Abstract
This review describes the Hierarchical Taxonomy of Psychopathology (HiTOP) model of psychosis-related psychopathology, the psychosis superspectrum. The HiTOP psychosis superspectrum was developed to address shortcomings of traditional diagnoses for psychotic disorders and related conditions including low reliability, arbitrary boundaries between psychopathology and normality, high symptom co-occurrence, and heterogeneity within diagnostic categories. The psychosis superspectrum is a transdiagnostic dimensional model comprising two spectra—psychoticism and detachment—which are in turn broken down into fourteen narrow components, and two auxiliary domains—cognition and functional impairment. The structure of the spectra and their components are shown to parallel the genetic structure of psychosis and related traits. Psychoticism and detachment have distinct patterns of association with urbanicity, migrant and ethnic minority status, childhood adversity, and cannabis use. The superspectrum also provides a useful model for describing the emergence and course of psychosis, as components of the superspectrum are relatively stable over time. Changes in psychoticism predict the onset of psychosis-related psychopathology, whereas changes in detachment and cognition define later course. Implications of the superspectrum for genetic, socio-environmental, and longitudinal research are discussed. A companion review focuses on neurobiology, treatment response, and clinical utility of the superspectrum, and future research directions.
Limitations of traditional diagnosis
Traditional diagnoses of psychotic disorders, codified in the 5th edition of Diagnostic and Statistical Manual (DSM-5)1 and 11th edition of International Classification of Diseases (ICD-11),2 face major limitations. First, reliability of diagnoses is low. Interrater kappa ranged from .40 to .56 for schizophrenia, schizoaffective disorder, and bipolar disorders in the DSM-5 Field Trials.3 Likewise, the temporal stability of these diagnoses is low, with kappa of .13 to .65.4 Reliability problems are inherent in the categorical diagnosis, because extensive evidence indicates that symptoms of psychotic disorders are distributed continuously in the general population, making any diagnostic boundary arbitrary.5–7 In particular, the modal case is just above the threshold, and even more people fall right below the threshold, so small shifts in symptom report change diagnosis for many people.8 Moreover, numerous people experiencing subthreshold symptoms are not captured by traditional diagnoses despite substantial symptom burden.
Second, symptoms of psychotic disorders often co-occur with mood, substance use, and personality pathology symptoms.9–11 Traditional systems deal with this problem by creating mutually-exclusive diagnoses. This leads to proliferation of diagnostic categories and obscures commonalities among them in etiology and treatment response.12 Moreover, reasons for the high co-occurrence are ignored. These cross-cutting relationships can be described by transdiagnostic models of psychopathology. Meta-analytic evidence indicates the co-occurrence symptoms of psychotic disorders reflects two underlying dimensions—psychoticism and detachment—that are interrelated and jointly form the psychosis superspectrum.11
Third, psychotic disorders are heterogeneous. For example, positive and negative symptoms are uncorrelated among individuals with psychotic disorders,8,13,14 implying these symptoms reflect different processes. Traditional subtypes proved ineffective in characterizing this heterogeneity,15 but dimensional models revealed a consistent set of homogeneous constructs within the psychosis superspectrum.16–20
These problems are unsurprising, given that the mutually-exclusive nature of psychotic disorders was assumed, rather than inferred from data,6 an assumption that accumulated evidence contradicts.21 In addition, the traditional strategy for developing diagnoses focuses on external validity, and the process for proposing diagnostic criteria is not specified.22,23 Consequently, a diagnosis may have external validity but lack internal coherence. For example, Emil Kraepelin sorted patients into groups to maximize schizophrenia’s (dementia praecox’s) prognostic value.24 The resulting criteria are useful for prediction, but capture a group heterogeneous in clinical presentation and etiology, which undermines the value of diagnosis for treatment selection and drug development.25–27
An alternative strategy for nosologic discovery is construct validation, which systematically considers both external and internal validity.28 Factor analysis and structural equation modeling were developed to implement this approach.29,30 It begins by examining empirical associations among elements of psychopathology (e.g., signs and symptoms) to identify coherent and distinct constructs, which are then related to external criteria to determine their utility. This approach helped to develop classifications of affect, personality, and cognitive abilities.31–33 In psychiatry, it produced influential models and measures, such as the Child Behavior Checklist and Positive and Negative Syndrome Scale.34–37
This review describes the application of a construct validation approach to the nosology of psychotic disorders. The emerging quantitative nosology has generated a substantial literature reviewed in a prior publication.12 This review provides major updates based on new literature, expands on prior publications by addressing cognitive and functional impairment in psychosis, and covers new topics such as lifespan development.
The psychosis superspectrum in the Hierarchical Taxonomy of Psychopathology (HiTOP)
Principles.
The main objective of the HiTOP consortium—a collaboration of 175 psychologists and psychiatrists—is to improve the utility of psychiatric nosology for clinicians and scientists. HiTOP resolves the problem of arbitrary categories by using tools such as factor analysis and structural equation modeling to identify constructs which most parsimoniously describe observed correlations among symptoms.38 This approach produces reliable descriptions of psychopathology. For example, it decomposes the heterogeneous schizophrenia category into multiple symptom components that are highly internally consistent (Cronbach’s α ranging from 0.76 to 0.90).14,39,19 Constructs in the HiTOP model are based on symptom-level data, so the model itself is descriptive and does not speak to the factors driving that structure. However, greater reliability of measurement facilitates research into psychopathology’s causes and consequences.
The HiTOP approach addresses the problem of comorbidity through a hierarchy of constructs, from narrow to broad (depicted in Figure 1). As an example, symptom components that are highly related (e.g., inexpressivity and avolition) are grouped into the higher-order detachment spectrum. Detachment is related to the psychoticism spectrum, and together they form the psychosis superspectrum. At the broadest level, comorbidity between the psychosis superspectrum and other psychopathology is reflected by a general factor, or p-factor. This hierarchical approach results in coherent constructs at multiple levels of specificity, capturing comorbidity through higher-order dimensions above the level of traditional diagnoses, as well as symptom heterogeneity within diagnoses through more specific constructs. For example, two individuals with severe detachment may be differentiated by their unique profiles of narrower components, such as romantic disinterest and anhedonia. Unlike the DSM hierarchy of chapters and diagnostic specifiers, HiTOP superspectra and components were identified through converging results from structural analyses of large, epidemiological and clinical datasets.
Figure 1. HiTOP model of psychosis superspectrum.
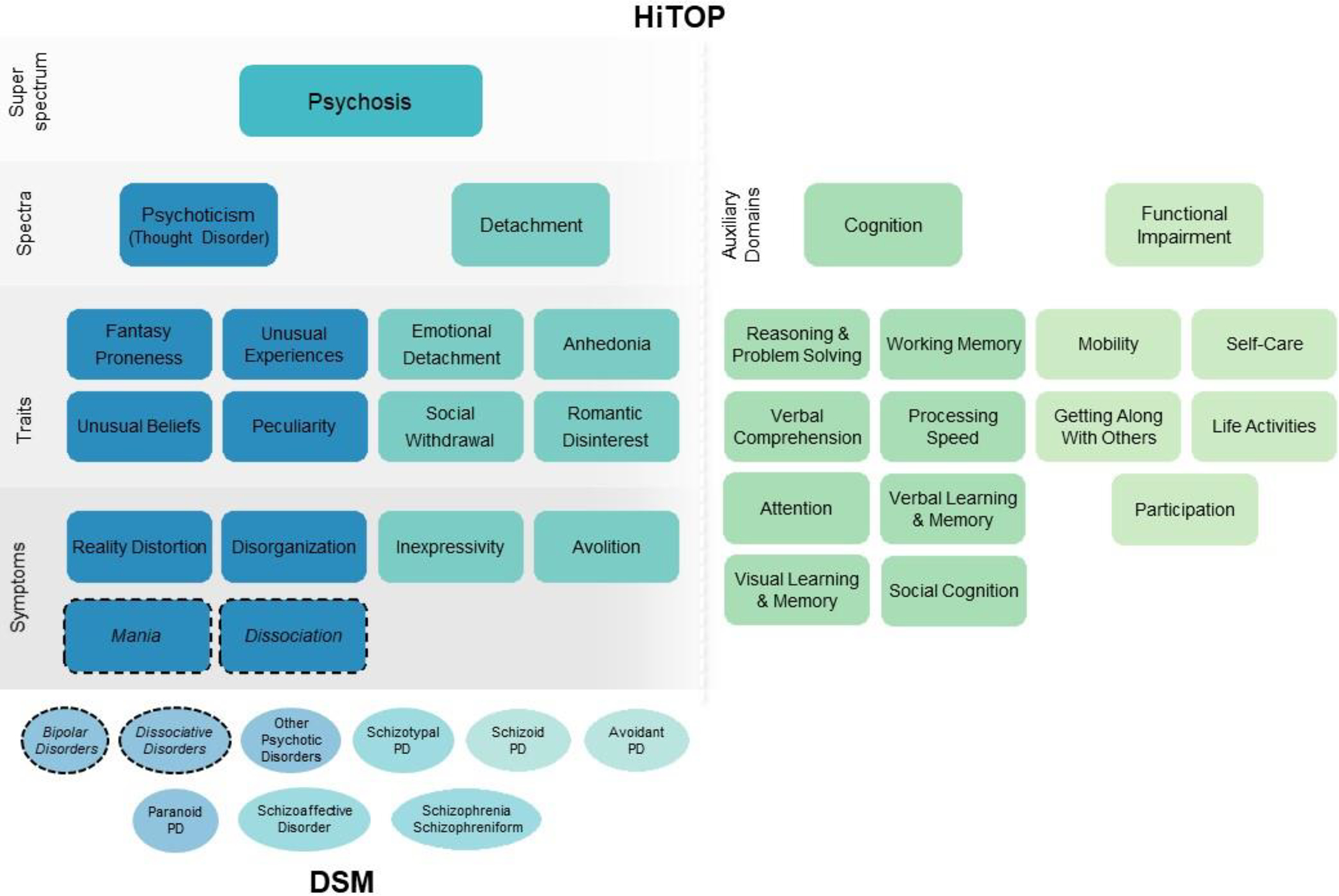
Note: Dashed circles indicate mania and dissociation are include in the model provisionally, because evidence supporting their inclusion is limited. Thought disorder is given in parentheses because it is the term currently used in HiTOP for the psychoticism spectrum.
HiTOP addresses arbitrary thresholds of severity through dimensional constructs. All HiTOP components are operationalized as continua, ranging from mild (e.g. social apprehension, unusual ideas) to severe (e.g. complete lack of social relationships, bizarre delusions). This facilitates the study and description of psychopathology along the same dimensions from the first signs observed in childhood, to first episode, to remission.
Model Content.
Figure 1 depicts the resulting model, a hierarchy of dimensional constructs. A meta-analysis of 35 structural studies comprising data from over 120,000 individuals describes the psychosis superspectrum defined by psychoticism and detachment, which together encompass psychotic disorders and some personality disorders, including schizotypal, schizoid, paranoid, and avoidant personality disorders.11
The HiTOP constructs outlined in Figure 1 are defined in Table 1. The superspectrum includes both maladaptive traits and symptom components. Usually components and traits parallel each other, and they are distinguished by timeframe: components capture psychological states (e.g., current or past month) and traits capture stable predispositions to these states.40 Indeed, evidence indicates clear alignment and continuity between positive schizotypy traits and positive symptoms as well as negative schizotypy traits and negative symptoms.12,41 However, some components (e.g., mania) and traits (e.g., fantasy proneness) do not have complements at the state or trait level. The superspectrum does not include depression because it falls within the emotional dysfunction superspectrum, and substance abuse because it is within the externalizing superspectrum.11,42,43 Instead, co-occurrence of psychosis with depression and substance misuse is captured by the general factor (p-factor), which reflects the tendency towards co-occurrence shared by most forms of psychopathology.44
Table 1.
Definitions of constructs within the psychosis superspectrum
| Construct | Definition |
|---|---|
|
| |
| Psychosis superspectrum | General individual difference in cognition and behavior that ranges from conventional and goal-oriented (low end) to bizarre and apathetic (high end). |
| Psychoticism (thought disorder) spectrum | General individual difference in perception, cognition, and behavior that ranges from conventional and uncreative (low end) to disconnected from reality (high end). |
| Fantasy Proneness trait | Tendency to fantasize, daydream, and become fully engrossed in one’s thoughts and experiences, sometimes becoming distracted and losing sight of reality. |
| Unusual Experiences trait | Tendency to experience perceptions that do not correspond to reality or impaired perception of self, including anomalous self-experiences, derealization, depersonalization, illusions, and frank hallucinations. |
| Unusual Beliefs trait | Tendency to hold unfounded and irrational thoughts, beliefs, and ideas about the world as well as frank delusions. This includes beliefs about the powers of oneself, others, and objects to control and influence others and the physical world. |
| Peculiarity trait | Tendency to exhibit behavior, speech, appearance, and mannerisms that are unusual, eccentric, or bizarre in person's culture. |
| Reality Distortion component | Acute experience of illusions, hallucinations, irrational thoughts, or frank delusions. |
| Disorganization component | Acute unusual or bizarre behavior, speech, mannerisms, or appearance. This includes formal thought disorder (i.e., impaired capacity to sustain coherent discourse). |
| Mania component | Acute euphoria and excessive goal-directed activity; hyperactive cognition and speech; overconfidence and grandiosity that may result in recklessness. |
| Dissociation component | Acute experience of depersonalization, derealization, dissociative amnesia, reduced awareness of the surroundings, or trance states |
| Detachment spectrum | General individual difference in volition, sociability, and affective expression that ranges from goal-oriented, sociable, and expressive behavior (low end) to apathy, disinterest in people, and blunted affect (high end) |
| Emotional Detachment trait | Tendency to be emotionally distant and reserved, impaired ability to experience, describe, and express feelings. |
| Anhedonia trait | Tendency to experience low levels of positive emotions (e.g., joy, confidence, alertness), energy, and interest in activities. |
| Social Withdrawal trait | Tendency to avoid interpersonal interactions or not initiate social contact, reticence in social situations, and a preference for being alone. |
| Romantic Disinterest trait | Tendency to lack of interest in, desire for, and enjoyment of sex, eroticism, and interpersonal intimacy. |
| Inexpressivity component | Acute deficit in vocal and facial expressions of affect, expressive gestures and eye contact, and alogia (e.g., poverty of speech, long latency of response) |
| Avolition component | Acute deficit in initiating or sustaining engagement in social, recreational, or occupational activities; remaining physically inert for long periods of time; deficit in positive emotions and energy; deficit in desire for intimacy or sex. |
Within the detachment spectrum, symptom components include inexpressivity and avolition, typically termed negative symptoms.12 Detachment also encompasses more stable traits and less severe forms of psychopathology via the maladaptive traits of emotional detachment, anhedonia, social withdrawal, and romantic disinterest (see Supplemental Table 1 in the companion paper for measures of detachment).45 At their most severe, these traits can also be considered negative symptoms, but commonly exist at lower levels of severity. Within the psychoticism spectrum, symptom components include reality distortion (hallucinations and delusions), disorganization, and provisionally, dissociation and mania. Mania’s placement is provisional because it shifts between psychoticism and internalizing spectra depending on the sample prevalence of mania and the assessment method.44,46–48 Dissociation’s placement is provisional because few structural studies included dissociation, although there is strong indirect evidence for its placement on the psychoticism spectrum.12,49 Dissociation encompasses anomalous self-experiences and other symptoms associated with disorders of self, which merge with dissociation in structural analyses.16 The spectrum also includes the maladaptive traits of fantasy proneness, unusual experiences, unusual beliefs, and peculiarity.12
Some structural studies propose more detailed models of symptoms and traits within these spectra.50–52 The HiTOP consortium is developing both a self-report measure and structured interview assessing detachment and thought disorder,16,53 which include many more maladaptive traits and symptom components. This work is ongoing, so the current model includes only the best-established lower-order constructs. The measure development process may validate more specific constructs, or may reveal that further differentiation is useful. Notably, the superspectrum is similar to the “psychosis spectrum” described by Guloksuz and van Os,54 with two small differences. First, Goluksuz and van Os’s spectrum includes depression, whereas depression is assigned to the internalizing spectrum in HiTOP. Second, the HiTOP psychosis superspectrum emphasizes the bifurcation of psychoticism and detachment, whereas Guloksuz and van Os focus on the broadest common factor of the psychosis superspectrum, rather than its elements.
Auxiliary domains.
While cognitive and functional deficits are central to the psychosis superspectrum, they have not yet been incorporated into the core HiTOP model. Accordingly, they are discussed here as auxiliary domains, with the intent that future revisions will formally include them.
The cognitive deficits associated with psychosis are broad, therefore the cognitive domain includes the same cognitive abilities in which the general population varies: processing speed, attention, working memory, verbal learning and memory, visual learning and memory, reasoning and problem solving, verbal comprehension, and social cognition.55 The HiTOP model of cognition vis-a-vis the psychosis superspectrum mirrors the domains identified by the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Neurocognition Committee.56 Note that while the MATRICS Committee concluded that verbal comprehension was impaired in schizophrenia, verbal comprehension was not included in the MATRICS battery, because the battery was designed to facilitate treatment development, and verbal comprehension is a very stable cognitive ability.56 However, since verbal comprehension is relevant to the description of psychosis,57 it is included here. Cognitive deficits associated with the psychosis superspectrum are largely trait-like.58,59
Cognitive deficits are a core feature of schizophrenia and related to most forms of psychopathology in the superspectrum. Cognitive decline precedes schizophrenia onset and is an important determinant of outcomes among people with schizophrenia.60 Cognitive abilities in other non-affective psychoses, bipolar disorder, and schizotypal personality disorder are intermediate between abilities observed in schizophrenia and healthy controls.61–64 Generalized cognitive impairment is particularly closely linked to the detachment spectrum, notably negative symptoms, and disorganization, but not reality distortion symptoms.65–67
Cognitive deficits are not limited to the superspectrum—they are observed in many other mental disorders,68 particularly disinhibited externalizing psychopathology69—but deficits are generally the largest in schizophrenia and bipolar disorder.68,70 How strongly cognition relates to underlying spectra is unknown. Cognitive deficits are listed as an auxiliary spectrum due to a lack of studies that examined the structure of psychopathology and cognitive functioning together. Further research is needed to determine which cognitive impairments are specific to the superspectrum, and which symptoms and traits within the psychosis superspectrum are most closely linked to various cognitive impairments. The HiTOP model can facilitate this research providing a comprehensive assessment of psychopathology across all severity levels, appropriate to both clinical and population samples. Box 1 illustrates an application of HiTOP to such a study design.
Box 1.
To illustrate application of HiTOP spectra to explication of connections between neurocognition and psychopathology, we leveraged the Philadelphia Neurodevelopmental Cohort (PNC). The PNC is a community sample of nearly 10,000 genotyped youth who were phenotyped in 2009–2011 as part of an ARRA Grand Opportunity (GO) project.248 Participants received a thorough clinical assessment using the GOASSESS, an adaptation of the K-SADS covering major DSM disorders. They also received a neurocognitive assessment, the Penn Computerized Neurocognitive Battery (PennCNB) that included 14 tests, providing measures of accuracy and speed on major neurocognitive domains.249
Psychoticism, externalizing, and two internalizing subdimensions (distress and fear) were linked to four cognitive abilities assessed by the PennCNB: general intelligence, social cognition, reasoning and problem solving, memory, and attention.250 A bifactor model was used to remove variance attributable to the p-factor from spectra, to focus on specific psychopathology-cognition links. The associations between HiTOP spectra and cognitive traits are reported in the Figure below.
Box Figure: Cognition by Spectrum.
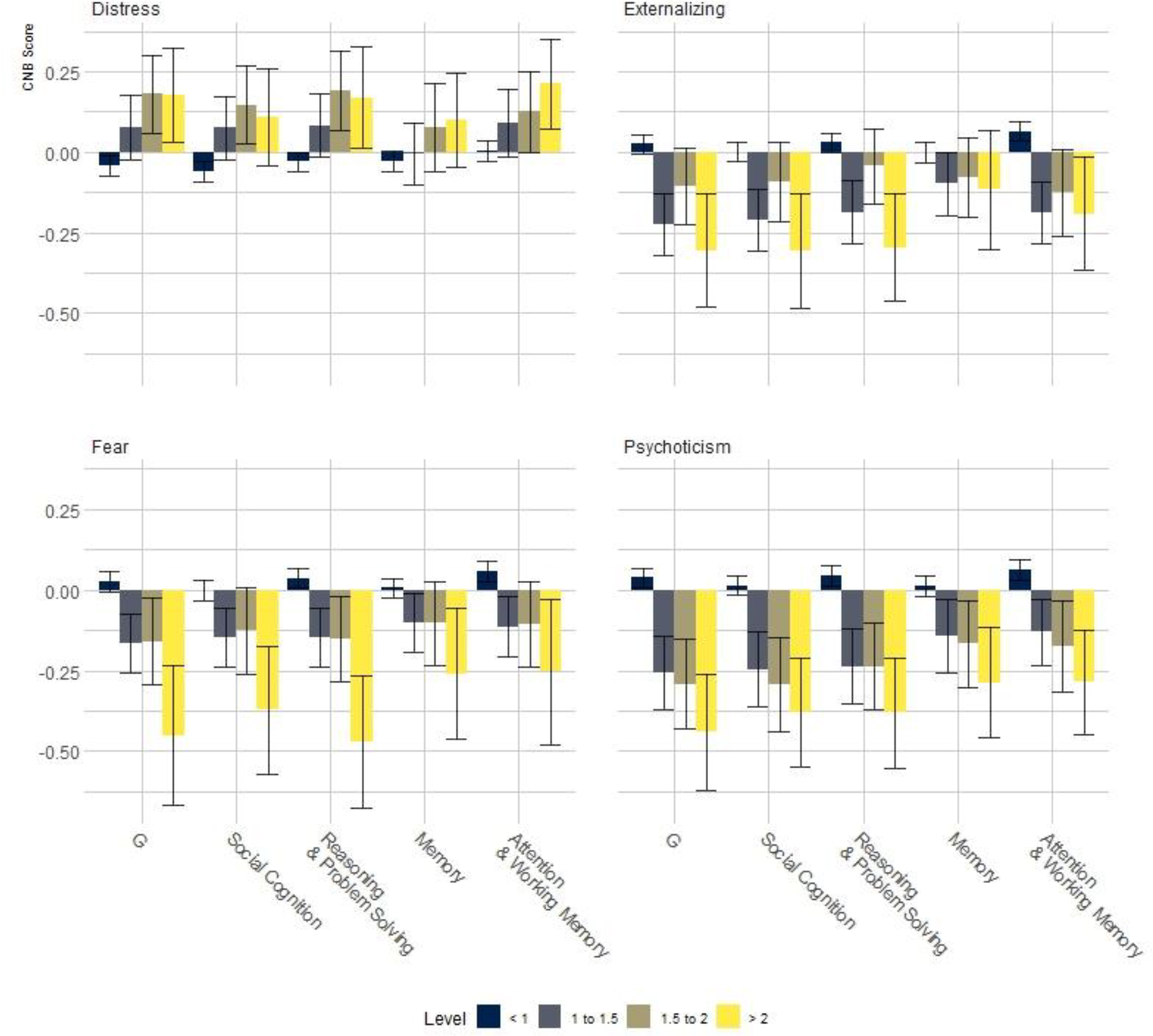
As can be seen in the Figure, the 200 males and 150 females in the highest percentile of psychoticism showed cognitive deficits with a moderate effect size (d>0.40), consistent with the magnitude of cognitive deficits observed in CHR-P and prodromal cohorts.251 The greatest deficits were observed in G, or the general cognitive factor, but deficits of similar magnitude were observed in social cognition, and reasoning & problem solving.
The effect sizes for externalizing are considerably smaller, but emerge at lower levels of severity. In contrast to all other psychopathology domains, the distress dimension was associated with better than average performance. This “paradoxical” effect of distress being positively associated with performance is likely due to these analyses controlling for the p-factor. In other words, psychopathology in general is associated with worse cognitive functioning, but when this effect is controlled, distress is associated with better functioning than psychosis or disinhibition.
The results presented here are sparse and designed to illustrate an approach for linking clinical with neurocognitive domains. We only show data concerning psychoticism, and not detachment. In addition, there could be scientifically and clinically meaningful links between specific traits and symptom components and cognitive abilities. There are other neurocognitive domains of high potential relevance to psychopathology dimension, like reward processing, or meta-cognitive traits such as insight, which may be informative.
Psychotic disorders are associated with functional decline. In the DSM, functional impairment or decline is incorporated into the diagnostic criteria. In the HiTOP model, functioning is assessed as a separate domain,71 and hence is included here as an auxiliary spectrum. This disentangles psychological dysfunction (e.g., paranoid ideation) from its sequelae for relationships, occupation, recreation, etc. The approach also facilitates tracking improvement and deterioration in functioning as well as in symptoms, as functional impairment may persist even in symptom remission.72 HiTOP captures classically deteriorating cases through elevations on both symptoms and functional impairment, but also describes people who diverge from this common pattern. This approach is consistent with the distinction that ICD-11 makes between disorder and disability.73
Functional impairment is multidimensional.74 The HiTOP model of functional impairment is based on the World Health Organization’s Disability Assessment Schedule (WHO-DAS),75 as the WHO-DAS was developed following the same principals of construct validation used to construct the HiTOP model.76 Functional impairment comprises mobility, self-care, getting along with others, life activities, and participation. The HiTOP model of functional impairment does not include a “cognition” domain, as this is well-described through the cognitive domain, and because self-reported cognitive ability is weakly correlated with psychometrically measured cognitive ability.77
The remainder of this review describes the genetic architecture, socio-environmental risk factors, and developmental trajectories of the superspectrum from the prodromal stage to late adulthood. The companion review covers neurobiology, animal research, and utility of the superspectrum model for treatment.45 Notably, diagnostic language is used to describe findings based on diagnostic phenotypes, which account for a majority of the extant literature. However, HiTOP allows us to move beyond the case-control design to multidimensional phenotypes, which describe continua of severity at various levels of specificity. Therefore, findings from diagnostic research are summarized in diagnostic terms, followed by statements describing their relevance to the psychosis superspectrum. Throughout both papers, implications for future research are discussed.
Genetic architecture of the superspectrum
The structure of genetic risk for psychosis and related psychopathology largely mirrors the phenotypic structure of the psychosis superspectrum. The estimated heritability of the psychosis superspectrum based on behavioral genetic research is 73%.78 Molecular genetic research also indicates common genetic risks underly psychotic disorders. The high genetic correlation between schizophrenia and bipolar disorder (rg=0.68)79 outlines a psychoticism dimension in genomic structural equation models.80 Within the superspectrum, behavioral genetic methods identify a genetic factor underpinning psychoticism,81–83 and a distinct factor underlying detachment.83–85 Schizophrenia GWAS appear to capture genetic variance underlying both psychoticism and detachment, as the schizophrenia polygenic risk score, which quantifies common genetic risk associated with the schizophrenia diagnosis, has sometimes been associated with measures of psychoticism,86 and sometimes with measures of detachment.87 In aggregate, however, polygenic risk for schizophrenia has more often been associated with detachment in both people with psychotic disorders88,89 and in population samples.90,91
Both behavioral and molecular genetic research identify strong genetic links between detachment and cognition. Behavioral genetic methods indicate that a large majority of the covariance between schizophrenia and cognition is driven by shared genetic risk factors.92–95 In molecular genetic approaches, shared genetic risk factors underlying the psychosis superspectrum and cognition are indicated by the significant genetic correlations between schizophrenia and intelligence. Genetic risk for schizophrenia has been shown to predict cognitive deficits in cases,87 nonpsychotic adults,96 and adolescents.89,97 The lack of a genetic correlation between bipolar disorder and intelligence (rg=−0.09)79 likely reflects that the association between detachment and cognition is stronger than that linking psychoticism and cognition, discussed above.
The genetic structure of symptom components is tentatively parallel to the phenotypic structure of the superspectrum. Behavioral genetic approaches in population samples estimate the heritability of symptom components ranges from 31–60%.98 A molecular genetic analysis of the Northern Finland Birth Cohort, a sample of non-psychotic individuals, identified a close genetic correlation between physical and social anhedonia (elements of detachment), moderate genetic correlations between hypomania and unusual experiences (elements of psychoticism), and weaker or even negative correlations between these two symptom clusters, mirroring the organization of the detachment and psychoticism spectra.99 While molecular genetic research has not yet identified replicable SNPs associated with specific symptoms of the psychosis superspectrum, a mega-analytic GWAS of three population-based samples of adolescents identified distinct, significant genetic liabilities for anhedonia, negative symptoms, and cognitive disorganization,100 the latter two symptom dimensions of which had significant genetic correlations with the schizophrenia diagnosis. Although evidence for specific genetic liabilities at narrower levels of the hierarchical model is weak, largely due to the difficulty of obtaining thorough phenotypic data on samples large enough for genetic analyses, the aforementioned analyses indicate the potential to parse genetic components of the psychosis superspectrum.
The analyses described above indicate common genetic risks underpin both subthreshold symptoms and traits and more severe symptoms. Behavioral genetic research identifies a dose-dependent association between familial history of psychotic disorders and psychotic symptoms across the continuum of severity, from no symptoms to subclinical forms of psychoticism, to psychotic disorders.101,102 Family transmission study showing siblings of probands with schizophrenia are more likely to have schizotypal personality disorder and other non-affective psychoses.103–105 Schizoid personality disorder is also more common among relatives of probands with schizophrenia and other non-affective psychoses106,107 compared to relatives of individuals with affective psychosis and controls. Genetic risk is specific to disorders within the psychosis superspectrum, as rates for affective and anxiety disorders among siblings were not elevated.
There is some evidence that psychotic experiences in the general population may be genetically more closely correlated to major depression (rg=0.46) and autism (rg=0.39) than schizophrenia (rg=0.21).108 However, comparisons of GWAS of subthreshold symptoms and case-control GWAS are confounded by differences in the mode of assessment (self-reported versus interviewer-rated symptoms), and by the exclusion of cases from these analyses, which truncates the dimension at its upper ranges. Its notable that parent-reported subthreshold symptoms reported in Pain and colleagues100 have significant genetic correlations with schizophrenia, and polygenic risk for self-reported psychotic experiences in the general population predict development of psychosis,108 supporting a link between subthreshold reality distortion and more severe degrees of psychoticism.
A HiTOP-based approach to gene discovery may help disentangle genetic liability for psychoticism, detachment, and cognition. Genetic risk for psychotic disorders is largely attributable to the cumulative effect of many common variants, and therefore approximates a normal distribution.109 Dimensional phenotypes, such as those described by the HiTOP psychosis superspectrum, most closely match the underlying distribution of genetic risk, and thereby increase statistical power for gene discovery.110 While the polygenic risk score for schizophrenia has often been associated with measures of detachment, indicating schizophrenia GWAS capture some degree of genetic variance underlying detachment, there has not yet been a GWAS with detachment itself as the target phenotype. A GWAS of psychoticism, detachment, and cognitive deficits has the potential to attenuate the high correlations among bipolar disorder, schizophrenia, and intelligence, and improve the specificity of genetic prediction. The hierarchical aspect of the model also has advantages for gene discovery. Directly phenotyping higher-order spectra has the potential to increase power for gene discovery, as suggested by novel genomic and transcriptomic hits identified via the psychoticism factor in genomic and transcriptomic structural equation models.80,111 As a complement, assessing narrower traits and states may increase the specificity of genetic findings and improve genetic prediction.112
In sum, both behavioral and molecular genetic methods indicate a common genetic liability underlies psychotic disorders, consistent with the HiTOP psychosis super-spectrum. More specific genetic risks underlying psychoticism and detachment have been identified in behavioral genetic research, but structural analyses of molecular data are limited by the lack of large-scale GWAS of the detachment spectrum. The fine-grained genetic structure of the super-spectrum remains unclear. Genetic research implementing assessments of the psychosis super-spectrum, or using the super-spectrum as a model for investigating genetic structure, can distinguish many of the factors confounded by case-control genome-wide association studies.
The superspectrum and socio-environmental risk factors
A wealth of evidence implicates socio-environmental factors in the etiology of the psychosis superspectrum. These factors include urbanicity,113–115 migrant and ethnic minority status,116–119 childhood adversity,120–123 and cannabis use.124–127 Meta-analyses on contributions of these socio-environmental factors suggest that each increases risk of psychotic disorder 2- to 4-fold,113,117,123,125,127 the effects show a dose-response gradient,124,125,127–129 and population attributable risk fractions are 20%–35%.125,130,131
Minority status increases risk of symptoms across the psychosis superspectrum, although evidence for the link between minority status and psychoticism are stronger than links with other spectra. Elevated incidence of non-affective and affective psychotic disorders has consistently been found in migrant and ethnic minority groups across several countries.116–119 The largest incidence study to date confirmed this effect in 6 European countries.116 Few studies have investigated associations between ethnic minority status and specific symptom components of the psychosis superspectrum, but those that have find more severe reality distortion in patients from ethnic minority than from majority groups.132–134 Ethnic minority status also has been linked to greater reality distortion in the general population.7,135,136 These effects can be explained, at least in part, by greater exposure to social adversity among minorities.118,119,137 Ethnic minority status is also associated with greater detachment132,133,138 and disorganization,133,134 albeit these effects are weaker and less consistent.
Associations between urbanicity and individual symptom components of the psychosis superspectrum are understudied. Available evidence suggests that urbanicity is associated with more severe detachment and disorganization among cases.134 In the general population, research generally focused on effects of urbanicity on reality distortion.115,139,140 Given the temporal priority and dose-response gradient in effect of urbanicity on the superspectrum,114 confounds such as social drift are unlikely to account for this effect. However, the association between urbanicity and psychotic disorders does not seem to hold in low- and middle-income countries, where urbanicity may index greater access to resources in some societies and greater exposure to adversity in others.141 Hence, the role of contextual and area-level factors is central for understanding the role of urbanicity in the superspectrum.
Extensive evidence supports the role of childhood adversity as an important risk factor for the psychosis superspectrum.120,121,123,142,143 The effect has been reported for both traits and symptom components. At trait level, consistent evidence indicates association with psychoticism, and emerging evidence supports an association with detachment.144,145 At symptom level, a clear link has been consistently reported for reality distortion, whereas associations with detachment and disorganization are substantially weaker.120,146 Childhood adversities are associated with various forms of psychopathology, but there is preliminary evidence that certain types of exposures are relatively specific to the psychosis superspectrum, including interpersonal violence, hostility, and threat.128,143 This potential specificity needs to be verified further by taking careful account of exposure timing and validity of symptom outcome measures.
Cannabis use has been consistently linked to psychopathology across the psychosis superspectrum,126,147–150 with the weight of evidence supporting a dose-dependent association between cannabis and psychoticism specifically.127,151 Furthermore, psychotic disorders were strongly associated with premorbid exposure to high-potency cannabis in a recent large study in 6 European countries.125 In the general population, numerous studies observed that cannabis use predicts future reality distortion symptoms.7 Some studies also found an increase in detachment, although the effect is much less consistent,152 and no data are available on disorganization. In patients, cannabis exposure has been linked to elevated reality distortion, whereas no consistent effect was observed for detachment and disorganization.153–155 Research on maladaptive traits found the strongest associations between cannabis use and unusual experiences and beliefs, whereas links to disorganization and detachment were weaker.124,156
Overall, some methodological issues remain in validating associations between socio-environmental exposures and the psychosis superspectrum. Especially notable is the relative emphasis in epidemiological research on identifying risks linked to psychoticism, rather than detachment. Other outstanding issues include possible reverse causality, confounding by genetic and other factors, and publication biases. Nevertheless, the available evidence indicates each factor contributes most strongly to reality distortion and related traits of unusual experiences and unusual beliefs. The effects on disorganization and detachment are weaker, and an important research priority is to identify socio-environmental factors specific to these elements of the superspectrum. The diathesis-stress model hypothesizes genetic vulnerabilities moderate effects of socio-environmental factors on psychopathology.157,158 While there are some promising results in large studies,159 most gene-environment interactions await replication.
Development of the Superspectrum
The psychosis superspectrum has its origins in childhood, long before the emergence of clinically significant symptoms. Below we review the trajectories of psychoticism, detachment, cognitive impairment, and functional impairment from childhood, through the clinical-high risk for psychosis (CHR-P) phase, into first episode and post-onset periods.
Development of psychoticism.
Individual differences in psychoticism are apparent by middle school.160 These traits show moderate rank-order stability from age 7 to age 12,161 from early adolescence to late adolescence,162–164 and from late adolescence to early adulthood (r=0.33–0.42).165 Early-life psychoticism predicts subsequent onset of psychotic disorders,166–168 and is a risk factor primarily for reality distortion symptoms.169–171
CHR-P can be considered an intermediate stage between subclinical manifestations of psychoticism observed in childhood and reality distortion typically emerging in early adulthood. The vast majority (85–95%) of CHR-P individuals meet criteria based on the presence of attenuated psychotic symptoms—that is, subthreshold reality distortion—and the rest either have brief, limited psychotic symptoms or genetic risk and functional deterioration.172,173 Although CHR-P status emphasizes the state of experiencing subthreshold psychoticism, risk traits are also apparent in this population. Paranoid and schizotypal personality disorders are common among CHR-P cases.174 Nevertheless, psychoticism abates among a sizable fraction of the CHR-P population,175 implying significant state variability.
CHR-P is a prodromal stage specific to the psychosis superspectrum. Although over half of CHR-P cases meet criteria for non-psychotic disorders,172,173 these rates of comorbidity are comparable to rates observed in schizophrenia,176,177 and CHR-P cases are not at an increased risk for onset or persistence of non-psychotic disorders.178,179 Additionally, most CHR-P individuals do not progress to threshold psychosis,180 consistent with the conceptualization of reality distortion as a continuum in which subthreshold experiences are more common than persistent psychosis.181
Over 2–3 years, reality distortion progresses to full psychotic intensity among 15–25% of CHR-P individuals.182 Reality distortion symptoms are the strongest predictor of transition to psychotic disorder in CHR-P,166,183 but personality disorders do not consistently predict transition.174 This may be due to their heterogeneity, as conditions such as schizotypal personality disorder encompass both psychoticism and detachment. Importantly, transition is defined as reality distortion reaching the intensity of frank and persistent hallucinations or delusions; hence, psychoticism is more relevant to predicting transition than detachment (i.e., for a person whose reality distortion is just short of frank psychosis, even a small exacerbation would result in transition).184 Were there a parallel concept of transition to pathological detachment, presumably milder manifestations of detachment would predict transition better than reality distortion.
Psychoticism is relatively stable in the post-onset period. Studies of people with personality disorders found a 10-year stability coefficient of r=0.66 for psychoticism.185 In cohort of individuals with psychotic disorders, psychoticism symptoms are somewhat less stable.14,186 For example, a 20-year follow-up of patients with first-admission psychosis found stability of psychoticism to be r=0.21.14
Mean-level symptom trajectories provide another perspective on course. However, trajectories can be affected by the choice of baseline, as studies beginning at first admission with psychosis observe an initial decrease in symptoms due to treatment initiation and regression to the mean (i.e., all participants are in a psychotic episode at baseline due to the sampling strategy, but many will be in remission at each follow-up). When the baseline is not tied to a common event (e.g., baseline is several months after first admission), trajectories of psychoticism are largely flat for the next 10 to 20 years.187–189 This indicates the average symptom burden remains consistent, although individual patients may improve or worsen over time. Likewise, schizotypal personality disorder follows a steady long-term symptom trajectory.190
In sum, psychoticism as a trait emerges in childhood. Those with elevated psychoticism in early adolescence are most likely to experience clinically-significant psychoticism in the future. However, psychoticism is more variable than other spectra in the HiTOP psychosis super-spectrum, such that it is difficult to predict who will transition into a more severe state. Furthermore, in samples ascertained on the basis of psychoticism, such as CHR-P and first-episode cohorts, the most common trajectory is one of regression toward the mean, and the abatement of psychoticism.
Development of detachment.
Individual differences in detachment are already apparent by preschool.160 Detachment has moderate rank-order stability in this period.191 Likewise, stability is moderate from age 16 to 22 for detachment (r=.51).165 Detachment in early adolescence predicts subsequent schizophrenia onset.192 Detachment is primarily a predictor of later negative symptoms.169–171,193
While CHR-P status privileges reality distortion, this population is heterogeneous and many experience elevated detachment.194 In CHR-P, detachment is associated with greater functional and cognitive impairment.195,196 Schizoid personality disorder is observed in CHR-P174 and is stable over time,197 indicating elevated detachment. Indeed, symptoms of detachment are largely explained by stable differences, with detachment being more trait-like than psychoticism.198
Detachment remains highly stable through first-episode and into the post-onset period. Among individuals with personality disorders, the 10-year stability of detachment is r=0.82.185 Among individuals with psychotic disorders,14,186 symptoms of detachment are nearly twice as stable as symptoms of psychoticism (detachment r=0.38, psychoticism r=0.21).14 These findings parallel the greater stability for detachment than psychoticism in CHR-P198 and general youth samples165 discussed above. Mean-levels of detachment, like psychoticism, are largely flat in the 10–20 years following first onset.187,188 In sum, the detachment spectrum emerges in childhood, with early individual differences predicting later development of more severe manifestations of the spectrum. Among those with psychotic disorders, detachment is remarkably stable into late adulthood.
Importantly, psychoticism and detachment develop largely independently with minimal (r<0.12) cross-lagged correlations between them.165 Both traits also predict future internalizing and externalizing problems, but these effects are weaker indicating psychoticism and detachment are relatively specific risk factors for psychotic disorders.162,164,167,171 Among those with psychotic disorders, the two spectra evolve largely independently.199,200 Autocorrelations within spectra are much higher than lagged correlations between spectra (r=0.08).14 In sum, the low magnitude of cross-lagged correlations, relative to auto-correlations, supports psychoticism and detachment as independent spectra with unique contributions to the psychosis superspectrum. Individuals largely progress along the spectrum (or spectra) on which they showed an initial elevation.
Development of cognitive impairment.
Neurodevelopmental evidence suggests cognitive trajectories among those described by the psychosis superspectrum are perturbed in early adolescence, if not childhood. However, the neurodevelopmental theory of psychosis is the subject of considerable interest and debate.201–203 The evidence is inconclusive, and limited by the lack of longitudinal data on less severe manifestations of these spectra, but some inferences can be drawn by comparing neurodevelopment in healthy, CHR-P, and psychosis cohorts.
Conceptualizing schizophrenia as a neurodevelopmental disorder implies cognitive development is consistently deficient, lagging, or declining among individuals who go on to develop both psychoticism and detachment. Some data suggest individuals who are later diagnosed with schizophrenia have slowed cognitive development in childhood, consistent with the neurodevelopmental hypothesis.204 However, cognitive trajectories are best described relative to psychosis onset, rather than chronological age.205 When described relative to psychosis onset, cognitive development is normal until 14 years before psychosis onset.205 Consequentially, individuals who experience psychosis onset in their early 20s may have already begun to experience cognitive decline at the chronological ages described by Reichenberg et al.204 Furthermore, while individuals who go on to develop schizophrenia may have lower cognitive abilities than controls, in childhood future cases seem indistinguishable from their unaffected siblings.206 This implies that childhood cognitive deficits perhaps are not risk factors for psychosis beyond a general familial liability. When measured relative to psychosis onset, cognitive development among individuals with psychotic disorders is normal, but diverges from controls more than a decade before psychosis onset, grows to about 0.50 standard deviations (SD) below population average by the CHR-P phase. By first onset, cognitive deficits have grown to more than 1.00 SD below average.59,205,207–209 Cognitive deficits predict transition in CHR-P cohorts, but to a lesser degree than reality distortion.166
Structural imaging data indicates neural development largely parallels cognitive function in the psychosis superspectrum. Notably, the structural differences observed in psychotic disorders are observed to a lesser degree in unaffected relatives,210 and with increasing prominence in clinical high risk individuals,211 adolescents who have experienced psychotic symptoms,212 individuals recently diagnosed with schizophrenia, and chronic schizophrenia.213 Unfortunately, it is not possible to disentangle the effects of severity and illness course in these studies. However, while structural differences have been shown to be associated with genetic liability for psychotic disorders,214 these links almost entirely explained by individual differences in cognitive function.210,215 These data and evidence that cognitive decline precedes psychosis onset indicate the centrality of cognitive development to the psychosis superspectrum.
Cognition is quite stable in the general population216 and in the psychosis superspectrum, meaning long follow-up periods are necessary in order to detect cognitive change in the post-onset period. Short-term follow-up studies of psychotic disorder found greater deficits in schizophrenia than in bipolar disorder but observed little change over time.58,61,217 However, studies following patients for 10–25 years observed gradual cognitive decline.57,205,218 Although small on a year-by-year basis, these declined accrue over the illness course, manifesting as a high dementia incidence later in life.219
To summarize, cognitive deficits associated with the psychosis super-spectrum emerge more than a decade before the emergence of clinically-significant psychoticism. The rate of cognitive decline is quite slow, but losses accrued over the lifespan are significant. There is some evidence that the rate of cognitive decline accelerates in early adulthood, with many individuals meeting criteria for dementia.
Although there is little data on how strongly cognitive change contributes to psychoticism versus detachment, premorbid cognitive decline is much larger for schizophrenia than bipolar and other psychotic disorders.205,220 Cognitive development appears to be unperturbed among those who do not develop detachment symptoms, consistent with a stronger link between cognition and detachment than with psychoticism. More nuanced dynamics of cognition, detachment, and psychoticism and their interplay are poorly understood, in part due to the long time-scales needed to detect cognitive change.
Development of functional impairment.
Functional impairment, like other spectra within the psychotic superspectrum, begins to emerge in childhood. Those who go on to develop psychotic disorders have impaired psychosocial function in childhood, with those later diagnosed with schizophrenia having greater impairment than those diagnosed with affective psychoses.221,222 Even individuals with subthreshold symptoms of the psychosis superspectrum experience impairment. In the general population, the full range of reality distortion, from subtle illusions to well-formed hallucinations, is associated with psychosocial impairment in a dose-dependent fashion.223 Real-world functioning is significantly impaired in CHR-P,224 and psychosocial impairment predicts transition, although to a lesser degree than reality distortion.166
In the post-onset period, functional impairment tends to be stable over time,188,221,225 with an apparent association between the burden of detachment and greater functional impairment. This is reflected in diagnostic data, as recovery rates are persistently lower (20 to 30%) in schizophrenia than in affective psychoses (85%).72,226 Likewise, employment rates are 30% in schizophrenia compared to 58% in affective psychoses.227 When detachment and psychoticism are measured directly, greater detachment predicts worse functional outcomes even decades later, whereas psychoticism is not predictive.228,229 In sum, functional impairments associated with the psychosis superspectrum emerge early, and are stable over time. The links between detachment and functional impairment are particularly strong.
Implications of the psychosis-superspectrum for studies of course.
The HiTOP model provides a framework for a transdiagnostic staging model that describes trajectories of psychoticism, detachment, cognition, psychosocial function, and other spectra across the lifespan.230–232 The superspectrum model may be most useful in childhood and adolescence, as the DSM-5 has no descriptors for the pre-clinical period aside from Attenuated Psychosis Syndrome, in Section III. However, this category privileges psychoticism over other symptom spectra, and does not include individuals with subthreshold symptoms without functional impairment. HiTOP identifies dimensions (Figure 1) for risk assessment prior to psychosis onset in youth. Moreover, repeated assessments can explicate progression from risk to clinical problems or course patterns in treated samples as individual trajectories. Fine-grained, reliable measures are available for such longitudinal tracing and have been validated in children, adolescents, and adults (see companion paper).45
The superspectrum model can also aid in the assessment and further evolution of CHR-P models, by expanding these paradigms beyond the transition to frank psychosis.184,233 First, dimensional conceptualization enables research on symptom exacerbations that have not reached full psychotic severity, thus expanding the scope beyond transition. Second, constructs in Figure 1 can facilitate research on other clinically-significant outcomes (e.g., worsening detachment, functional deterioration). Multidimensional risk and outcome assessments can provide critical evidence on the heterotypic (pluripotent) versus homotypic continuity between clinical risk and full-blown disorders. For example, psychopathology other than CHR-P also increases psychosis risk (although much less than CHR-P),54,234 and CHR-P often leads to non-psychotic disorders.180 This heterotypy may reflect correlations between different spectra, a hypothesis that could be tested within the HiTOP framework. Hence, clinical staging and CHR-P concepts describe common patterns of illness course that, overlaid onto HiTOP dimensions, offer a heuristic guide to treatment decisions. In return, HiTOP offers staging models a multidimensional characterization of stages based on extensive structural and psychometric research. Moreover, profiling at-risk individuals on lower-order dimensions within the two spectra is expected to increase prediction accuracy and specificity.
The psychosis superspectrum also has advantages for describing post-onset course. In diagnostic frameworks, course is typically characterized in terms of remission, the criteria for which combine psychoticism and detachment symptoms.235 Lower rates of remission in schizophrenia (56%) and schizotypal personality disorder (46%) compared to affective psychosis (79%) are consistent with the higher burden of detachment in these disorders.72,236 Similarly, definitions of recovery combine psychoticism, detachment, and functional impairment.237 These traditional operationalizations of course provide useful clinical benchmarks but miss many details. The HiTOP psychosis superspectrum offers advantages over existing operationalizations by specifying remission for each spectrum, and distinguishing functional recovery from symptomatic remission. In addition, dimensional models can capture subtle changes in symptom severity that are missed by dichotomous constructs, because small changes rarely move an individual across a threshold. Topics such as the impact of repeated psychotic episodes on antipsychotic efficacy238,239 can be studied as interactions of time, treatment, and symptom severity.
The psychosis superspectrum provides a useful framework for studying the course of psychoticism, detachment, cognition, and functional impairment. However, it does not yet include temporal patterns themselves. Patterns such as psychosis recurrence or co-occurrence between psychosis and mania over time have major implications for treatment and prognosis.240,241 Traditional nosologies attempt to capture this information using heuristic categories, such as course specifiers (e.g., first episode vs. multiple episodes) and diagnoses (e.g., bipolar disorder with psychosis vs. schizoaffective disorder vs. schizophrenia). Research is ongoing to identify empirical and quantitative course characteristics.242–244 For example, recurrence frequency can be represented by within-person symptom variability of co-occurrence by within-person symptoms correlations. The number of potential quantitative characteristics is large, including intercept (propensity to experience symptoms), slope of the trajectory (direction and rate of change), autoregressive effects (symptom stability), cross-lagged effects (shifts in presentation), and many others. The literature on the quantitative characteristics of course is growing, but to incorporate these features into HiTOP, structural analyses needed to consider course-based constructs together with existing HiTOP constructs, and explicate relations between them. Such integrative studies are rare,245 but as science advances it will become possible to include course features in the superspectrum model. At present, the model captures the distinction between symptoms and traits (i.e., current acute state and persistent problems). This distinction offers only a limited characterization of course but already has proven to increase utility of the model beyond symptoms alone.246
Conclusions
The HiTOP psychosis superspectrum, a hierarchical, transdiagnostic model of psychosis-related psychopathology, shows promise as an alternative to DSM and ICD diagnoses. Derived through construct validation,28 the psychosis superspectrum captures both the comorbidity and heterogeneity through a hierarchical structure, with constructs reflecting varying degrees of breadth versus specificity. The model accounts for heterogeneity by distinguishing psychoticism—which includes hallucinations, delusions, disorganization, and maladaptive traits such as unusual beliefs and peculiarity—from detachment—which includes avolition, inexpressivity, and traits such as anhedonia and social withdrawal. Auxiliary domains include cognitive and functional deficits, completing the profile of psychotic disorders and related psychopathologies.
The psychosis superspectrum’s higher-order structure, including psychoticism, detachment, and cognitive deficits, mirrors the structure of genetic risk described by behavioral and molecular genetic research. Although genetic data on narrower components of the superspectrum are less common, existing evidence hints at the potential for identifying unique genetic liabilities underlying traits and symptom components. The psychosis superspectrum also informs the understanding of environmental and societal risk factors associated with psychotic disorders, as established risks are more strongly associated with psychoticism than detachment. Together, the interaction of genetic risk and environmental exposures may increase risk for schizophrenia spectrum disorders,159 although these findings need to be replicated. A HiTOP approach to quantifying genetic risk and environmental stressors may facilitate more precise, powerful tests of the diathesis-stress model.
The HiTOP model’s dimensional nature is particularly useful for studies of the development of psychosis, facilitating models of developmental trajectories leading to clinical problems. Transdiagnostic elements facilitate the comprehensive investigation of outcomes of at-risk states, beyond the onset of frank psychosis. While longitudinal studies of psychotic disorders have illustrated the course of psychoticism and detachment, as well as cognition and functioning, more research is needed on the longitudinal course of maladaptive traits and subthreshold symptoms.
The HiTOP model is intended to reflect currently available structural and validation evidence. As such, it is continually under development. The provisional status of numerous elements, including mania, dissociation, cognitive deficits, and functional impairment, are notable limitations. The model also is limited in how it captures illness course. Efforts to address these limitations are ongoing.
The companion paper completes this review by considering data on neurobiology, treatment response, clinical utility, and measurement.45
Declaration of Interest:
This research was supported by the National Institute of Mental Health: R21MH123908 (KG Jonas) and R01MH122537 (R Kotov).
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). (American Psychiatric Pub, 2013). [Google Scholar]
- 2.World Health Organization. International statistical classification of diseases and related health problems. (2019). [Google Scholar]
- 3.Regier DA et al. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am. J. Psychiatry 170, 59–70 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Bromet EJ et al. Diagnostic Shifts During the Decade Following First Admission for Psychosis. Am. J. Psychiatry 168, 1186–1194 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haslam N, McGrath MJ, Viechtbauer W & Kuppens P Dimensions over categories: A meta-analysis of taxometric research. Psychol. Med. 50, 1418–1432 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Krueger RF et al. Progress in achieving quantitative classification of psychopathology. World Psychiatry 17, 282–293 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linscott RJ & van Os J An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol. Med. 43, 1133–1149 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Kotov R, Jonas K, Lian W, Docherty AR & Carpenter WT Reconceptualizing schizophrenia in the Hierarchical Taxonomy Of Psychopathology (HiTOP). Schizophr. Res. 242, 73–77 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotov R et al. New dimensions in the quantitative classification of mental illness. Arch. Gen. Psychiatry 68, 1003–1011 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Markon KE Modeling psychopathology structure: a symptom-level analysis of Axis I and II disorders. Psychol. Med. 40, 273–288 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Ringwald WR, Forbes MK & Wright AG Meta-analysis of structural evidence for the Hierarchical Taxonomy of Psychopathology (HiTOP) model. Psychol. Med. 1–14 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Kotov R et al. Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): I. Psychosis superspectrum. World Psychiatry 19, 151–172 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuesta M & Peralta V Integrating psychopathological dimensions in functional psychoses: a hierarchical approach. Schizophr. Res. 52, 215–229 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Kotov R et al. Validating dimensions of psychosis symptomatology: Neural correlates and 20-year outcomes. J. Abnorm. Psychol. 125, 1103 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tandon R et al. Definition and description of schizophrenia in the DSM-5. Schizophr. Res. 150, 3–10 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Cicero DC et al. Development of the Thought Disorder Measure for the Hierarchical Taxonomy of Psychopathology. Assessment 29, 46–61 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crego C & Widiger TA Convergent and discriminant validity of alternative measures of maladaptive personality traits. Psychol. Assess. 28, 1561–1575 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Grube BS, Bilder RM & Goldman RS Meta-analysis of symptom factors in schizophrenia. Schizophr. Res. 31, 113–120 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Kring AM, Gur RE, Blanchard JJ, Horan WP & Reise SP The clinical assessment interview for negative symptoms (CAINS): final development and validation. Am. J. Psychiatry 170, 165–172 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longenecker JM, Haas GL & Salisbury DF Hierarchical Symptom Components in Early Psychosis. Schizophr. Bull. (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotov R et al. The Hierarchical Taxonomy of Psychopathology (HiTOP): a dimensional alternative to traditional nosologies. J. Abnorm. Psychol. 126, 454 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Andrews G et al. Exploring the feasibility of a meta-structure for DSM-V and ICD-11: could it improve utility and validity? Psychol. Med. 39, 1993–2000 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Robins E & Guze SB Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. Am. J. Psychiatry 126, 983–987 (1970). [DOI] [PubMed] [Google Scholar]
- 24.Jablensky A Living in a Kraepelinian world: Kraepelin’s impact on modern psychiatry. Hist. Psychiatry 18, (2007). [DOI] [PubMed] [Google Scholar]
- 25.Carpenter WT & Koenig JI The evolution of drug development in schizophrenia: past issues and future opportunities. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 33, 2061–2079 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lally J & MacCabe JH Antipsychotic medication in schizophrenia: a review. Br. Med. Bull. 114, 169–179 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Young JW, Zhou X & Geyer MA Animal models of schizophrenia. Behav. Neurobiol. Schizophr. Its Treat. 391–433 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Cronbach LJ & Meehl. Construct validity in psychological tests. Psychol. Bull. 52, 281–302 (1955). [DOI] [PubMed] [Google Scholar]
- 29.Clark LA & Watson D Constructing validity: New developments in creating objective measuring instruments. Psychol. Assess. (2019) doi: 10.1037/pas0000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loevinger J Objective tests as instruments of psychological theory. Psychol. Rep. 3, 635–694 (1957). [Google Scholar]
- 31.Costa PT & McCrae RR The Revised NEO Personality Inventory (NEO-PI-R). (Sage Publications, Inc, 2008). [Google Scholar]
- 32.McGrew KS CHC theory and the human cognitive abilities project: Standing on the shoulders of the giants of psychometric intelligence research. Intelligence 37, 1–10 (2009). [Google Scholar]
- 33.Watson D Mood and temperament. (Guilford Press, 2000). [Google Scholar]
- 34.Achenbach TM The classification of children’s psychiatric symptoms: a factor-analytic study. Psychol. Monogr. Gen. Appl. 80, 1–37 (1966). [DOI] [PubMed] [Google Scholar]
- 35.Kay SR, Fiszbein A & Opler LA The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 13, 261–276 (1987). [DOI] [PubMed] [Google Scholar]
- 36.Lorr M, Klett CJ & McNair DM Syndromes of psychosis. (Pergamon Press, 1963). [Google Scholar]
- 37.Moore T The empirical determination of certain syndromes underlying praecox and manic-depressive psychoses. in (1929). [Google Scholar]
- 38.Jonas KG & Markon KE A descriptivist approach to trait conceptualization and inference. Psychol. Rev. 123, 90 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Emsley R, Rabinowitz J, Torreman M, Group, R.-I.−35 E. P. G. W. & others. The factor structure for the Positive and Negative Syndrome Scale (PANSS) in recent-onset psychosis. Schizophr. Res. 61, 47–57 (2003). [DOI] [PubMed] [Google Scholar]
- 40.DeYoung CG et al. The distinction between symptoms and traits in the Hierarchical Taxonomy of Psychopathology (HiTOP). J. Pers. 90, 20–33 (2022). [DOI] [PubMed] [Google Scholar]
- 41.Cicero DC, Jonas KG, Li K, Perlman G & Kotov R Common taxonomy of traits and symptoms: linking schizophrenia symptoms, schizotypy, and normal personality. Schizophr. Bull. 45, 1336–1348 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krueger RF et al. Validity and utility of hierarchical taxonomy of psychopathology (HiTOP): II. Externalizing superspectrum. World Psychiatry 20, 171–193 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watson D et al. Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): III. Emotional dysfunction superspectrum. World Psychiatry 21, 26–54 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caspi A et al. The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clin. Psychol. Sci. 2, 119–137 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kotov R et al. Psychosis Superspectrum II: Neurobiology, Treatment, and Implications. (in review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotov et al. Schizophrenia in the Internalizing-Externalizing Framework: A Third Dimension? Schizophr. Bull. 37, 1168–1178 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krabbendam L et al. Dimensions of depression, mania and psychosis in the general population. Psychol. Med. 34, 1177–1186 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Wright A et al. The Structure of Psychopathology: Toward an Expanded Quantitative Empirical Model. J. Abnorm. Psychol. 281 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Longden E et al. The Relationship Between Dissociation and Symptoms of Psychosis: A Meta-analysis. Schizophr. Bull. 46, 1104–1113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peralta V, Moreno-Izco L, Calvo-Barrena L & Cuesta MJ The low-and higher-order factor structure of symptoms in patients with a first episode of psychosis. Schizophr. Res. 147, 116–124 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Strauss GP et al. The Latent Structure of Negative Symptoms in Schizophrenia. JAMA Psychiatry (2018) doi: 10.1001/jamapsychiatry.2018.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Widiger TA, Lynam DR, Miller JD & Oltmanns TF Measures to Assess Maladaptive Variants of the Five-Factor Model. J. Pers. Assess. 94, 450–455 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Zimmermann J, Widiger TA, Oeltjen L, Conway CC & Morey LC Developing preliminary scales for assessing the HiTOP detachment spectrum. Assessment 29, 75–87 (2022). [DOI] [PubMed] [Google Scholar]
- 54.Guloksuz S & Van Os J The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol. Med. 48, 229 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Dickinson D, Ragland JD, Calkins ME, Gold JM & Gur RC A comparison of cognitive structure in schizophrenia patients and healthy controls using confirmatory factor analysis. Schizophr. Res. 85, 20–29 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nuechterlein KH et al. Identification of separable cognitive factors in schizophrenia. Schizophr. Res. 72, 29–39 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Zanelli J et al. Dynamic and Static Cognitive Deficits in Schizophrenia and Bipolar Disorder After the First Episode. Schizophr. Bull. sbab150 (2022) doi: 10.1093/schbul/sbab150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bora E & Özerdem A Meta-analysis of longitudinal studies of cognition in bipolar disorder: comparison with healthy controls and schizophrenia. Psychol. Med. 47, 2753–2766 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Keefe R The longitudinal course of cognitive impairment in schizophrenia: an examination of data from premorbid through posttreatment phases of illness. J. Clin. Psychiatry 75, 8–13 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Kahn RS & Keefe RSE Schizophrenia Is a Cognitive Illness: Time for a Change in Focus. JAMA Psychiatry 70, 1107–1112 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Bora E & Pantelis C Meta-analysis of cognitive impairment in first-episode bipolar disorder: comparison with first-episode schizophrenia and healthy controls. Schizophr. Bull. 41, 1095–1104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fett A-KJ et al. Long-term changes in cognitive functioning in individuals with psychotic disorders: findings from the Suffolk County mental health project. JAMA Psychiatry 77, 387–396 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mitropoulou V et al. Neuropsychological Performance in Schizotypal Personality Disorder: Importance of Working Memory. Am. J. Psychiatry 162, 1896–1903 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Bora E & Pantelis C Social cognition in schizophrenia in comparison to bipolar disorder: A meta-analysis. Schizophr. Res. 175, 72–78 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Dominguez M. de G., Viechtbauer W, Simons CJP, van Os J & Krabbendam L. Are psychotic psychopathology and neurocognition orthogonal? A systematic review of their associations. Psychol. Bull. 135, 157–171 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Ventura J, Thames AD, Wood RC, Guzik LH & Hellemann GS Disorganization and reality distortion in schizophrenia: A meta-analysis of the relationship between positive symptoms and neurocognitive deficits. Schizophr. Res. 121, 1–14 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dibben CRM, Rice C, Laws K & McKenna PJ Is executive impairment associated with schizophrenic syndromes? A meta-analysis. Psychol. Med. 39, 381–392 (2009). [DOI] [PubMed] [Google Scholar]
- 68.Abramovitch A, Short T & Schweiger A The C Factor: Cognitive dysfunction as a transdiagnostic dimension in psychopathology. Clin. Psychol. Rev. 86, 102007 (2021). [DOI] [PubMed] [Google Scholar]
- 69.Michelini G, Palumbo IM, DeYoung CG, Latzman RD & Kotov R Linking RDoC and HiTOP: A new interface for advancing psychiatric nosology and neuroscience. Clin. Psychol. Rev. 86, 102025 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meier MH et al. Neuropsychological Decline in Schizophrenia From the Premorbid to the Postonset Period: Evidence From a Population-Representative Longitudinal Study. Am. J. Psychiatry 171, 91–101 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kotov R et al. The Hierarchical Taxonomy of Psychopathology (HiTOP): A quantitative nosology based on consensus of evidence. Annu. Rev. Clin. Psychol. 17, 83–108 (2021). [DOI] [PubMed] [Google Scholar]
- 72.Lally J et al. Remission and recovery from first-episode psychosis in adults: systematic review and meta-analysis of long-term outcome studies. Br. J. Psychiatry 211, 350–358 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Clark LA, Cuthbert B, Lewis-Fernández R, Narrow WE & Reed GM Three approaches to understanding and classifying mental disorder: ICD-11, DSM-5, and the National Institute of Mental Health’s Research Domain Criteria (RDoC). Psychol. Sci. Public Interest 18, 72–145 (2017). [DOI] [PubMed] [Google Scholar]
- 74.World Health Organization. IFC: International Classification of Functioning, Disability and Health. (2001). [Google Scholar]
- 75.World Health Organization. WHO psychiatric disability assessment schedule (WHODAS). (WHO, 1988). [Google Scholar]
- 76.Üstün TB et al. Developing the World Health Organization disability assessment schedule 2.0. Bull. World Health Organ. 88, 815–823 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Freund PA & Kasten N How smart do you think you are? A meta-analysis on the validity of self-estimates of cognitive ability. Psychol. Bull. 138, 296 (2012). [DOI] [PubMed] [Google Scholar]
- 78.Hilker R et al. Heritability of Schizophrenia and Schizophrenia Spectrum Based on the Nationwide Danish Twin Register. Biol. Psychiatry 83, 492–498 (2018). [DOI] [PubMed] [Google Scholar]
- 79.Mullins N et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat. Genet. 53, 817–829 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grotzinger AD et al. Genetic architecture of 11 major psychiatric disorders at biobehavioral, functional genomic and molecular genetic levels of analysis. Nat. Genet. 54, 548–559 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cardno AG & Owen MJ Genetic relationships between schizophrenia, bipolar disorder, and schizoaffective disorder. Schizophr. Bull. 40, 504–515 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pettersson E, Larsson H & Lichtenstein P Common psychiatric disorders share the same genetic origin: a multivariate sibling study of the Swedish population. Mol. Psychiatry 21, 717–721 (2016). [DOI] [PubMed] [Google Scholar]
- 83.South SC et al. A population based twin study of DSM–5 maladaptive personality domains. Personal. Disord. Theory Res. Treat. 8, 366–375 (2017). [DOI] [PubMed] [Google Scholar]
- 84.Kendler KS et al. The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and all axis II disorders. Am. J. Psychiatry 168, 29–39 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kendler KS et al. The Structure of Genetic and Environmental Risk Factors for DSM-IV Personality Disorders: A Multivariate Twin Study. Arch. Gen. Psychiatry 65, 1438–1446 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Legge SE et al. Associations Between Schizophrenia Polygenic Liability, Symptom Dimensions, and Cognitive Ability in Schizophrenia. JAMA Psychiatry 78, 1143–1151 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jonas K et al. Schizophrenia polygenic risk score and 20-year course of illness in psychotic disorders. Transl. Psychiatry 9, 1–8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Legge SE et al. Genetic architecture of schizophrenia: a review of major advancements. Psychol. Med. 51, 2168–2177 (2021). [DOI] [PubMed] [Google Scholar]
- 89.Mistry S, Harrison JR, Smith DJ, Escott-Price V & Zammit S The use of polygenic risk scores to identify phenotypes associated with genetic risk of schizophrenia: Systematic review. Schizophr. Res. 197, 2–8 (2018). [DOI] [PubMed] [Google Scholar]
- 90.Jones HJ et al. Phenotypic manifestation of genetic risk for schizophrenia during adolescence in the general population. JAMA Psychiatry 73, 221–228 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Docherty AR et al. Molecular genetic risk for psychosis is associated with psychosis risk symptoms in a population-based UK cohort: Findings from generation Scotland. Schizophr. Bull 46, 1045–1052 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Toulopoulou T et al. Substantial genetic overlap between neurocognition and schizophrenia: genetic modeling in twin samples. Arch. Gen. Psychiatry 64, 1348–1355 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Toulopoulou T et al. Impaired intellect and memory: a missing link between genetic risk and schizophrenia? Arch. Gen. Psychiatry 67, 905–913 (2010). [DOI] [PubMed] [Google Scholar]
- 94.Fowler T, Zammit S, Owen MJ & Rasmussen F A population-based study of shared genetic variation between premorbid IQ and psychosis among male twin pairs and sibling pairs from Sweden. Arch. Gen. Psychiatry 69, 460–466 (2012). [DOI] [PubMed] [Google Scholar]
- 95.Kendler KS, Ohlsson H, Sundquist J & Sundquist K IQ and schizophrenia in a Swedish national sample: their causal relationship and the interaction of IQ with genetic risk. Am. J. Psychiatry 172, 259–265 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liebers DT et al. Polygenic Risk of Schizophrenia and Cognition in a Population-Based Survey of Older Adults. Schizophr. Bull. 42, 984–991 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hubbard L et al. Evidence of Common Genetic Overlap Between Schizophrenia and Cognition. Schizophr. Bull. 42, 832–842 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taylor MJ, Freeman D, Lundström S, Larsson H & Ronald A Heritability of psychotic experiences in adolescents and interaction with environmental risk. JAMA Psychiatry 79, 889–897 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ortega-Alonso A et al. Genome-Wide Association Study of Psychosis Proneness in the Finnish Population. Schizophr. Bull. 43, 1304–1314 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pain O et al. Genome-wide analysis of adolescent psychotic-like experiences shows genetic overlap with psychiatric disorders. Am. J. Med. Genet. 177, 416–425 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Binbay T et al. Testing the psychosis continuum: differential impact of genetic and nongenetic risk factors and comorbid psychopathology across the entire spectrum of psychosis. Schizophr. Bull. 38, 992–1002 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zavos HM et al. Consistent etiology of severe, frequent psychotic experiences and milder, less frequent manifestations: a twin study of specific psychotic experiences in adolescence. JAMA Psychiatry 71, 1049–1057 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Battaglia M, Bernardeschi L, Franchini L, Bellodi L & Smeraldi E A family study of schizotypal disorder. Schizophr. Bull. 21, 33–45 (1995). [DOI] [PubMed] [Google Scholar]
- 104.Kendler KS, McGuire M, Gruenberg AM & Walsh D Schizotypal symptoms and signs in the Roscommon Family Study. Their factor structure and familial relationship with psychotic and affective disorders. Arch. Gen. Psychiatry 52, 296–303 (1995). [DOI] [PubMed] [Google Scholar]
- 105.Kendler KS & Walsh D Schizotypal personality disorder in parents and the risk for schizophrenia in siblings. Schizophr. Bull. 21, 47–52 (1995). [DOI] [PubMed] [Google Scholar]
- 106.Kendler KS et al. The Roscommon Family Study: III. Schizophrenia-Related Personality Disorders in Relatives. Arch. Gen. Psychiatry 50, 781–788 (1993). [DOI] [PubMed] [Google Scholar]
- 107.Chang C-J et al. Morbidity Risk of Psychiatric Disorders Among the First Degree Relatives of Schizophrenia Patients in Taiwan. Schizophr. Bull. 28, 379–392 (2002). [DOI] [PubMed] [Google Scholar]
- 108.Legge SE et al. Association of Genetic Liability to Psychotic Experiences With Neuropsychotic Disorders and Traits. JAMA Psychiatry 76, 1256–1265 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gottesman II & Shields J A polygenic theory of schizophrenia. Int. J. Ment. Health 1, 107–115 (1972). [Google Scholar]
- 110.Yang J, Wray NR & Visscher PM Comparing apples and oranges: equating the power of case-control and quantitative trait association studies. Genet. Epidemiol. 34, 254–257 (2010). [DOI] [PubMed] [Google Scholar]
- 111.Grotzinger AD et al. Transcriptome-Wide Structural Equation Modeling of 13 Major Psychiatric Disorders for Cross-Disorder Risk and Drug Repurposing. JAMA Psychiatry (2023) doi: 10.1001/jamapsychiatry.2023.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Waszczuk MA et al. Dimensional and transdiagnostic phenotypes in psychiatric genome-wide association studies. Mol. Psychiatry 1–11 (2023) doi: 10.1038/s41380-023-02142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Castillejos M, Martín-Pérez C & Moreno-Küstner B A systematic review and meta-analysis of the incidence of psychotic disorders: the distribution of rates and the influence of gender, urbanicity, immigration and socio-economic level. Psychol. Med. 48, 2101–2115 (2018). [DOI] [PubMed] [Google Scholar]
- 114.Heinz A, Deserno L & Reininghaus U Urbanicity, social adversity and psychosis. World Psychiatry 12, 187–197 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Newbury J et al. Association between genetic and socioenvironmental risk for schizophrenia during upbringing in a UK longitudinal cohort. Psychol. Med. 1–11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jongsma HE et al. Treated incidence of psychotic disorders in the multinational EU-GEI study. JAMA Psychiatry 75, 36–46 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jongsma HE, Turner C, Kirkbride JB & Jones PB International incidence of psychotic disorders, 2002–17: a systematic review and meta-analysis. Lancet Public Health 4, e229–e244 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leaune E et al. Ethnic minority position and migrant status as risk factors for psychotic symptoms in the general population: a meta-analysis. Psychol. Med. 49, 545–558 (2019). [DOI] [PubMed] [Google Scholar]
- 119.Morgan C, Knowles G & Hutchinson G Migration, ethnicity and psychoses: evidence, models and future directions. World Psychiatry 18, 247–258 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alameda L et al. Association between specific childhood adversities and symptom dimensions in people with psychosis: systematic review and meta-analysis. Schizophr. Bull. 47, 975–985 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hjern A, Palacios J & Vinnerljung B Early childhood adversity and non-affective psychosis: a study of refugees and international adoptees in Sweden. Psychol. Med. 1–10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Matheson S, Shepherd AM, Pinchbeck R, Laurens K & Carr VJ Childhood adversity in schizophrenia: a systematic meta-analysis. Psychol. Med. 43, 225–238 (2013). [DOI] [PubMed] [Google Scholar]
- 123.Trotta A, Murray R & Fisher H The impact of childhood adversity on the persistence of psychotic symptoms: a systematic review and meta-analysis. Psychol. Med. 45, 2481–2498 (2015). [DOI] [PubMed] [Google Scholar]
- 124.Davis GP, Compton MT, Wang S, Levin FR & Blanco C Association between cannabis use, psychosis, and schizotypal personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Schizophr. Res. 151, 197–202 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Di Forti M et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatry 6, 427–436 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gage SH, Hickman M & Zammit S Association Between Cannabis and Psychosis: Epidemiologic Evidence. Biol. Psychiatry 79, 549–556 (2016). [DOI] [PubMed] [Google Scholar]
- 127.Robinson T et al. Risk-thresholds for the association between frequency of cannabis use and the development of psychosis: a systematic review and meta-analysis. Psychol. Med. 1–11 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morgan C et al. Threat, hostility and violence in childhood and later psychotic disorder: population-based case–control study. Br. J. Psychiatry 217, 575–582 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vassos E, Agerbo E, Mors O & Pedersen CB Urban–rural differences in incidence rates of psychiatric disorders in Denmark. Br. J. Psychiatry 208, 435–440 (2016). [DOI] [PubMed] [Google Scholar]
- 130.Dhondt N, Staines L, Healy C & Cannon M Childhood adversity and recurrence of psychotic experiences during adolescence: the role of mediation in an analysis of a population-based longitudinal cohort study. Psychol. Med. 1–9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kirkbride JB & Jones PB The prevention of schizophrenia—what can we learn from eco-epidemiology? Schizophr. Bull. 37, 262–271 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Perlman G et al. Symptoms of psychosis in schizophrenia, schizoaffective disorder, and bipolar disorder: A comparison of African Americans and Caucasians in the Genomic Psychiatry Cohort. Am. J. Med. Genet. B Neuropsychiatr. Genet. 171, 546–555 (2016). [DOI] [PubMed] [Google Scholar]
- 133.Reininghaus U et al. Transdiagnostic dimensions of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). World Psychiatry 18, 67–76 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Quattrone D et al. Transdiagnostic dimensions of psychopathology at first episode psychosis: findings from the multinational EU-GEI study. Psychol. Med. 49, 1378–1391 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Heuvelman H, Nazroo J & Rai D Investigating ethnic variations in reporting of psychotic symptoms: a multiple-group confirmatory factor analysis of the psychosis screening questionnaire. Psychol. Med. 48, 2757–2765 (2018). [DOI] [PubMed] [Google Scholar]
- 136.Murphy D, Vallières F, Murphy J, McElroy E & Hyland P Risk factors associated with general and specific dimensions of psychosis in a nationally representative sample of adults from the United States. Psychosis 12, 303–313 (2020). [Google Scholar]
- 137.Bardol O et al. Perceived ethnic discrimination as a risk factor for psychotic symptoms: a systematic review and meta-analysis. Psychol. Med. 50, 1077–1089 (2020). [DOI] [PubMed] [Google Scholar]
- 138.Veling W, Selten J-P, Mackenbach JP & Hoek HW Symptoms at first contact for psychotic disorder: comparison between native Dutch and ethnic minorities. Schizophr. Res. 95, 30–38 (2007). [DOI] [PubMed] [Google Scholar]
- 139.Scott J, Chant D, Andrews G & McGRATH J Psychotic-like experiences in the general community: the correlates of CIDI psychosis screen items in an Australian sample. Psychol. Med. 36, 231–238 (2006). [DOI] [PubMed] [Google Scholar]
- 140.Solmi F, Lewis G, Zammit S & Kirkbride JB Neighborhood characteristics at birth and positive and negative psychotic symptoms in adolescence: findings from the ALSPAC birth cohort. Schizophr. Bull. 46, 581–591 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.DeVylder JE et al. Association of urbanicity with psychosis in low-and middle-income countries. JAMA Psychiatry 75, 678–686 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Monshouwer K et al. Prevalence, incidence, and persistence of psychotic experiences in the general population: results of a 9-year follow-up study. Psychol. Med. 1–12 (2022). [DOI] [PubMed] [Google Scholar]
- 143.Morgan C & Gayer-Anderson C Childhood adversities and psychosis: evidence, challenges, implications. World Psychiatry 15, 93–102 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Back SN, Flechsenhar A, Bertsch K & Zettl M Childhood traumatic experiences and dimensional models of personality disorder in DSM-5 and ICD-11: Opportunities and challenges. Curr. Psychiatry Rep. 23, 1–10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Velikonja T, Fisher H, Mason O & Johnson S Childhood trauma and schizotypy: a systematic literature review. Psychol. Med. 45, 947–963 (2015). [DOI] [PubMed] [Google Scholar]
- 146.Gibson LE, Alloy LB & Ellman LM Trauma and the psychosis spectrum: A review of symptom specificity and explanatory mechanisms. Clin. Psychol. Rev. 49, 92–105 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Anglin DM et al. Early cannabis use and schizotypal personality disorder symptoms from adolescence to middle adulthood. Schizophr. Res. 137, 45–49 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hasan A et al. Cannabis use and psychosis: a review of reviews. Eur. Arch. Psychiatry Clin. Neurosci. 270, 403–412 (2020). [DOI] [PubMed] [Google Scholar]
- 149.Mustonen A et al. Adolescent cannabis use, baseline prodromal symptoms and the risk of psychosis. Br. J. Psychiatry 212, 227–233 (2018). [DOI] [PubMed] [Google Scholar]
- 150.Sideli L, Quigley H, La Cascia C & Murray RM Cannabis use and the risk for psychosis and affective disorders. J. Dual Diagn. 16, 22–42 (2020). [DOI] [PubMed] [Google Scholar]
- 151.Marconi A, Di Forti M, Lewis CM, Murray RM & Vassos E Meta-analysis of the association between the level of cannabis use and risk of psychosis. Schizophr. Bull. 42, 1262–1269 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ragazzi TC, Shuhama R, Menezes PR & Del-Ben CM Cannabis use as a risk factor for psychotic-like experiences: a systematic review of non-clinical populations evaluated with the Community Assessment of Psychic Experiences. Early Interv. Psychiatry 12, 1013–1023 (2018). [DOI] [PubMed] [Google Scholar]
- 153.Foti DJ, Kotov R, Guey LT & Bromet EJ Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am. J. Psychiatry 167, 987–993 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Quattrone D et al. Daily use of high-potency cannabis is associated with more positive symptoms in first-episode psychosis patients: the EU-GEI case–control study. Psychol. Med. 51, 1329–1337 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Seddon JL et al. Cannabis use is associated with increased psychotic symptoms and poorer psychosocial functioning in first-episode psychosis: a report from the UK national EDEN study. Schizophr. Bull. 42, 619–625 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Szoke A et al. Association between cannabis use and schizotypal dimensions–a meta-analysis of cross-sectional studies. Psychiatry Res. 219, 58–66 (2014). [DOI] [PubMed] [Google Scholar]
- 157.Stilo SA & Murray RM Non-genetic factors in schizophrenia. Curr. Psychiatry Rep. 21, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tsuang MT, Stone WS & Faraone SV Genes, environment and schizophrenia. Br. J. Psychiatry 178, s18–s24 (2001). [DOI] [PubMed] [Google Scholar]
- 159.Guloksuz S et al. Examining the independent and joint effects of molecular genetic liability and environmental exposures in schizophrenia: results from the EUGEI study. World Psychiatry 18, 173–182 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bettes BA & Walker E Positive and negative symptoms in psychotic and other psychiatrically disturbed children. J. Child Psychol. Psychiatry 28, 555–568 (1987). [DOI] [PubMed] [Google Scholar]
- 161.Gregersen M et al. Developmental pathways and clinical outcomes of early childhood psychotic experiences in preadolescent children at familial high risk of schizophrenia or bipolar disorder: a prospective, longitudinal cohort study-the Danish High Risk and Resilience Study, VIA 11. Am. J. Psychiatry 179, 628–639 (2022). [DOI] [PubMed] [Google Scholar]
- 162.Healy C, Coughlan H, Clarke M, Kelleher I & Cannon M What mediates the longitudinal relationship between psychotic experiences and psychopathology? J. Abnorm. Psychol. 129, 505–516 (2020). [DOI] [PubMed] [Google Scholar]
- 163.Isaksson J, Angenfelt M, Frick MA, Olofsdotter S & Vadlin S Psychotic-like experiences from adolescence to adulthood: A longitudinal study. Schizophr. Res. 248, 1–7 (2022). [DOI] [PubMed] [Google Scholar]
- 164.Trotta A et al. Mental health and functional outcomes in young adulthood of children with psychotic symptoms: a longitudinal cohort study. Schizophr. Bull. 46, 261–271 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Havers L, von Stumm S, Cardno AG, Freeman D & Ronald A Psychotic experiences and negative symptoms from adolescence to emerging adulthood: developmental trajectories and associations with polygenic scores and childhood characteristics. Psychol. Med. 1–13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cannon TD et al. An Individualized Risk Calculator for Research in Prodromal Psychosis. Am. J. Psychiatry 173, 980–988 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Healy C et al. Childhood and adolescent psychotic experiences and risk of mental disorder: a systematic review and meta-analysis. Psychol. Med. 49, 1589–1599 (2019). [DOI] [PubMed] [Google Scholar]
- 168.Sullivan SA et al. A population-based cohort study examining the incidence and impact of psychotic experiences from childhood to adulthood, and prediction of psychotic disorder. Am. J. Psychiatry 177, 308–317 (2020). [DOI] [PubMed] [Google Scholar]
- 169.Debbané M et al. Developing psychosis and its risk states through the lens of schizotypy. Schizophr. Bull. 41, S396–S407 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kwapil TR, Gross GM, Silvia PJ & Barrantes-Vidal N Prediction of psychopathology and functional impairment by positive and negative schizotypy in the Chapmans’ ten-year longitudinal study. J. Abnorm. Psychol. 122, 807 (2013). [DOI] [PubMed] [Google Scholar]
- 171.Lenzenweger MF Schizotypy 17 years on: Psychotic symptoms in midlife. J. Abnorm. Psychol. 130, 399 (2021). [DOI] [PubMed] [Google Scholar]
- 172.Addington J et al. North American prodrome longitudinal study (NAPLS 3): methods and baseline description. Schizophr. Res. 243, 262–267 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fusar-Poli P et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry 77, 755–765 (2020). [DOI] [PubMed] [Google Scholar]
- 174.Boldrini T et al. Comorbid personality disorders in individuals with an at-risk mental state for psychosis: a meta-analytic review. Front. Psychiatry 10, 429 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Allswede DM et al. Characterizing covariant trajectories of individuals at clinical high risk for psychosis across symptomatic and functional domains. Am. J. Psychiatry 177, 164–171 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Michail M & Birchwood M Social anxiety disorder in first-episode psychosis: incidence, phenomenology and relationship with paranoia. Br. J. Psychiatry 195, 234–241 (2009). [DOI] [PubMed] [Google Scholar]
- 177.Upthegrove R, Marwaha S & Birchwood M Depression and schizophrenia: cause, consequence, or trans-diagnostic issue? Schizophr. Bull. 43, 240–244 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Webb JR et al. Specificity of incident diagnostic outcomes in patients at clinical high risk for psychosis. Schizophr. Bull. 41, 1066–1075 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Woods SW et al. Lack of diagnostic pluripotentiality in patients at clinical high risk for psychosis: specificity of comorbidity persistence and search for pluripotential subgroups. Schizophr. Bull. 44, 254–263 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Oliver D et al. Prognostic accuracy and clinical utility of psychometric instruments for individuals at clinical high-risk of psychosis: a systematic review and meta-analysis. Mol. Psychiatry 1–9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Van Os J & Reininghaus U Psychosis as a transdiagnostic and extended phenotype in the general population. World Psychiatry 15, 118–124 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Salazar de Pablo G et al. Probability of Transition to Psychosis in Individuals at Clinical High Risk: An Updated Meta-analysis. JAMA Psychiatry (2021) doi: 10.1001/jamapsychiatry.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Carrion RE et al. Personalized prediction of psychosis: external validation of the NAPLS-2 psychosis risk calculator with the EDIPPP project. Am. J. Psychiatry 173, 989–996 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.van Os J & Guloksuz S A critique of the “ultra-high risk” and “transition” paradigm. World Psychiatry 16, 200–206 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hopwood CJ et al. Ten-Year Rank-Order Stability of Personality Traits and Disorders in a Clinical Sample. J. Pers. 81, 335–344 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Reichenberg A, Rieckmann N & Harvey PD Stability in schizophrenia symptoms over time: findings from the Mount Sinai Pilgrim Psychiatric Center Longitudinal Study. J. Abnorm. Psychol. 114, 363 (2005). [DOI] [PubMed] [Google Scholar]
- 187.Kotov R et al. Declining clinical course of psychotic disorders over the two decades following first hospitalization: evidence from the Suffolk County Mental Health Project. Am. J. Psychiatry 174, 1064–1074 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Secher RG et al. Ten-Year Follow-up of the OPUS Specialized Early Intervention Trial for Patients With a First Episode of Psychosis. Schizophr. Bull. 41, 617–626 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Morgan C et al. Rethinking the course of psychotic disorders: modelling long-term symptom trajectories. Psychol. Med. 1–10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sanislow CA et al. Ten-year stability and latent structure of the DSM–IV schizotypal, borderline, avoidant, and obsessive-compulsive personality disorders. J. Abnorm. Psychol. 118, 507 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Schreck MC et al. Empirically Derived Subtypes of Youth Withdrawn Behavior Across Eight Years: A Latent Class and Latent Transition Analysis. J. Child Fam. Stud. 30, 1736–1751 (2021). [Google Scholar]
- 192.Matheson SL et al. Systematic meta-analysis of childhood social withdrawal in schizophrenia, and comparison with data from at-risk children aged 9–14 years. J. Psychiatr. Res. 47, 1061–1068 (2013). [DOI] [PubMed] [Google Scholar]
- 193.Cohen AS, Couture SM & Blanchard JJ Social anhedonia and clinical outcomes in early adulthood: a three-year follow-up study within a community sample. Schizophr. Res. 223, 213–219 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cowan HR, Mittal VA, Allen DN, Gold JM & Strauss GP Heterogeneity of emotional experience in schizophrenia: Trait affect profiles predict clinical presentation and functional outcome. J. Abnorm. Psychol. 129, 760 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Devoe D et al. Persistent negative symptoms in youth at clinical high risk for psychosis: a longitudinal study. Schizophr. Res. 227, 28–37 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cowan HR & Mittal VA Transdiagnostic Dimensions of Psychiatric Comorbidity in Individuals at Clinical High Risk for Psychosis: A Preliminary Study Informed by HiTOP. Front. Psychiatry 11, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Deng W et al. Characterizing sustained social anxiety in individuals at clinical high risk for psychosis: trajectory, risk factors, and functional outcomes. Psychol. Med. 1–8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hochstrasser L et al. Latent state-trait structure of BPRS subscales in clinical high-risk state and first episode psychosis. Sci. Rep. 12, 6652 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Eaton WW, Thara R, Federman B, Melton B & Liang K Structure and Course of Positive and Negative Symptoms in Schizophrenia. Arch Gen Psychiatry 52, 127–134 (1995). [DOI] [PubMed] [Google Scholar]
- 200.Carrà G et al. Positive and negative symptoms in schizophrenia: A longitudinal analysis using latent variable structural equation modelling. Schizophr. Res. 204, 58–64 (2019). [DOI] [PubMed] [Google Scholar]
- 201.Rapoport JL, Giedd JN & Fernandes D Neurodevelopmental model of schizophrenia: update 2012. Mol. Psychiatry 17, 1228–1238 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Stone WS et al. Neurodegenerative model of schizophrenia: Growing evidence to support a revisit. Schizophr. Res. 243, 154–162 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Murray R, Bora E, Modinos G & Vernon A Schizophrenia: A developmental disorder with a risk of non-specific but avoidable decline. Schizophr. Res. 243, 181–186 (2022). [DOI] [PubMed] [Google Scholar]
- 204.Reichenberg A et al. Static and Dynamic Cognitive Deficits in Childhood Preceding Adult Schizophrenia: A 30-Year Study. Am. J. Psychiatry 167, 160–169 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jonas K et al. The Course of General Cognitive Ability in Individuals With Psychotic Disorders. JAMA Psychiatry 79, 659–666 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cannon TD et al. Childhood Cognitive Functioning in Schizophrenia Patients and Their Unaffected Siblings: A Prospective Cohort Study. Schizophr. Bull. 26, 379–393 (2000). [DOI] [PubMed] [Google Scholar]
- 207.Catalan A et al. Neurocognitive Functioning in Individuals at Clinical High Risk for Psychosis: A Systematic Review and Meta-analysis. JAMA Psychiatry (2021) doi: 10.1001/jamapsychiatry.2021.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mollon J, David AS, Zammit S, Lewis G & Reichenberg A Course of cognitive development from infancy to early adulthood in the psychosis spectrum. JAMA Psychiatry 75, 270–279 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sheffield JM, Karcher NR & Barch DM Cognitive Deficits in Psychotic Disorders: A Lifespan Perspective. Neuropsychol. Rev. 28, 509–533 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.de Zwarte SMC et al. Running in the Family? Structural Brain Abnormalities and IQ in Offspring, Siblings, Parents, and Co-twins of Patients with Schizophrenia. Schizophr Bull vol. 45 1209–1217 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vissink CE et al. Structural Brain Volumes of Individuals at Clinical High Risk for Psychosis: A Meta-analysis. Biol Psychiatry Glob Open Sci vol. 2 147–152 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Satterthwaite TD et al. Structural Brain Abnormalities in Youth With Psychosis Spectrum Symptoms. JAMA Psychiatry vol. 73 515–24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liloia D et al. Updating and characterizing neuroanatomical markers in high-risk subjects, recently diagnosed and chronic patients with schizophrenia: A revised coordinate-based meta-analysis. Neurosci Biobehav Rev vol. 123 83–103 (2021). [DOI] [PubMed] [Google Scholar]
- 214.Hulshoff Pol HE et al. Overlapping and segregating structural brain abnormalities in twins with schizophrenia or bipolar disorder. Arch Gen Psychiatry vol. 69 349–59 (2012). [DOI] [PubMed] [Google Scholar]
- 215.Toulopoulou T et al. Reciprocal causation models of cognitive vs volumetric cerebral intermediate phenotypes for schizophrenia in a pan-European twin cohort. Mol Psychiatry vol. 20 1386–96 (2015). [DOI] [PubMed] [Google Scholar]
- 216.Deary IJ The Stability of Intelligence From Childhood to Old Age. Curr. Dir. Psychol. Sci. 23, 239–245 (2014). [Google Scholar]
- 217.Watson AJ, Harrison L, Preti A, Wykes T & Cella M Cognitive trajectories following onset of psychosis: a meta-analysis. Br. J. Psychiatry 1–8 (2022). [DOI] [PubMed] [Google Scholar]
- 218.Zanelli J et al. Cognitive change in schizophrenia and other psychoses in the decade following the first episode. Am. J. Psychiatry 176, (2019). [DOI] [PubMed] [Google Scholar]
- 219.Stroup TS et al. Age-Specific Prevalence and Incidence of Dementia Diagnoses Among Older US Adults With Schizophrenia. JAMA Psychiatry (2021) doi: 10.1001/jamapsychiatry.2021.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Trotta A, Murray R & MacCabe J Do premorbid and post-onset cognitive functioning differ between schizophrenia and bipolar disorder? A systematic review and meta-analysis. Psychol. Med. 45, 381–394 (2015). [DOI] [PubMed] [Google Scholar]
- 221.Velthorst E et al. The 20-Year Longitudinal Trajectories of Social Functioning in Individuals With Psychotic Disorders. Am. J. Psychiatry 174, 1075–1085 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cannon M et al. Premorbid Social Functioning in Schizophrenia and Bipolar Disorder: Similarities and Differences. Am. J. Psychiatry 154, 1544–1550 (1997). [DOI] [PubMed] [Google Scholar]
- 223.Jonas KG & Markon KE A model of psychosis and its relationship with impairment. Soc. Psychiatry Psychiatr. Epidemiol. 48, 1367–1375 (2013). [DOI] [PubMed] [Google Scholar]
- 224.Fusar-Poli P et al. Disorder, not just state of risk: meta-analysis of functioning and quality of life in people at high risk of psychosis. Br. J. Psychiatry 207, 198–206 (2015). [DOI] [PubMed] [Google Scholar]
- 225.O’Keeffe D, Kinsella A, Waddington JL & Clarke M 20-year prospective, sequential follow-up study of heterogeneity in associations of duration of untreated psychosis with symptoms, functioning, and quality of life following first-episode psychosis. Am. J. Psychiatry 179, 288–297 (2022). [DOI] [PubMed] [Google Scholar]
- 226.Hansen HG et al. Clinical recovery among individuals with a first-episode schizophrenia an updated systematic review and meta-analysis. Schizophr. Bull. (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ajnakina O et al. Duration of Untreated Psychosis in First-Episode Psychosis is not Associated With Common Genetic Variants for Major Psychiatric Conditions: Results From the Multi-Center EU-GEI Study. Schizophr. Bull. (2021) doi: 10.1093/schbul/sbab055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Austin SF et al. Predictors of recovery in first episode psychosis: the OPUS cohort at 10 year follow-up. Schizophr. Res. 150, 163–168 (2013). [DOI] [PubMed] [Google Scholar]
- 229.Hegelstad W et al. Long-term follow-up of the TIPS early detection in psychosis study: effects on 10-year outcome. Am. J. Psychiatry 169, 374–380 (2012). [DOI] [PubMed] [Google Scholar]
- 230.Hickie IB et al. Applying clinical staging to young people who present for mental health care. Early Interv. Psychiatry 7, 31–43 (2013). [DOI] [PubMed] [Google Scholar]
- 231.Nelson B, McGorry PD & Fernandez AV Integrating clinical staging and phenomenological psychopathology to add depth, nuance, and utility to clinical phenotyping: a heuristic challenge. Lancet Psychiatry 8, 162–168 (2021). [DOI] [PubMed] [Google Scholar]
- 232.Shah JL et al. Transdiagnostic clinical staging in youth mental health: a first international consensus statement. World Psychiatry 19, 233–242 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.McGorry PD, Hartmann JA, Spooner R & Nelson B Beyond the “at risk mental state” concept: transitioning to transdiagnostic psychiatry. World Psychiatry 17, 133–142 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lee TY, Lee J, Kim M, Choe E & Kwon JS Can we predict psychosis outside the clinical high-risk state? A systematic review of non-psychotic risk syndromes for mental disorders. Schizophr. Bull 44, 276–285 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Andreasen NC et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am. J. Psychiatry 162, 441–449 (2005). [DOI] [PubMed] [Google Scholar]
- 236.Skodol AE et al. Positive childhood experiences: Resilience and recovery from personality disorder in early adulthood. J. Clin. Psychiatry 68, 1102 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Liberman RP & Kopelowicz A Recovery from schizophrenia: a concept in search of research. Psychiatr. Serv. 56, 735–742 (2005). [DOI] [PubMed] [Google Scholar]
- 238.Takeuchi H et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology 44, 1036–1042 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Emsley R, Oosthuizen P, Koen L, Niehaus D & Martinez L Comparison of Treatment Response in Second-Episode Versus First-Episode Schizophrenia. J. Clin. Psychopharmacol. 33, 80 (2013). [DOI] [PubMed] [Google Scholar]
- 240.Ciompi L & Clemens S Catamnestic Long-term Study on the Course of Life and Aging of Schizophrenics. Schizophr. Bull. 6, 606–618 (1980). [DOI] [PubMed] [Google Scholar]
- 241.Kotov R et al. Boundaries of schizoaffective disorder: revisiting Kraepelin. JAMA Psychiatry 70, 1276–1286 (2013). [DOI] [PubMed] [Google Scholar]
- 242.Jin J, Zeidman P, Friston KJ & Kotov R Inferring trajectories of psychotic disorders using dynamic causal modeling. 7, 60–75 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Nelson B, McGorry PD, Wichers M, Wigman JTW & Hartmann JA Moving From Static to Dynamic Models of the Onset of Mental Disorder: A Review. JAMA Psychiatry 74, 528–534 (2017). [DOI] [PubMed] [Google Scholar]
- 244.Wright AGC & Woods WC Personalized Models of Psychopathology. Annu. Rev. Clin. Psychol. 16, 49–74 (2020). [DOI] [PubMed] [Google Scholar]
- 245.Jonas K et al. Where is Mania in the Hierarchical Taxonomy of Psychopathology: Internalizing, Thought Disorder, or Novel Spectrum? at 10.31234/osf.io/nrgav (2023). [DOI]
- 246.Waszczuk MA et al. The prognostic utility of personality traits versus past psychiatric diagnoses: Predicting future mental health and functioning. Clin. Psychol. Sci. J. Assoc. Psychol. Sci. 10, 734–751 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Krueger RF & Markon KE The role of the DSM-5 personality trait model in moving toward a quantitative and empirically based approach to classifying personality and psychopathology. Annu. Rev. Clin. Psychol. 10, 477–501 (2014). [DOI] [PubMed] [Google Scholar]
- 248.Calkins ME et al. The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. J. Child Psychol. Psychiatry 56, 1356–1369 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Gur RC et al. Neurocognitive growth charting in psychosis spectrum youths. JAMA Psychiatry 71, 366–374 (2014). [DOI] [PubMed] [Google Scholar]
- 250.Moore TM, Reise SP, Gur RE, Hakonarson H & Gur RC Psychometric properties of the Penn Computerized Neurocognitive Battery. Neuropsychology 29, 235 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Woodberry KA, Giuliano AJ & Seidman LJ Premorbid IQ in Schizophrenia: A Meta-Analytic Review. Am. J. Psychiatry 165, 579–587 (2008). [DOI] [PubMed] [Google Scholar]


