Abstract
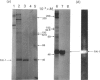
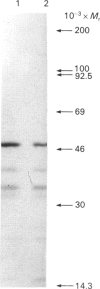
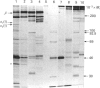
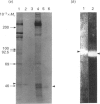
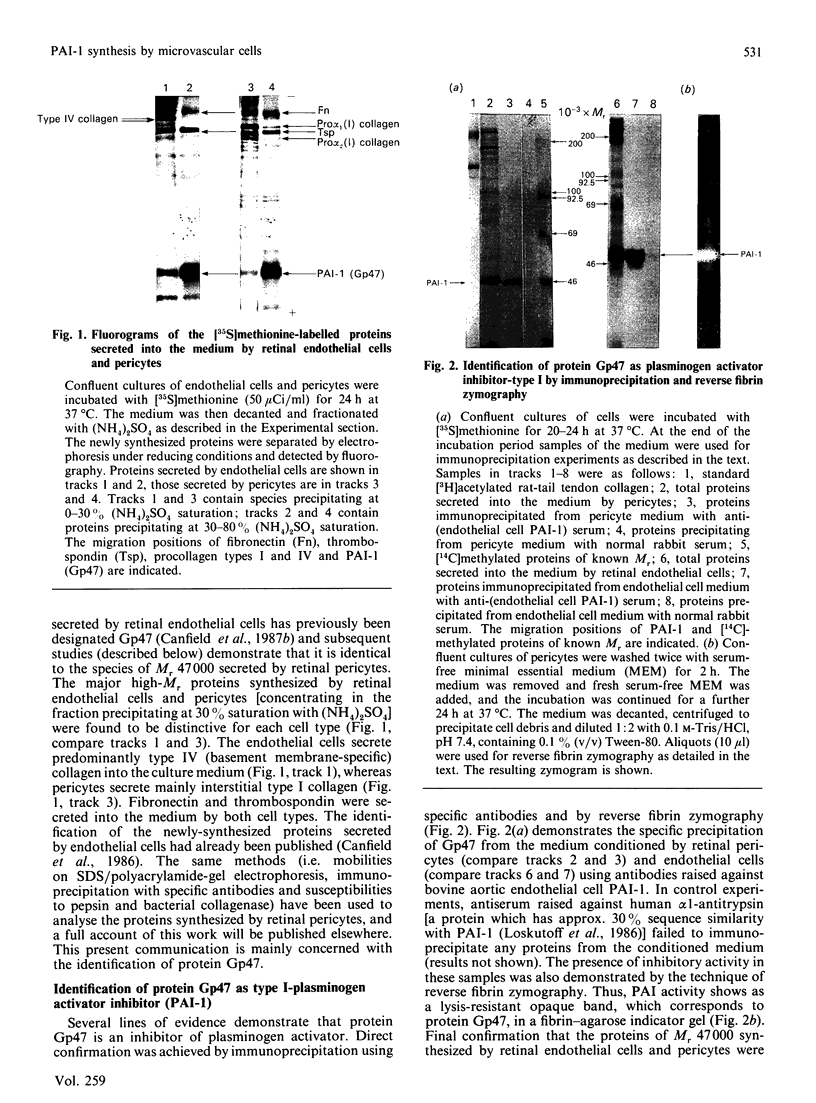
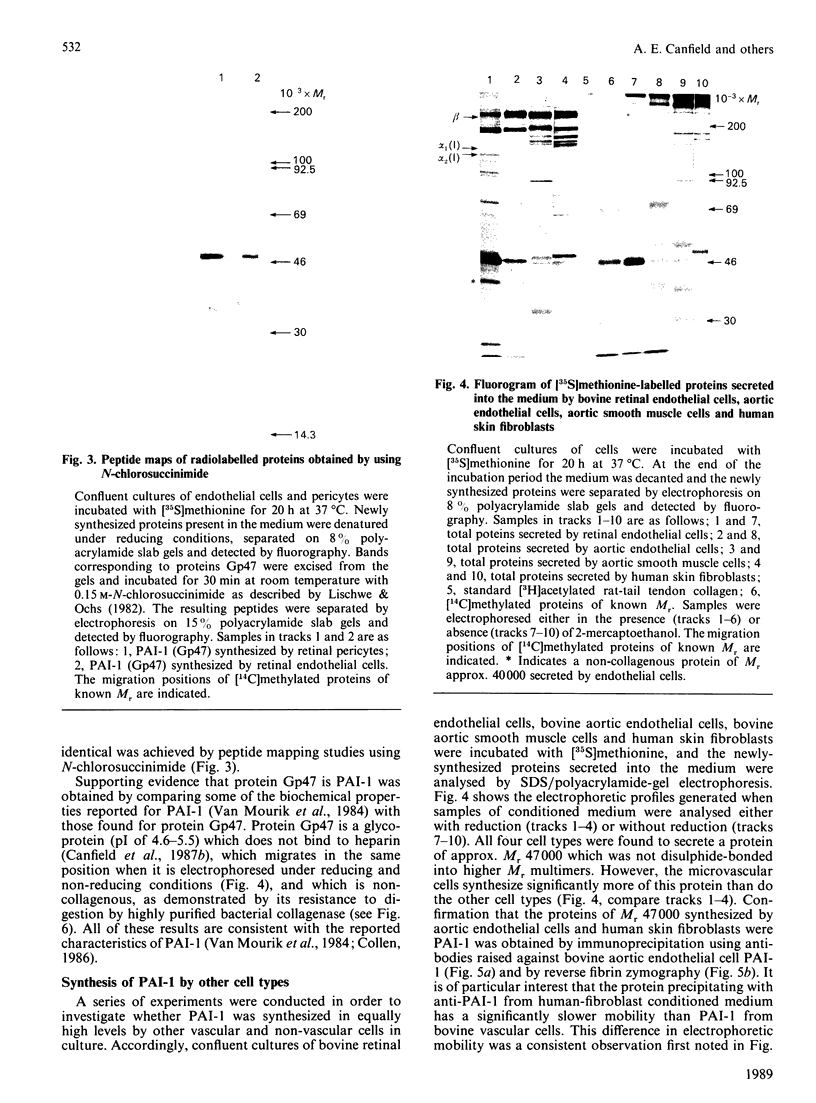
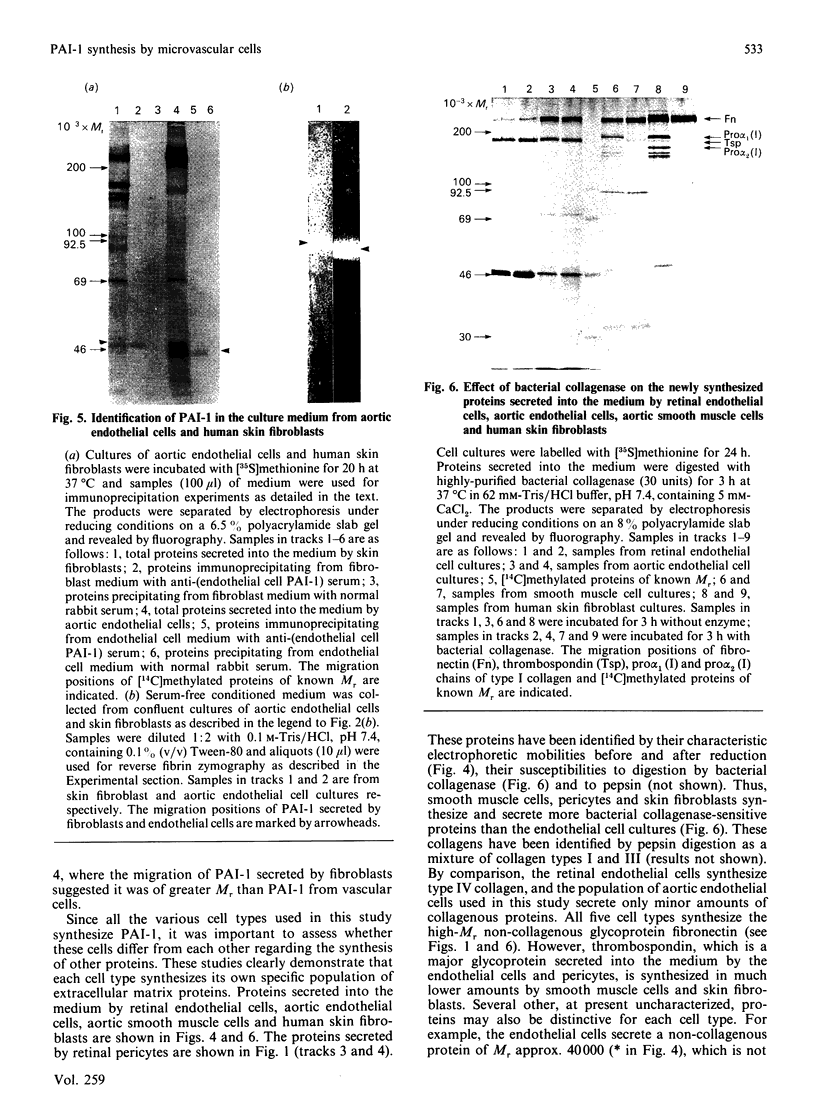
Previous studies have shown that a glycoprotein of Mr 47,000 (designated Gp47) is a major biosynthetic product of retinal endothelial cells in vitro (Canfield, Schor, West, Schor & Grant (1987) Biochem. J. 246, 121-129). We now present data indicating that (a) an identical protein is secreted by bovine retinal pericytes, (b) this protein is plasminogen activator inhibitor-type I (PAI-1), as revealed by immunoprecipitation with specific antibodies and reverse fibrin zymography, and (c) retinal endothelial cells and pericytes synthesize different species of matrix macromolecules, that is: type IV collagen is the major collagen secreted by endothelial cells, whereas pericytes produce predominantly type I collagen; fibronectin and thrombospondin are synthesized by both cell types. Our studies also indicate that PAI-1 is produced, albeit at considerably lower levels, by large vessel vascular cells (aortic endothelial and smooth muscle cells) and human skin fibroblasts. PAI-1 produced by human skin fibroblasts appears to be a distinct molecular species compared to its bovine counterpart as assessed by its slower mobility on SDS/polyacrylamide-gel electrophoresis. The potential significance of elevated PAI-1 production by retinal endothelial cells and pericytes, as well as their distinctive patterns of matrix biosynthesis, is discussed in terms of the involvement of these cells in the maintenance and remodelling of microvessel basement membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton N. Vascular basement membrane changes in diabetic retinopathy. Montgomery lecture, 1973. Br J Ophthalmol. 1974 Apr;58(4):344–366. doi: 10.1136/bjo.58.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi F., Vassalli J. D., Danø K. Urokinase-type plasminogen activator: proenzyme, receptor, and inhibitors. J Cell Biol. 1987 Apr;104(4):801–804. doi: 10.1083/jcb.104.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Booth N. A., Anderson J. A., Bennett B. Platelet release protein which inhibits plasminogen activators. J Clin Pathol. 1985 Jul;38(7):825–830. doi: 10.1136/jcp.38.7.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth N. A., MacGregor I. R., Hunter N. R., Bennett B. Plasminogen activator inhibitor from human endothelial cells. Purification and partial characterization. Eur J Biochem. 1987 Jun 15;165(3):595–600. doi: 10.1111/j.1432-1033.1987.tb11481.x. [DOI] [PubMed] [Google Scholar]
- Booyse F. M., Osikowicz G., Feder S., Scheinbuks J. Isolation and characterization of a urokinase-type plasminogen activator (Mr = 54,000) from cultured human endothelial cells indistinguishable from urinary urokinase. J Biol Chem. 1984 Jun 10;259(11):7198–7205. [PubMed] [Google Scholar]
- Brownlee M., Cerami A. The biochemistry of the complications of diabetes mellitus. Annu Rev Biochem. 1981;50:385–432. doi: 10.1146/annurev.bi.50.070181.002125. [DOI] [PubMed] [Google Scholar]
- Canfield A. E., Schor A. M., Schor S. L., Grant M. E. The biosynthesis of extracellular-matrix components by bovine retinal endothelial cells displaying distinctive morphological phenotypes. Biochem J. 1986 Apr 15;235(2):375–383. doi: 10.1042/bj2350375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield A. E., Schor A. M., West D. C., Schor S. L., Grant M. E. Identification and partial characterization of two major proteins of Mr 47,000 synthesized by bovine retinal endothelial cells in culture. Biochem J. 1987 Aug 15;246(1):121–129. doi: 10.1042/bj2460121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. R., Kurkinen M., Taylor A., Hogan B. L. Studies on the biosynthesis of laminin by murine parietal endoderm cells. Eur J Biochem. 1981 Sep;119(1):189–197. doi: 10.1111/j.1432-1033.1981.tb05593.x. [DOI] [PubMed] [Google Scholar]
- Erickson L. A., Hekman C. M., Loskutoff D. J. The primary plasminogen-activator inhibitors in endothelial cells, platelets, serum, and plasma are immunologically related. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8710–8714. doi: 10.1073/pnas.82.24.8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson L. A., Lawrence D. A., Loskutoff D. J. Reverse fibrin autography: a method to detect and partially characterize protease inhibitors after sodium dodecyl sulfate--polyacrylamide gel electrophoresis. Anal Biochem. 1984 Mar;137(2):454–463. doi: 10.1016/0003-2697(84)90113-1. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Furcht L. T. Critical factors controlling angiogenesis: cell products, cell matrix, and growth factors. Lab Invest. 1986 Nov;55(5):505–509. [PubMed] [Google Scholar]
- Gelehrter T. D., Sznycer-Laszuk R. Thrombin induction of plasminogen activator-inhibitor in cultured human endothelial cells. J Clin Invest. 1986 Jan;77(1):165–169. doi: 10.1172/JCI112271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg D., Zeheb R., Yang A. Y., Rafferty U. M., Andreasen P. A., Nielsen L., Dano K., Lebo R. V., Gelehrter T. D. cDNA cloning of human plasminogen activator-inhibitor from endothelial cells. J Clin Invest. 1986 Dec;78(6):1673–1680. doi: 10.1172/JCI112761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. L., Moscatelli D., Jaffe E. A., Rifkin D. B. Plasminogen activator and collagenase production by cultured capillary endothelial cells. J Cell Biol. 1982 Dec;95(3):974–981. doi: 10.1083/jcb.95.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. H., Sheldon L. A., Fletcher C. F., Brinckerhoff C. E. Isolation of a collagenase cDNA clone and measurement of changing collagenase mRNA levels during induction in rabbit synovial fibroblasts. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1981–1985. doi: 10.1073/pnas.81.7.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham R. G. Dermal fibroblasts. Methods Cell Biol. 1980;21A:255–276. [PubMed] [Google Scholar]
- Hekman C. M., Loskutoff D. J. Endothelial cells produce a latent inhibitor of plasminogen activators that can be activated by denaturants. J Biol Chem. 1985 Sep 25;260(21):11581–11587. [PubMed] [Google Scholar]
- Kooistra T., Sprengers E. D., van Hinsbergh V. W. Rapid inactivation of the plasminogen-activator inhibitor upon secretion from cultured human endothelial cells. Biochem J. 1986 Nov 1;239(3):497–503. doi: 10.1042/bj2390497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laiho M., Saksela O., Andreasen P. A., Keski-Oja J. Enhanced production and extracellular deposition of the endothelial-type plasminogen activator inhibitor in cultured human lung fibroblasts by transforming growth factor-beta. J Cell Biol. 1986 Dec;103(6 Pt 1):2403–2410. doi: 10.1083/jcb.103.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Laug W. E. Secretion of plasminogen activators by cultured bovine endothelial cells: partial purification, characterization and evidence for multiple forms. Thromb Haemost. 1981 Jun 30;45(3):219–224. [PubMed] [Google Scholar]
- Laug W. E. Vascular smooth muscle cells inhibit the plasminogen activators secreted by endothelial cells. Thromb Haemost. 1985 Apr 22;53(2):165–169. [PubMed] [Google Scholar]
- Lawrence D. A., Loskutoff D. J. Inactivation of plasminogen activator inhibitor by oxidants. Biochemistry. 1986 Oct 21;25(21):6351–6355. doi: 10.1021/bi00369a001. [DOI] [PubMed] [Google Scholar]
- Levin E. G. Quantitation and properties of the active and latent plasminogen activator inhibitors in cultures of human endothelial cells. Blood. 1986 May;67(5):1309–1313. [PubMed] [Google Scholar]
- Levin E. G., Santell L. Association of a plasminogen activator inhibitor (PAI-1) with the growth substratum and membrane of human endothelial cells. J Cell Biol. 1987 Dec;105(6 Pt 1):2543–2549. doi: 10.1083/jcb.105.6.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischwe M. A., Ochs D. A new method for partial peptide mapping using N-chlorosuccinimide/urea and peptide silver staining in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1982 Dec;127(2):453–457. doi: 10.1016/0003-2697(82)90203-2. [DOI] [PubMed] [Google Scholar]
- Loskutoff D. J., Ny T., Sawdey M., Lawrence D. Fibrinolytic system of cultured endothelial cells: regulation by plasminogen activator inhibitor. J Cell Biochem. 1986;32(4):273–280. doi: 10.1002/jcb.240320404. [DOI] [PubMed] [Google Scholar]
- Loskutoff D. J., Sawdey M., Mimuro J. Type 1 plasminogen activator inhibitor. Prog Hemost Thromb. 1989;9:87–115. [PubMed] [Google Scholar]
- Loskutoff D. J., van Mourik J. A., Erickson L. A., Lawrence D. Detection of an unusually stable fibrinolytic inhibitor produced by bovine endothelial cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2956–2960. doi: 10.1073/pnas.80.10.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund L. R., Riccio A., Andreasen P. A., Nielsen L. S., Kristensen P., Laiho M., Saksela O., Blasi F., Danø K. Transforming growth factor-beta is a strong and fast acting positive regulator of the level of type-1 plasminogen activator inhibitor mRNA in WI-38 human lung fibroblasts. EMBO J. 1987 May;6(5):1281–1286. doi: 10.1002/j.1460-2075.1987.tb02365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D., Rifkin D. B. Membrane and matrix localization of proteinases: a common theme in tumor cell invasion and angiogenesis. Biochim Biophys Acta. 1988 Aug 3;948(1):67–85. doi: 10.1016/0304-419x(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Moscatelli D. Urokinase-type and tissue-type plasminogen activators have different distributions in cultured bovine capillary endothelial cells. J Cell Biochem. 1986;30(1):19–29. doi: 10.1002/jcb.240300104. [DOI] [PubMed] [Google Scholar]
- Ny T., Sawdey M., Lawrence D., Millan J. L., Loskutoff D. J. Cloning and sequence of a cDNA coding for the human beta-migrating endothelial-cell-type plasminogen activator inhibitor. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6776–6780. doi: 10.1073/pnas.83.18.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek H., Veerman H., Lambers H., Diergaarde P., Verweij C. L., van Zonneveld A. J., van Mourik J. A. Endothelial plasminogen activator inhibitor (PAI): a new member of the Serpin gene family. EMBO J. 1986 Oct;5(10):2539–2544. doi: 10.1002/j.1460-2075.1986.tb04532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöllänen J., Saksela O., Salonen E. M., Andreasen P., Nielsen L., Danø K., Vaheri A. Distinct localizations of urokinase-type plasminogen activator and its type 1 inhibitor under cultured human fibroblasts and sarcoma cells. J Cell Biol. 1987 Apr;104(4):1085–1096. doi: 10.1083/jcb.104.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich R., Thompson E. W., Iwamoto Y., Martin G. R., Deason J. R., Fuller G. C., Miskin R. Effects of inhibitors of plasminogen activator, serine proteinases, and collagenase IV on the invasion of basement membranes by metastatic cells. Cancer Res. 1988 Jun 15;48(12):3307–3312. [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Rifkin D. B. The opposing effects of basic fibroblast growth factor and transforming growth factor beta on the regulation of plasminogen activator activity in capillary endothelial cells. J Cell Biol. 1987 Aug;105(2):957–963. doi: 10.1083/jcb.105.2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O. Plasminogen activation and regulation of pericellular proteolysis. Biochim Biophys Acta. 1985 Nov 12;823(1):35–65. doi: 10.1016/0304-419x(85)90014-9. [DOI] [PubMed] [Google Scholar]
- Schor A. M., Schor S. L. The isolation and culture of endothelial cells and pericytes from the bovine retinal microvasculature: a comparative study with large vessel vascular cells. Microvasc Res. 1986 Jul;32(1):21–38. doi: 10.1016/0026-2862(86)90041-5. [DOI] [PubMed] [Google Scholar]
- Schor A. M., Schor S. L. Tumour angiogenesis. J Pathol. 1983 Nov;141(3):385–413. doi: 10.1002/path.1711410315. [DOI] [PubMed] [Google Scholar]
- Sprengers E. D., Kluft C. Plasminogen activator inhibitors. Blood. 1987 Feb;69(2):381–387. [PubMed] [Google Scholar]
- van Mourik J. A., Lawrence D. A., Loskutoff D. J. Purification of an inhibitor of plasminogen activator (antiactivator) synthesized by endothelial cells. J Biol Chem. 1984 Dec 10;259(23):14914–14921. [PubMed] [Google Scholar]