as well as patients receiving a permanent pacemaker (PPM) or implantable cardioverter-defibrillator (ICD) on the day of PVI were also excluded. Patients were stratified into patients with heart failure with reduced ejection fraction (HFrEF), heart failure with preserved ejection fraction (HFpEF), and no history of heart failure (HF).
Otherwise, when comparing patients with HFrEF with those with HFpEF, specifically patients with HFrEF were more likely to have persistent AF (49% vs 44%; P < .01). However, there were no significant differences in the percentage of patients with paroxysmal (17% vs 19%; P = .05), permanent (0.1% vs 0.1%; P = .57), or unspecified (35% vs 37%; P = .08) AF. In addition, when comparing patients with HFrEF with those with HFpEF, there were no significant differences in the percentage of patients who underwent intracardiac catheter ablation of an arrhythmia mechanism discrete from the primary ablation mechanism (39% vs 37%; P = .39) or in the percentage of patients who underwent additional linear or focal intracardiac catheter ablation of the left and right atrium for the treatment of AF remaining after completion of PVI (43% vs 42%; P = .58).
Outcomes in unmatched cohorts
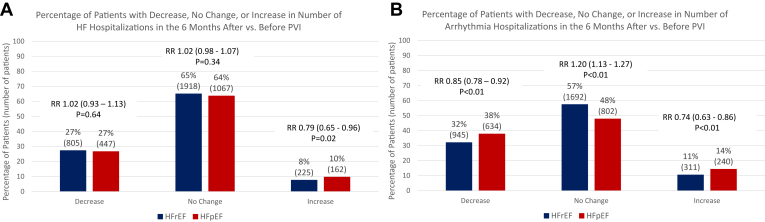
When analyzing individual patient outcomes, 27% of patients with HFrEF and 27% of patients with HFpEF had a decrease in the cumulative number of HFHs in the 6 months after vs 6 months before PVI (P = .64). Meanwhile, 65% of patients with HFrEF and 64% of patients with HFpEF had no change in the number of HFH (P = .34) and 14% of patients with HFrEF and 10% of patients with HFpEF had an increase in the number of HFHs (P = .02) (Figure 2A). Otherwise, 32% of patients with HFrEF and 38% of patients with HFpEF had a decrease in AF/atrial flutter hospitalizations in the 6 months after vs 6 months before PVI (P < .01). Meanwhile, 57% of patients with HFrEF and 48% of patients with HFpEF had no change in arrhythmia hospitalization (P < .01) and 11% of patients with HFrEF and 14% of patients with HFpEF had an increase in the number of arrhythmia hospitalizations (P < .01) (Figure 2B).
Figure 2.
Percentages of patients with a decrease, no change, or increase in heart failure (HF) and arrhythmia hospitalizations. Percentage of patients (with overall number in parentheses) with a decrease, no change, or increase in the number of HF (A) and atrial fibrillation hospitalizations (B) when comparing the 6-month period after pulmonary vein isolation (PVI) vs the 6-month period before PVI. HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; RR = relative risk.
Compared with those with no history of HF, both patients with HFrEF (HR 7.27; 95% confidence interval [CI] 6.64–7.96; P < .01) and patients with HFpEF (HR 9.46; 95% CI 8.53–10.49; P < .01) were more likely to be hospitalized for HF after PVI ablation. Similarly, patients with HFrEF (HR 1.17; 95% CI 1.10–1.25; P < .01) and patients with HFpEF (HR 1.74; 95% CI 1.62–1.88; P < .01) were more likely to be hospitalized for AF or atrial flutter after PVI than patients with no history of HF. However, when comparing patients with HFpEF and those with HFrEF, patients with HFpEF were both more likely to have HFH (HR 1.30; 95% CI 1.16–1.45; P < .01) and arrhythmia hospitalization (HR 1.50; 95% CI 1.37–1.65; P < .01) after PVI compared with patients with HFrEF (Figure 3). This effect was noted early on and diverged further in longer-term follow-up, particularly notable after 1 year. Median follow-up for the cohorts was 1.96 years (IQR 1.16–3.34 years) for patients without HF and 1.81 years (IQR 1.09–3.02 years) for all patients with HF: 1.84 years (IQR 1.10–3.06 years) for HFrEF and 1.76 years (IQR 1.08–2.93 years) for HFpEF.
Figure 3.
Heart failure (HF) and arrhythmia hospitalization free survival in the unmatched cohort. In unmatched analysis, patients with no history of HF are less likely to have any HF hospitalization (HFH) and any arrhythmia hospitalization after pulmonary vein isolation than patients with HF with reduced ejection fraction (HFrEF). Patients with HFrEF are less likely to have HFH or arrhythmia hospitalization than patients with HF with preserved ejection fraction (HFpEF) (P < .01). Univariable hazard ratios (HRs) are reported. CI = confidence interval.
Multivariable analysis of significant covariates for HF and arrhythmia hospitalizations is summarized in Online Supplemental Tables 3 and 4.
Propensity score–matched cohort analysis
The matched cohorts included 2020 patients matched on 37 characteristics using propensity scores: 1010 with HFrEF and 1010 with HFpEF (Online Supplemental Table 5). Importantly, patients were also matched according to all-cause, noncardiovascular disease, HF, and arrhythmia hospitalizations within 6 months of PVI. In addition, patients were matched according to the history of medical therapy, including antiarrhythmic medications, β-blockers, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker, mineralocorticoid receptor antagonist, angiotensin receptor neprilysin inhibitor, sodium-glucose cotransporter-2 inhibitor, and hydralazine/isosorbide dinitrate at any time before PVI in an effort to account for possible differences in arrhythmia and HF medication regimen. Finally, patients were matched according to AF type (paroxysmal, persistent, permanent, or unspecified AF) as well as additional procedural characteristics (additional ablation of an arrhythmia mechanism discrete from primary ablation mechanism and additional linear or focal intracardiac ablation of the left and right atrium for the treatment of AF remaining after completion of PVI).
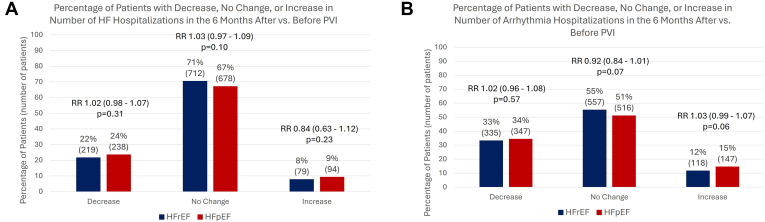
When analyzing individual patients, 22% of patients with HFrEF and 24% of patients with HFpEF had a decrease in the number of HFHs 6 months after vs 6 months before PVI (P = .31); 71% of patients with HFrEF and 67% of patients with HFpEF had no change in the number of hospitalizations (P = .10); and 8% of patients with HFrEF and 9% of patients with HFpEF had an increase (P = .23). Meanwhile, 33% of patients with HFrEF and 34% of patients with HFpEF had individual decreases in arrhythmia rehospitalizations in the 6 months after vs 6 months before PVI (P = .57); 55% of patients with HFrEF and 51% of patients with HFpEF had no change in the number of arrhythmia hospitalizations (P = .07); and 12% of patients with HFrEF and 15% of patients with HFpEF had an increase (P = .06) (Figures 4A and 4B).
Figure 4.
Percentages of patients with a decrease, no change, or increase in heart failure (HF) and arrhythmia hospitalizations in the matched cohort analysis. Percentage of patients (with overall number in parentheses) with a decrease, no change, or increase in the number of HF (A) and arrhythmia hospitalizations (B) when comparing the 6-month period after pulmonary vein isolation (PVI) vs the 6-month period before PVI. There are no significant differences between the 2 groups. HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; RR = relative risk.
In the matched cohort analysis, patients with HFpEF were more likely to have HF rehospitalization (HR 1.30; 95% CI 1.10–1.55; P < .01) and atrial flutter or AF rehospitalization (HR 1.19; 95% CI 1.04–1.37; P = .01) than patients with HFrEF (Figure 5) in longer-term follow-up.
Figure 5.
Heart failure (HF) and arrhythmia hospitalization free survival in the matched cohort. In the matched cohort analysis, patients with HF with reduced ejection fraction (HFrEF) are significantly less likely to have any HF (A) and any atrial flutter or atrial fibrillation hospitalizations after pulmonary vein isolation (B) than patients with HF with preserved ejection fraction (HFpEF). Univariable hazard ratios (HRs) are reported. CI = confidence interval; HFH = heart failure hospitalization.
Discussion
This study used a large nationally representative data set to assess HF and arrhythmia rehospitalizations after PVI for AF in patients with HFpEF. The main findings of this study include the following:
-
1.
Compared with those with HFrEF, patients with HFpEF demonstrated a higher incidence of comorbid conditions at baseline and a higher risk of HF or arrhythmia hospitalization after PVI in unmatched analysis. This increased risk persisted in matched analysis even after controlling for baseline comorbidities, including AF type as well as additional procedural characteristics.
-
2.
After matching for baseline covariates, PVI was associated with a reduction in HF or arrhythmia hospitalization early after the procedure, with a similar proportion of patients with HFpEF and HFrEF showing arrhythmia hospitalization and HFH in the 6 months after compared with the 6 months before PVI.
-
3.
When followed over the longer-term (particularly >1 year), patients with HFpEF demonstrated a higher risk of arrhythmia hospitalization and HFH relative to patients with HFrEF. This increased risk persisted in matched analysis even after controlling for baseline comorbidities.
PVI is now a well-established nonpharmacological therapy for rhythm control in patients with symptomatic paroxysmal or persistent AF who have been intolerant to at least 1 class I or III antiarrhythmic medication. The 2023 American Heart Association, American College of Cardiology, and Heart Rhythm Society and 2020 European Society of Cardiology guidelines recently extended indications for PVI to patients with symptomatic AF and HFrEF to reduce the risk of HFH and possibly improve survival.1,2 Fewer data are available regarding the outcome of PVI in patients with HFpEF, with small cohort studies reporting reduced efficacy. For example, Ichijo et al11 and Black-Maier et al12 examined the rates of AF rehospitalization between patients with HFrEF and those with HFpEF in single-center retrospective studies and did not find significant differences in arrhythmia-free survival between these groups. Furthermore, other studies have found worse outcomes for patients with HFpEF after PVI. In a prospective study comparing 1-year antiarrhythmic drug–free AF recurrence in patients with systolic dysfunction to patients with isolated diastolic dysfunction, Cha et al13 found a trend toward higher rates of AF recurrence in patients with diastolic dysfunction, although they found no statistically significant difference in long-term (5-year) recurrence on or off antiarrhythmic drugs. Similarly, in a retrospective study of the UC San Diego Ablation Registry comparing the recurrence of atrial arrhythmias between patients with HFpEF and those with HFrEF, Aldaas et al14 reported a trend toward increased recurrence in the HFpEF subgroup, although the difference also did not reach statistical significance.
Our study extends the observations of prior work to a large real-world population. We found that PVI did appear to improve HF and arrhythmia rehospitalizations in the 6-month periprocedural period, similarly for patients with HFpEF vs those with HFrEF, but that increased rates of arrhythmia and HF recurrence were noted in longer-term follow-up, with divergence noted at 1 year and extending later during follow-up. It is interesting to note that the majority of patients with HF post PVI demonstrated either no change or a reduction in HF or arrhythmia hospitalization in the 6 months after the procedure. The overall increased risk of rehospitalization was driven by a relatively small cohort of patients in the longer-term. The fact that this risk remains significantly higher in patients with HFpEF than in those with HFrEF may be driven in part because of the underlying clinical heterogeneity of patients with HFpEF. Indeed, the complexity of describing clinical phenogroups in HFpEF has been alluded to by multiple investigators.15, 16, 17 Supporting this notion is that HFpEF remained a significant independent predictor for both HF and arrhythmia rehospitalizations even after adjusting for numerous other comorbidities (see Online Supplemental Tables 4 and 5).
The differential impact of PVI in patients with HFpEF may well reflect the underlying pathophysiology. For example, in a prospective study comparing clinical, functional, and echocardiographic outcomes of 24 patients with AF and left ventricular ejection fraction ≥ 50% to 78 patients with AF without HF, Zylla et al18 found progression of adverse left atrial remodeling and no relevant improvement in diastolic function in patients with HFpEF after PVI, with persistence of a lower physical component summary score.
With that noted, durable sinus rhythm may still have a benefit. In a retrospective observational study comparing HFH in patients who achieved maintenance of sinus rhythm by ablation with or without antiarrhythmic medications and patients with HFpEF treated with rate control, Machino-Ohtsuka et al19 demonstrated that maintenance of sinus rhythm was associated with a lower risk of hospitalization for HF in patients with HFpEF and AF. In addition, in their study comparing HF rehospitalization between patients with HFpEF undergoing catheter ablation and patients with HFpEF treated with conventional pharmacotherapy, Fukui et al20 demonstrated reduced HFH in the catheter ablation group, which was also correlated with increased time in sinus rhythm. Our study, meanwhile, differs from the above studies in that it compares HF rehospitalization in patients with HFpEF and HFrEF undergoing catheter ablation. While our results show similar 6-month periprocedural HF rehospitalization rates between the 2 groups, we found that hospitalization rates diverged later during follow-up. While one focus may be due to our inadequate taxonomy of HFpEF, another possibility is that the outcome simply reflects different rates of durability of sinus rhythm. Greater insight into the burden of AF or atrial flutter after ablation by group may help to better parse whether worse outcomes after PVI in HFpEF is due to progression of the underlying cardiomyopathy and disease progression vs ineffective arrhythmia suppression.
Limitations
Because MarketScan is an administrative claims database, there are certain limitations in study design: for example, there is no access to specific left ventricular ejection fraction. Instead, patients were categorized into groups on the basis of ICD codes indicating systolic or diastolic dysfunction. As a result, we excluded patients with diagnoses of both systolic and diastolic function to maintain as much accuracy in categorization of HF. In addition, although index PVI was identified according to the first PVI identified in the data set, it is possible that a patient may have had PVI before enrollment in the MarketScan database. This clinical information is not available. Importantly, our study does include median (IQR) of pre-PVI enrollment (3.84 years for no HF, 4.27 years HFrEF, and 4.51 years in HFpEF; see Table 1). The pre-PVI median enrollment interval may assuage concerns about whether index PVI is truly the first PVI for a patient, although it does not exclude this possibility with certainty.
Because MarketScan is a billing claims data set, there are also limitations in available data regarding procedural information and assessment of AF burden in follow-up. For example, the data set does not provide information regarding energy source used for AF ablation, and thus analyses cannot differentiate radiofrequency ablation vs cryoballoon, laser balloon, or other techniques (notably, pulsed field ablation was not commercially available in the United States during this study period). Similarly, there is limited ability to distinguish the etiopathology of HFpEF from claims data. The ability to identify specific HFpEF phenogroups may be particularly helpful in understanding the observed differences that a relatively small group of patients appear to drive worse outcomes over the longer-term in patients with HFpEF. Finally, the success of PVI at achieving and maintaining sinus rhythm, as assessed by the burden of AF or atrial flutter, could not be determined. Altogether, higher coding granularity would be beneficial for more nuanced understanding of observed differences.
There was a large proportion of patients in this study with “unspecified AF” as their listed diagnosis, and it is possible that differences in AF type between HFpEF and HFrEF groups could account for differences in outcomes. In particular, data regarding the proportion of patients with long-standing persistent AF were not specifically coded. However, it is notable that paroxysmal vs persistent AF was not a statistically significant predictor of HF or arrhythmia rehospitalization. Moreover, AF type was used in the creation of the matched cohort, and increased risk of arrhythmia and HF rehospitalizations persisted even when AF type was equally distributed among the HF groups.
Conclusion
Compared with those with HFrEF or those with no history of HF, patients with HFpEF selected for PVI demonstrate a higher degree of comorbid illness and a higher incidence of noncardiovascular disease, arrhythmia hospitalizations, and all-cause hospitalizations at baseline. After PVI, patients with HFpEF and HFrEF both demonstrate improvements in rehospitalizations for HF and arrhythmia early in the first 6 months after PVI, although HFpEF does worse overall in longer-term follow-up. Differences in outcomes between HFpEF and HFrEF are driven by a relatively small proportion of all patients with HFpEF and persist even after matching for multiple comorbidities. Together, these findings suggest that increased vigilance and closer follow-up are required in the management of patients with HFpEF after PVI.
Acknowledgments
Funding Sources
The authors have no funding sources to disclose.
Disclosures
Zaid Aziz reports speaking for Biotronik. Andrew D. Beaser reports speaking for Abbott. Gaurav A. Upadhyay reports speaking or consulting for Abbott, Biotronik, Boston Scientific, GE Medical, Medtronic, Philips, Rhythm Science, and ZOLL Medical. The other authors report no relevant disclosures.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Patient consent not required because of the use of deidentified data.
Ethics Statement
The present study used existing large deidentified databases for which institutional review board exemption was obtained (IRB22-1859). Ethical guidelines have been followed according to the Helsinki Declaration on human research.
Appendix. Supplementary Data
References
- 1.Hindricks G., Potpara T., Kirchhof P., et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 2.Joglar J.A., Chung M.K., Armbruster A.L., et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:E1–E156. doi: 10.1161/CIR.0000000000001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Biase L., Mohanty P., Mohanty S., et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637–1644. doi: 10.1161/CIRCULATIONAHA.115.019406. [DOI] [PubMed] [Google Scholar]
- 4.Packer D.L., Piccini J.P., Monahan K.H., et al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation. 2021;143:1377–1390. doi: 10.1161/CIRCULATIONAHA.120.050991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prabhu S., Taylor A.J., Costello B.T., et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI study. J Am Coll Cardiol. 2017;70:1949–1961. doi: 10.1016/j.jacc.2017.08.041. [DOI] [PubMed] [Google Scholar]
- 6.Kuck K.H., Merkely B., Zahn R., et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA trial. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.119.007731. [DOI] [PubMed] [Google Scholar]
- 7.Parkash R., Wells G.A., Rouleau J., et al. Randomized ablation-based rhythm-control versus rate-control trial in patients with heart failure and atrial fibrillation: results from the RAFT-AF trial. Circulation. 2022;145:1693–1704. doi: 10.1161/CIRCULATIONAHA.121.057095. [DOI] [PubMed] [Google Scholar]
- 8.Marrouche N.F., Brachmann J., Andresen D., et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 9.MarketScan Research Databases for life sciences researchers [internet] 2022. https://www.merative.com/content/dam/merative/documents/brief/marketscan-research-databases-for-life-sciences-researchers.pdf pp. 1–15. Available from:
- 10.Parsons L. Reducing bias in a propensity score matched-pair sample using greedy matching techniques [internet]. Ovation Research Group. https://support.sas.com/resources/papers/proceedings/proceedings/sugi26/p214-26.pdf pp. 214–226. Available from:
- 11.Ichijo S., Miyazaki S., Kusa S., et al. Impact of catheter ablation of atrial fibrillation on long-term clinical outcomes in patients with heart failure. J Cardiol. 2018;72:240–246. doi: 10.1016/j.jjcc.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Black-Maier E., Ren X., Steinberg B.A., et al. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm. 2018;15:651–657. doi: 10.1016/j.hrthm.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Cha Y.M., Wokhlu A., Asirvatham S.J., et al. Success of ablation for atrial fibrillation in isolated left ventricular diastolic dysfunction: a comparison to systolic dysfunction and normal ventricular function. Circ Arrhythm Electrophysiol. 2011;4:724–732. doi: 10.1161/CIRCEP.110.960690. [DOI] [PubMed] [Google Scholar]
- 14.Aldaas O.M., Malladi C.L., Mylavarapu P.S., et al. Comparison of outcomes after ablation of atrial fibrillation in patients with heart failure with preserved versus reduced ejection fraction. Am J Cardiol. 2020;136:62–70. doi: 10.1016/j.amjcard.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah S.J., Katz D.H., Selvaraj S., et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131:269–279. doi: 10.1161/CIRCULATIONAHA.114.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segar M.W., Patel K.V., Ayers C., et al. Phenomapping of patients with heart failure with preserved ejection fraction using machine learning-based unsupervised cluster analysis. Eur J Heart Fail. 2020;22:148–158. doi: 10.1002/ejhf.1621. [DOI] [PubMed] [Google Scholar]
- 17.Cohen J.B., Schrauben S.J., Zhao L., et al. Clinical phenogroups in heart failure with preserved ejection fraction: detailed phenotypes, prognosis, and response to spironolactone. JACC Heart Fail. 2020;8:172–184. doi: 10.1016/j.jchf.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zylla M.M., Leiner J., Rahm A.K., et al. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2022;15 doi: 10.1161/CIRCHEARTFAILURE.121.009281. [DOI] [PubMed] [Google Scholar]
- 19.Machino-Ohtsuka T., Seo Y., Ishizu T., et al. Relationships between maintenance of sinus rhythm and clinical outcomes in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Cardiol. 2019;74:235–244. doi: 10.1016/j.jjcc.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Fukui A., Tanino T., Yamaguchi T., et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2020;31:682–688. doi: 10.1111/jce.14369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.