Abstract
The novel structure of Hg(II) complexes including the pyridinium ylide C5H5NCHC(O)C6H4-m-Br (Y) were synthesized and reported in this study. In the first step, the pyridinium salt C5H5NCH2C(O)C6H4-m-Br (S) was produced by reacting 2,3′-dibromoactophenone and pyridine. then, treatment of S with K2CO3 gave the related pyridinium ylide Y. Finally, the reaction of Y with HgX2 and Hg(NO3)2·H2O leads to the formation of novel binuclear [HgY2][HgX4] (X=Cl (1); X=Br (2); X=I (3)) and polymeric [HgY(NO3)2]n (4) complexes. The structure of complex 2 was also determined by X-ray diffraction analysis. The obtained analyses proved the coordination through the ylidic carbon to metallic center. Additionally, Natural Bond Orbital (NBO), Energy Decomposition Analysis (EDA), and EDA-NOCV studies are also used to investigate the nature of metal–ligand bonding in the complexes. Finally, the antibacterial activity of 1–4 was also examined against Gram positive and negative represented significant levels of inhibitory potency respected to used standards.
Keywords: Pyridinium ylide, Mercury(ii) complex, X-ray diffraction technique, Theoretical studies, Antibacterial activity
Subject terms: Ligands, Organometallic chemistry, Inorganic chemistry
Introduction
One of the widely used compounds in organic chemistry are ylides, a type of ligands with a sigma bond between a negative carbon and a positive heteroatom1–3. Although, different methods have yet been used to synthesis such compounds, most of them common in two steps: (1) synthesis of pyridinium salt [RCH2-EZn]+X− by reaction between an alkyl/aryl halide RCH2X and a nucleophile EZn (PR3, AsR3, NR3, SR2, etc.); (2) dehydrohalogenation of pyridinium salt to obtain the desired ylide RCH = EZn4–6. Among ylides, pyridinium ones studied by Kronkhe in 19407 have aroused considerable attention as ambidentate ligands in organometallic chemistry fields8–11. There are two modes of coordination in these compounds, Cα- and O-coordinated modes12–15. These differences in coordination are due to the presence of the carbonyl group and carbanion together which causes a resonance delocalization of the electron density according to the Hard-Soft-Acid–Base principle (HSAB) (Fig. 1)16. Soft metal ions like Hg, Pd, and Cu can receive electrons more easily from the carbanion atom, which leads to the creation of stable metal–carbon bonds in ylidic complexes.
Fig. 1.

The resonance structure of pyridinium ylides.
The absence of occupied d orbitals in the nitrogen of the pyridinium ylides causes a significant difference in these compounds compared to the phosphorous and sulfur derivatives17. Therefore, stability of such compounds arises from the resonance in their heterocyclic ring (Fig. 1)18. The synthesis of mercury (II) complexes derived from pyridinium ylides was first published in 1975 by Weleski19. They proposed symmetric and binuclear structure with halide-bridged form for such complexes. Although our group have recently synthesized and characterized phosphonium and sulfonium ylidic derivatives and their transition metal complexes13. These synthesized complexes in the present study are including pyridinium ylidic derivatives, which can be widely used in organometallic chemistry fields.
In this project, we synthesized new binuclear and polymeric Hg(II) complexes bearing pyridinium ylide as an monodentate ligand. The structure of obtained compounds was investigated by NMR (1 H, 13C), IR and element analysis spectroscopic methods. The structure of complex 2 was clearly determined by X-ray crystallographic method. The obtained data confirmed the proposed structures which pyridinium ylide coordinated to Hg through ylidic carbon atom. For further studies, the proposed structures of 1–3 were also studied by theoretical methods.
Results and discussion
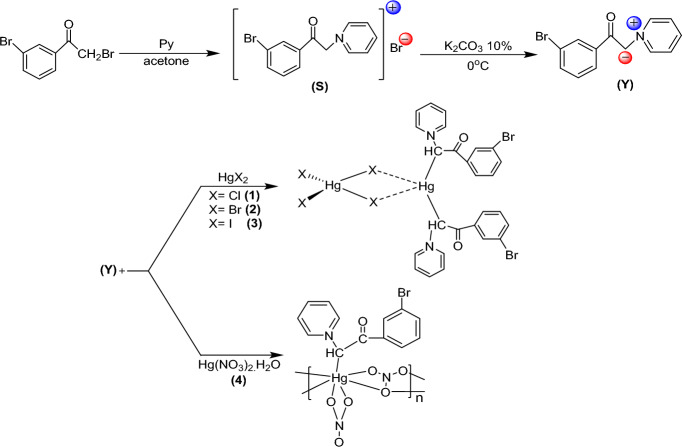
The mentioned Hg(II) complexes were synthesized by an equimolar reaction between Hg(II) salts and desired ylide Y, which is obtained with elimination of HBr from related pyridinium salt S by K2CO3 10% solution. The final reaction gave three dimeric complexes 1–3 and polymeric complex 4 (Fig. 2). All compounds were characterized by IR, NMR, and element analyses methods. The exact structure of 2 was also determined by X-ray crystallographic analysis.
Fig. 2.
Synthetic route for the preparation of complexes 1–4.
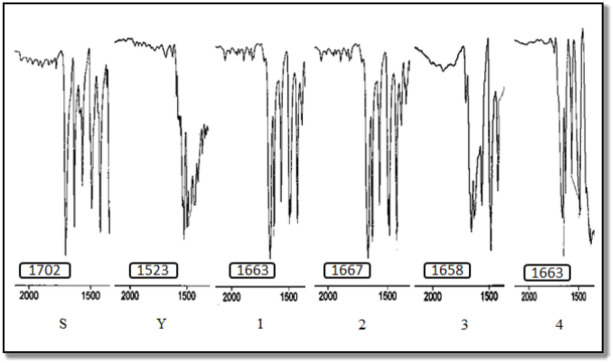
Table 1 summarizes the characteristic IR, 1H and 13C NMR data of S, Y, and Hg(II) complexes 1–4.
Table 1.
Selected spectroscopic data for compounds 1–4.
| Compound | IR; ν(CO) cm−1 | 1H NMR; δ(CH) ppm | 13C NMR; δ(CO) ppm |
|---|---|---|---|
| S | 1702 | 6.58 | 190.3 |
| Y | 1588 | 4.49 | 170.5 |
| 1 | 1663 | 7.01 | 185.6 |
| 2 | 1667 | 6.98 | 184.3 |
| 3 | 1658 | 7.00 | 184.4 |
| 4 | 1663 | 6.94 | 189.6 |
As can be inferred from the IR spectra, the ν(CO) is more sensitive peaks related to others because of changing in electron density between carbonyl group and ylidic bond N+‒C− during successive reactions (Fig. 1). Because of delocalized negative charge on carbon atom in ylidic form, there are two main resonance structures, N+‒C−‒C=O and N+‒C=C‒O−, which causes lower frequency of ν(CO) in the Y compared to the related salt and complexes 1–420. Therefore, coordination through the ylidic carbon to metal leads to disruption of resonance and increasing of ν(CO) (Fig. 3)15.
Fig. 3.
The ν(CO) peak of compounds S, Y, and 1–4 in IR spectra.
The other conventional method to characterize of the synthesized compounds is NMR studies (1H and 13C NMR). By comparing the 1H NMR spectra of S and Y, a clear shift to lower frequency is observed for the ylidic proton from 6.58 to 4.49 ppm. Also, the expected downfield shifts upon complexation in the case of C-coordination were also observed in complexes (around 7 ppm) respected to Y, resulted of the inductive effect of metal center21.
The 13C NMR spectra is also confirmed the proposed structure of synthesized complexes based on significance shifts for two peaks related to ylidic carbon and carbonyl group. The coordination through ylidic carbon causes an up-field shift for the corresponding signals in complexes 1–4. Also, shifting the peak assigned to carbonyl group to higher frequency in complexes confirms the coordination through ylidic carbon which can be seen in 13C NMR spectra.
X-ray crystallography
Figure 4 depicts the precise molecular structure of complex 2 obtained from X-ray analysis labeling by rwg2018_15Gau. Additionally, Table 2 presents the bond lengths (Å) and angles (°) selection for the corresponding structure.
Fig. 4.
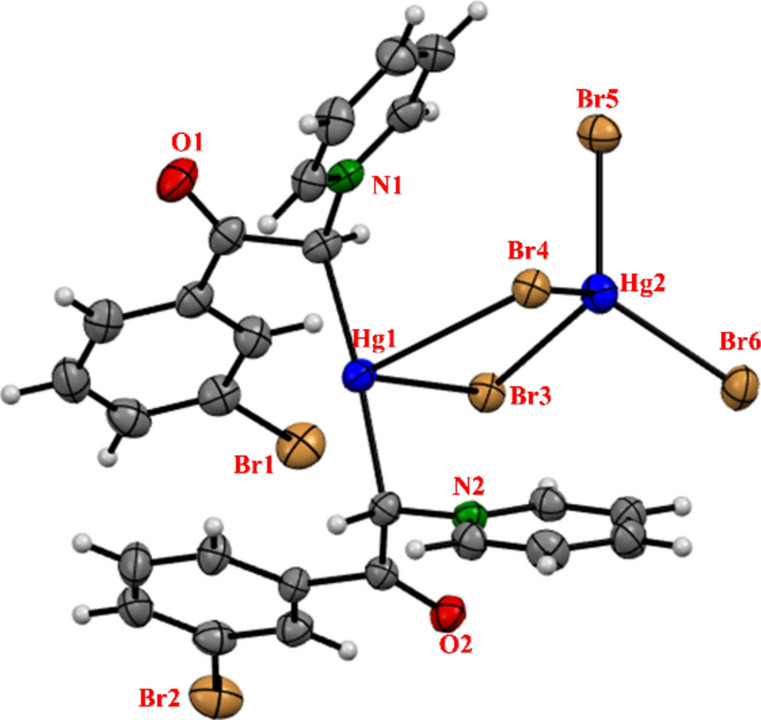
The molecular crystal structure of the synthesized complex 2 (rwg2018_15Gau).
Table 2.
Some of the bond lengths and bond angles of the crystal structure complex 2.
| Bond type | Bond Lengths/Å | Angle type | Angle/˚ |
|---|---|---|---|
| Hg1-C1 | 2.156(5) | C14-Hg1-C1 | 175.39(18) |
| Hg1-C14 | 2.155(4) | C9-N1-C1 | 119.7(4) |
| Br1-C5 | 1.897(6) | C13-N1-C1 | 119.5(4) |
| Br2-C18 | 1.893(5) | N1-C1-Hg1 | 109.8(3) |
| O1-C2 | 1.220(7) | N1-C1-C2 | 111.1(4) |
| O2-C15 | 1.223(6) | C2-C1-Hg1 | 115.8(3) |
| N1-C1 | 1.477(6 | O1-C2-C1 | 121.8(5) |
| C1-C2 | 1.499(7) | O1-C2-C3 | 120.4(5) |
| C2-C3 | 1.487(7) | C3-C2-C1 | 117.7(4) |
| C3-C4 | 1.401(8) | C4-C3-C2 | 121.1(5) |
| C3-C8 | 1.400(8) | C8-C3-C2 | 119.7(5) |
| C4-C5 | 1.385(7) | C8-C3-C4 | 119.2(5) |
The crystals were grown by the direct diffusion of methanol in the dimethyl sulfoxide solution including complex 2 over several days. Crystals were good quality. Dark orange crystals with cream amorphous material. Crystals were clustered together, but easily separated. The X‐ray analysis shows that complex 2 adopts a binuclear structure with two Hg(II) metal center. As shown in Fig. 2, the Hg1 atom in each unit coordinated in an almost linear coordination environment to two ylidic ligands and Hg2 through ylidic carbons and two Br bridging atoms, respectively. The coordination number in this complex is four (two of ylides and two bridged bromides). The geometry is similar to that found for other two coordinate ylide complexes such as bis(bis(methoxycarbonyl)methyl)-mercury(ii)5. The distance of Hg1 to ylidic carbon (Hg1–C1 and Hg1–C14) is almost the same about 2.156 Å. The ligands lie in an almost parallel orientation, with the N1-C1…C14-N2 torsion angle being − 41.2(5)° with the dihedral angle between the pyridine groups being 57.25(14)°. While the carbonyls are oriented in an almost trans-configuration [O1-C2…C15-O2 -156.4(7)°], the bromo-phenyl rings adopt similar orientations [Br1-C5…C18-Br2 -40.3(3)°], the dihedral angle being the two rings is 21.2(2)°. The carbonyl groups are oriented at 27.6(3)° and 13.6(3)°, respectively, to the bromo-phenyl rings. It is interesting that both ylide carbons in each molecule adopt the same chirality, the crystal being composed of equal numbers of SS & RR molecules. There are weak interactions between the Hg atom and two bromine atoms from the [HgBr4]2− anion [Hg-Br3: 3.2213(6)Å, Hg1-Br4: 3.1649(5)Å, Br3-Hg1-Br4: 83.112(13)°], while shorter than the sum of the vdW radii (3.55 Å) 22, they are significantly longer than terminal Hg-Br single bonds (~ 2.5 Å) and bridging Hg-Br bonds (~ 2.8 Å). Weak C-H…O, C-H…Br, Br…Br [Br2…Br5iii 3.5955(9)°, C18-Br2-Briii 54.05(17)°, symm code iii: 0.5 + x, 0.5 − y, − 0.5 + z] and π…π interactions between the aromatic rings [C23-C23iv 3.368 (10)Å, C24-C23…C23iv 81.0(3)°; symm code iv: − x, 1 − y, − z] link the moieties into a 3D network. On the other hand, bond angles around the Hg1 including Br1–Hg1–C1, C1–Hg1–C14, C14–Hg1–Br2, and Br1–Hg1–Br2 show that the complex has cubic structure. Although The Hg1–Br1 and Hg1–Br2 distances in complex 2 are longer than the aforementioned bonds Hg1–C1 and Hg1–C14.
Theoretical studies
The obtained structure of complex 2 by X-ray analysis (see Fig. 3) was also optimized at the M06-d3/def2-TZVP level of theory. The structure of [HgY2][HgBr4] was also used as a basis for DFT calculations of [HgY2][HgX4] (X=Cl, I) complexes. Table 3 shows selected bond lengths and bond angles of [HgY2][HgX4] (X=Cl, Br, and I) complexes. As it shown the values of Hg1 − C(1 and 14) and Hg2 − Br(3 and 4) are greater and the values of Hg1 − Br(3 and 4) and Hg2 − Br(5 and 6) are smaller than corresponding experimental values (Table 3).
Table 3.
The important bond length (Å) and bond angle (˚) and WBI of [HgY2][HgX4] complexes at M06-d3/def2-TZVP level of theory.
| [HgY2][HgCl4] | [HgY2][HgBr4] | [HgY2][HgI4] | ||||||
|---|---|---|---|---|---|---|---|---|
| Bond Lengths (Å ) | WBI | Bond Lengths (Å ) | WBI | Bond Lengths (Å ) | WBI | |||
| Hg1-C1 | 2.25 | 0.40 | Hg1-C1 | 2.30 (2.15) | 0.34 | Hg1-C1 | 2.38 | 0.30 |
| Hg1-C14 | 2.25 | 0.41 | Hg1-C14 | 2.25 (2.16) | 0.40 | Hg1-C14 | 2.35 | 0.33 |
| Hg1-Cl1 | 2.69 | 0.30 | Hg1-Br3 | 2.92 (3.22) | 0.27 | Hg1-I1 | 2.92 | 0.50 |
| Hg1-Cl2 | 2.71 | 0.30 | Hg1- Br4 | 2.77 (3.16) | 0.36 | Hg1-I2 | 2.88 | 0.55 |
| Hg2-Cl1 | 2.80 | 0.24 | Hg2-Br3 | 2.97 (2.69) | 0.25 | Hg2-I1 | 3.24 | 0.24 |
| Hg2-Cl2 | 2.87 | 0.20 | Hg2-Br4 | 3.01 (2.61) | 0.22 | Hg2-I2 | 3.26 | 0.23 |
| Hg2-Cl3 | 2.40 | 0.61 | Hg2-Br5 | 2.51 (2.57) | 0.71 | Hg2-I3 | 2.70 | 0.79 |
| Hg2-Cl4 | 2.38 | 0.64 | Hg2-Br6 | 2.53 (2.56) | 0.65 | Hg2-I4 | 2.71 | 0.76 |
| Bond Angels (˚) | Bond Angels (˚) | Bond Angels (˚) | ||||||
|---|---|---|---|---|---|---|---|---|
| C1-Hg1-C14 | 155.91 | C1-Hg1-C14 | 153.94 (175.35) | C1-Hg1-C14 | 135.95 | |||
| C1-Hg1-Cl1 | 98.28 | C1-Hg1-Br3 | 86.88 (86.91) | C1-Hg1-I1 | 97.57 | |||
| C1-Hg1-Cl2 | 97.81 | C1-Hg1-Br4 | 96.08 (88.94) | C1-Hg1-I2 | 102.84 | |||
| C14-Hg1-Cl1 | 99.80 | C14-Hg1-Br3 | 93.49 (95.12) | C14-Hg1-I1 | 102.39 | |||
| C14-Hg1-Cl2 | 97.24 | C14-Hg1-Br4 | 109.39 (95.46) | C14-Hg1-I2 | 106.88 | |||
| Cl1-Hg1-Cl2 | 92.27 | Br3-Hg1-Br4 | 100.75 (83.11) | I1-Hg1-I2 | 109.18 | |||
| Cl1-Hg2-Cl2 | 86.78 | Br3-Hg2-Br4 | 94.37 (106.22) | I1-Hg2-I2 | 93.20 | |||
| Cl1-Hg2-Cl3 | 98.11 | Br3-Hg2-Br5 | 98.61 (107.60) | I1-Hg2-I3 | 99.21 | |||
| Cl1-Hg2-Cl4 | 107.85 | Br3-Hg2-Br6 | 99.87 (97.36) | I1-Hg2-I4 | 101.88 | |||
| Cl2-Hg2-Cl3 | 104.43 | Br4-Hg2-Br5 | 100.51 (108.73) | I2-Hg2-I3 | 100.89 | |||
| Cl2-Hg2-Cl4 | 99.66 | Br4-Hg2-Br6 | 94.96 (118.25) | I2-Hg2-I4 | 97.39 | |||
| Cl3-Hg2-Cl4 | 145.35 | Br5-Hg2-Br6 | 154.84 (116.96) | I3-Hg2-I4 | 151.13 | |||
Two interacting fragments, Hg2X4 and Y fragments in [HgY2][HgX4] complexes, are considered, and the nature of bonds between fragments is investigated using NBO calculations (Fig. 5). Data showed that by changing the X atom form Cl to I the values of WBI’s of Hg1-C1 slightly decreased. The natural charges on Hg metal ion and each of X (Cl, Br, I) and C atoms in ligand were also been determined (Table 4). Results revealed the positive and negative natural charges on Hg metal ion, and X and C atoms respectively.
Fig. 5.
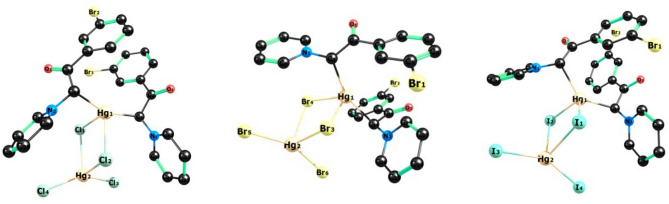
Optimized structures of [HgY2][HgX4] complexes at M06-d3/def2-TZVP level of theory.
Table 4.
Natural charges of Hg, C and X atoms of [HgY2][HgX4] complexes as well as the value of charge transfer in latter complexes at M06-d3/def2-TZVP level of theory.
| Natural charge (q) [HgY2][HgCl4] | Natural charge (q) [HgY2][HgBr4] | Natural charge (q) [HgY2][HgI4] | |||
|---|---|---|---|---|---|
| Hg1 | 0.88 | Hg1 | 0.85 | Hg1 | 0.65 |
| Hg2 | 0.92 | Hg2 | 0.75 | Hg2 | 0.49 |
| C1 | − 0.47 | C1 | − 0.45 | C1 | − 0.43 |
| C14 | − 0.47 | C14 | − 0.48 | C14 | − 0.45 |
| Cl1 | − 0.64 | Br3 | − 0.64 | I1 | − 0.45 |
| Cl2 | − 0.66 | Br4 | − 0.59 | I2 | − 0.43 |
| Cl3 | − 0.58 | Br5 | − 0.49 | I3 | − 0.37 |
| Cl4 | − 0.57 | Br6 | − 0.50 | I4 | − 0.38 |
In recent years, Morokuma23 and Ziegler24 introduced the EDA technique, which is a quantitative computational model for elucidating the bonding properties, the σ donation of L → M and back bonding M → L strengths in the metallic complexes25–31. In this case, to gain a better understanding of the bond nature between Hg2X4 fragment and ligand Y in the complex, we performed quantum chemical calculations of EDA under the (TPSS-D3/TZ2P(ZORA)//M06-d3/def2-TZVP) level considering C1 as the fixed symmetry for all complexes.
The ΔEint value can be divided into different components for simplify the analysis. Specifically, the Pauli repulsion ΔEPauli and the three attractive components contribute to this breakdown. It is shown that approximately 48.9–50.9% of the ΔEint value is attributed to the electrostatic attraction (ΔEelstat), while 39.8–43.2% is derived from the orbital term (ΔEorb). Additionally, 7.0–10.1% of the ΔEint value is accounted for by the dispersion term (ΔEdisp) in 1–3 (Table 5). Therefore, it may be inferred that the link between the two examined pieces in 1–3 is rather more electrostatic. The covalent connection between the X ligand and Hg metal ions in 1–3 is made apparent through the computed deformation densities Δρ. These densities are result of significant orbital interactions between the respective pieces. Fig. S15 shows the important deformation densities Δρ. Visual examination of the deformation densities of the latter confirms the donation of electrons from the ligand to the metal (M ← L) in the σ bond, as well as the back bonding from the metal to the ligand (M → L) in the complexes. Please observe that the color in Fig. S15 represents the direction of the charge flow, specifically from the red region to the blue region.
Table 5.
EDA analysis (TPSS-D3/TZ2P(ZORA)//M06-d3/def2-TZVP) of the complexes 1–3 with the C1 symmetrya.
| [HgY2][HgCl4] | [HgY2][HgBr4] | [HgY2][HgI4] | |
|---|---|---|---|
| ∆Eint | − 117.57 | − 120.66 | − 95.10 |
| ∆EPauli | 273.62 | 253.15 | 193.19 |
| ∆Eelstat | − 199.15 (50.91%) | − 182.69 (48.87%) | − 144.50 (50.12%) |
| ∆Eorb | − 164.52 (42.06%) | − 161.67 (43.25%) | − 114.67 (39.78%) |
| ∆Edisper | − 27.51 (7.03%) | − 29.44 (7.88%) | − 29.11 (10.10%) |
aThe energy values are at kcal mol−1.
Biological investigation
The antimicrobial efficacy of the novel compounds was assessed against four strains of both Gram-positive and Gram-negative bacteria groups, and subsequently compared to routine standards. The results are presented in Tables 6 and 7. The compounds were dissolved in various quantities in DMSO, and this solvent was also tested against all bacteria included in this investigation. However, no activity was observed. Most of the samples had the highest antibacterial activity against Pseadomonas aeroginesa and E. coli but in contrast, Bacillus thuringiensis and Staphylococcus aureus were resistant (Table 6). Although, the samples 1–4 showed significant antibacterial activity against all tested bacteria as well as standard antibiotics, sample 2 has lower antibacterial activity than other against bacteria species (Experiments were performed in triplicate and expressed as mean ± SD).
Table 6.
Antibacterial activity of the synthesized compounds.
| Inhibition zone (mm) | |||||
|---|---|---|---|---|---|
| Sample | Concentration (mg/L) | S. aureus | B. thuringiensis | E. coli | P. aeroginesa |
| 1 | 100 | 23.83 ± 0.57 | 21.16 ± 1.25 | 23.5 ± 0.5 | 25.16 ± 0.76 |
| 1 | 200 | 26.33 ± 0.76 | 27.33 ± 1.52 | 27.33 ± 1.52 | 28.5 ± 1.32 |
| 2 | 100 | 9.5 ± 0.5 | 9.76 ± 0.25 | 13.33 ± 0.76 | 18.16 ± 1.25 |
| 2 | 200 | 13.16 ± 1.04 | 11.83 ± 0.28 | 14.16 ± 0.76 | 21.83 ± 1.75 |
| 3 | 100 | 20.4 ± 0.96 | 16.16 ± 1.25 | 28.5 ± 1.32 | 24.16 ± 1.04 |
| 3 | 200 | 28.5 ± 1.5 | 19.66 ± 0.57 | 32.33 ± 1.52 | 28.03 ± 0.45 |
| 4 | 100 | 18.83 ± 0.76 | 17.36 ± 0.32 | 21.16 ± 1.04 | 29.16 ± 0.76 |
| 4 | 200 | 24.16 ± 0.76 | 21 ± 1 | 24.16 ± 1.04 | 32.83 ± 0.76 |
Table 7.
Antibacterial activity of the antibiotics as positive controls and DMSO as negative control.
| Microorganism | Inhibition zone (mm) | |||
|---|---|---|---|---|
| Positive controls | Negative control | |||
| Cephalexin | Penicillin | Gentamicin | DMSO | |
| E. coli | Na | Na | Na | Na |
| P. aeroginesa | 25 ± 0.57 | Na | 10 ± 0.18 | Na |
| S. aureus | 25 ± 0.44 | Na | Na | Na |
| B. thuringiensis | 20 ± 0.66 | Na | 30 ± 0.22 | Na |
Na No active.
General procedure of synthesis
All the chemical substances used in this study was purchased from Merck company. All of the reactions were performed in air. The NMR spectra were obtained by Joel and broker spectrometer (90 and 250 MHz, respectively) in CDCl3 and DMSO-d6 as the NMR solvent. The melting points were determined using a Stuart SMPI equipment. The Fourier Transform IR spectra were obtained using the Shimadzu 435-U-04 spectrophotometer, and the samples were also produced as KBr pellets.
Synthesis of S
0.08 ml of pyridine (1 mmol) was added to 20 ml of acetone solution included 0.28 g of 2,3′-dibromoactophenone (1 mmol) and stirred in the room temperature. After 12 h stirring at 25 °C, the produced precipitate (S) was separated off and washed with acetone. Yield: 88%; M.p.: 147–150 °C. Elm. Anal. % for C13H11ONBr2: C, 43.73; H, 3.11; N, 3.92. Exp.: C, 43.89; H, 3.11; N, 3.89. IR (KBr disk): υ (cm−1) 1702 (C=O). 1H NMR (CDCl3): δ (ppm) 6.58 (2H, s, CH2), 7.64–9.07 (16H, m, Ph). 13C NMR (CDCl3): δ (ppm) 66.7 (s, CH2); 122.7–146.9 (m, Ph); 190.3 (CO).
Synthesis of Y
0.35 g, 1 mmol of S was dissolved in 20 ml of 10% distilled water/K2CO3. The reaction was carried out in the ice bath for 45 min. The yellow precipitate was dried and weighted. Yield: 52%; M.p.: 95–97 °C. Elm. Anal. % for C13H10ONBr: C, 56.55; H, 3.65; N, 5.07. Exp.: C, 56.70; H, 3.92; N, 5.11. IR (KBr disk): υ(cm−1) 1588 (C=O). 1H NMR (CDCl3): δ (ppm) 4.49 (1H, s, CH); 7.27–8.60 (9H, m, Ph). 13C NMR (CDCl3): δ (ppm) 98.8 (s, CH); 121.7–149.7 (m, Ph); 170.5 (CO).
Synthesis of complexes 1–4
0.12 gr of Y (0.5 mmol) was added to 20 ml of methanol solution including 0.5 mmol of Hg(II) salt. The precipitate that formed after 6 h of stirring was isolated and cleaned with diethyl ether.
Data for 1
Yield: 89%; M.p.: 183–185 °C. Elm. Anal. % for C26H20O2N2Hg2Cl4Br2: C, 28.51; H, 1.84; N, 2.56. Exp.: C, 28.20; H, 1.80; N, 2.43. IR (KBr disk): υ(cm−1) 1663 (C=O). 1H NMR (DMSO-d6): δ (ppm) 7.01 (1H, s, CH); 7.33–9.10 (9H, m, Ph). 13C NMR (DMSO-d6): δ (ppm) 86.4 (s, CH3); 122.4–146.7 (m, Ph); 185.6 (CO).
Data for 2
Yield: 80%; M.p.: 190–192 °C. Anal. Calc. % for C26H20O2N2Hg2Br6: C, 24.53; H, 1.58; N, 2.20. Exp.: C, 24.15; H, 1.78; N, 2.31. IR (KBr disk): υ(cm−1) 1667 (C=O). 1H NMR (DMSO-d6): δ (ppm) 6.98 (1H, s, CH); 7.14–9.10 (9H, m, Ph). 13C NMR (DMSO-d6): δ (ppm) 88.0 (s, CH); 122.4–144.1 (m, Ph); 184.3 (CO).
Data for 3
Yield: 78%; M.p.: 183–185 °C. Elm. Anal. % for C26H20O2N2Hg2Br2I4: C, 21.37; H, 1.38; N, 1.92. Exp.: C, 21.89; H, 1.45; N, 2.02. IR (KBr disk): υ(cm−1) 1658 (C=O). 1H NMR (DMSO-d6): δ (ppm) 7.00 (1H, s, CH); 7.39–9.10 (9H, m, Ph). 13C NMR (DMSO-d6): δ (ppm) 88.5 (s, CH); 122.4–144.1 (m, Ph); 184.4 (CO).
Data for 4
Yield: 73%; M.p.: 150–155 °C. IR (KBr disk): υ(cm−1) 1663 (C=O). 1H NMR (DMSO-d6): δ (ppm) 6.94 (1H, s, CH); 7.32–9.13 (9H, m, Ph). 13C NMR (DMSO-d6): δ (ppm) 86.1 (s, CH); 122.5–147.1 (m, Ph); 189.6 (CO).
X-ray crystallography
Crystals of C26H20Br6Hg2N2O2 ([C26H20Br2HgN2O2][HgBr4]) with a dark orange color were formed using the process of solvent diffusion, where methanol was introduced into DMSO-d6. An Oxford Diffraction SuperNova diffractometer was applied for data collection of compounds using mirror monochromated Cu Kα radiation (λ = 1.54184 Å) at 130 K. Using Olex232. The structure was resolved using the ShelXT33 software employing Intrinsic Phasing, and further refined using the ShelXL34 refinement package utilizing Least Squares minimization on F2. Data was corrected by Gaussian absorption method. The refinement process involved assigning anisotropic displacement parameters to all atoms except for hydrogen atoms, which were positioned based on geometric estimates and refined using the riding model.
Computational methods
The M06-d3 method35–38, in combination with the def2-TZVP basis set39–41, was selected to investigate the complexes 1–4. Quantum mechanical calculations were also performed using the Gaussian 09 program42. All measurements are performed in the gas phase. Harmonic vibrational frequency measurements are computed using the same theoretical framework to guarantee the absence of imaginary frequencies. Then, optimized geometries were used for theoretical analyses at the mentioned theory level. The crystal structure obtained from X-ray analysis of complex 2 was used as a starting point for the calculations. NBO43 analysis was also performed using the internal Gaussian 09 module. Bond analysis in terms of EDA was also performed in (TPSS-D3/TZ2P(ZORA)//M06-d3/def2-TZVP) with C1 symmetry consideration. The basis sets for all elements have triple quality augmented by the functions ADF with basis set TZ2P(ZORA) using the program package ADF2009.0144.
Antibacterial assays
To evaluate the antibacterial activities of the synthesized compounds, we employed two categories of bacteria: Gram-positive and Gram-negative bacteria. The selected approach for evaluation was the disc diffusion technique45. Initially, all compounds were dissolved in different concentrations of 50, 100, and 200 mg/L in the DMSO, and the experiments were performed using a 10 m bacterial suspension encompassing 1.5 × 108 bacteria/mL for the negative controls, a DMSO solution was used. Additionally, gentamicin, penicillin, and cephalosporin were employed as positive reference standards. Ultimately, a statistical analysis was performed utilizing the Student's t-Test through the SPSS software, and the data was presented as the mean value plus or minus the standard deviation.
Conclusion
In this study, the novel Hg(II) complexes including pyridinium ylide was synthesized and characterized by spectroscopic methods. The X-ray analysis proved that the novel structure for such complexes including two parts. In the one part, Hg metal coordinated directly to pyridinium ylide through ylidic carbon ([HgY2]). While, in another one, Hg atom surrounded by four bromine ions ([HgX4]) which two of them located as bridging atom. The structures of synthesized complexes were also studied with theoretical methods. The NBO, EDA, and EDA-NOCV analyses were applied to investigate the nature of metal–ligand bonding in the complexes. Lastly, the antibacterial activity of complexes 1–4 was examined against Gram positive (B. thuringiensis and S. aureus) and negative (P. aeroginesa and E. coli) bacteria represented significant levels of inhibitory potency respected to used standards.
Supplementary Information
Acknowledgements
We gratefully acknowledge the support of this work by the Bu-Ali Sina University.
Author contributions
Z. B.: data curation, formal analysis, writing-original draft. A. Y.: data curation, formal analysis, writing-original draft, writing-review and editing, project administration. S. J. S.: corresponding, supervision, resources, conceptualization, data curation, funding acquisition, administration. Z. A.: theoretical section, data curation, software, validation. M. B.: theoretical section, validation. M. A.K.: biological section, validation. R.W. G.: X-ray section, validation, software.
Data availability
The authors confirm that all relevant data are included in the article and/or its Supplementary information files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-71896-0.
References
- 1.Morris, D. G. Recent advances in the chemistry of ylides. Surv. Progress Chem.10, 189–257 (1983). 10.1016/B978-0-12-610510-0.50010-9 [DOI] [Google Scholar]
- 2.Bachrach, S. M. Molecular structure of phosphonium ylides. J. Org. Chem.57, 4367–4373 (1992). 10.1021/jo00042a012 [DOI] [Google Scholar]
- 3.Chauvin, R. & Canac, Y. Transition Metal Complexes of Neutral Eta1-Carbon Ligands. Vol. 30 (Springer Science & Business Media, 2010).
- 4.Johnson, A. with special contributions by WC Kaska, KAO Statzewski and DA Dixon, Ylides and Imines of Phosphorus. Wiley, New York, chapters6, 153-220 (1993).
- 5.Coyne, E., Gilheany, D., Katritzky, A., Meth-Cohn, O. & Rees, C. Comprehensive Organic Functional Group Transformation (Pergamon Press, Elsevier, Oxford, 1995). [Google Scholar]
- 6.Clark, J. S., Dossetter, A. G. & Whittingham, W. G. Stereoselective synthesis of the bicyclic core structure of the highly oxidised sesquiterpene neoliacinic acid. Tetrahedron Lett.37, 5605–5608 (1996). 10.1016/0040-4039(96)01136-7 [DOI] [Google Scholar]
- 7.Krohnke, F. & Timmler, H. Ber.69, 674 (1930).
- 8.Navarro, R. & Urriolabeitia, E. P. α-Stabilized phosphoylides as versatile multifunctional ligands. J. Chem. Soc. Dalton Trans.23, 4111–4122 (1999). 10.1039/a906116i [DOI] [Google Scholar]
- 9.Spannenberg, A., Baumann, W. & Rosenthal, U. Palladium (II) complexes of α-stabilized phosphorus ylides. Organometallics19, 3991–3993 (2000). 10.1021/om000429z [DOI] [Google Scholar]
- 10.Ebrahim, M. M., Stoeckli-Evans, H. & Panchanatheswaran, K. Reactivity of mercury (II) halides with the unsymmetrical phosphorus ylide Ph2PCH2CH2PPh2C(H)C(O)Ph: Crystal structure of {HgI2 [PPh2CH2CH2PPh2C(H)C(O)Ph]}n. Polyhedron26, 3491–3495 (2007). 10.1016/j.poly.2007.03.059 [DOI] [Google Scholar]
- 11.Sabounchei, S. J. et al. Synthesis and characterization of novel simultaneous C and O-coordinated and nitrate-bridged complexes of silver (I) with carbonyl-stabilized sulfonium ylides and their antibacterial activities. Dalton Trans.42, 2520–2529 (2013). 10.1039/C2DT31902K [DOI] [PubMed] [Google Scholar]
- 12.Sabounchei, S. J. et al. Pd(II) and Pt(II) complexes of α-keto stabilized sulfur ylide: Synthesis, structural, theoretical and catalytic activity studies. J. Mol. Struct.1135, 174–185 (2017). 10.1016/j.molstruc.2017.01.063 [DOI] [Google Scholar]
- 13.Bravo, P., Fronza, G., Ticozzi, C. & Gaudiano, G. Palladium (II) complexes with sulphonium ylides. J. Organomet. Chem.74, 143–154 (1974). 10.1016/S0022-328X(00)83771-7 [DOI] [Google Scholar]
- 14.Deuerlein, S., Leusser, D., Flierler, U., Ott, H. & Stalke, D. [(thf)Li2{H2CS(Nt-Bu)2}]2: Synthesis, polymorphism, and experimental charge density to elucidate the bonding properties of a lithium sulfur ylide. Organometallics27, 2306–2315 (2008). 10.1021/om800046n [DOI] [Google Scholar]
- 15.Sabounchei, S. J. et al. Reactivity of mercury(II) halides with the α-keto stabilized sulfonium ylides: Crystal structures of two new polymer and binuclear complexes and in vitro antibacterial study. Polyhedron53, 1–7 (2013). 10.1016/j.poly.2012.10.054 [DOI] [Google Scholar]
- 16.Pearson, R. G. Hard and soft acids and bases. J. Am. Chem. soc.85, 3533–3539 (1963). 10.1021/ja00905a001 [DOI] [Google Scholar]
- 17.Fronza, G., Bravo, P. & Ticozzi, C. Carbon-13 nuclear magnetic resonance studies of some phosphonium, arsonium, sulfonium and pyridinium keto-stabilized salts, and ylides and of their palladium(II) complexes. J. Organomet. Chem.157, 299–310 (1978). 10.1016/S0022-328X(00)91713-3 [DOI] [Google Scholar]
- 18.Dega-Szafran, Z. et al. Experimental and quantum chemical evidences for C-H⋯ N hydrogen bonds involving quaternary pyridinium salts and pyridinium ylides. J. Mol. Struct.555, 31–42 (2000). 10.1016/S0022-2860(00)00585-8 [DOI] [Google Scholar]
- 19.Sabounchei, S. et al. A new Pd(II) complex of a sulfur ylide; Synthesis, X-ray characterization, theoretical study and catalytic activity toward the Suzuki-Miyaura reaction. Polyhedron117, 273–282 (2016). 10.1016/j.poly.2016.05.046 [DOI] [Google Scholar]
- 20.Sabounchei, S. J., Gharacheh, M. A. & Hosseinzadeh, M. Synthesis and multinuclear NMR study of novel complexes of Zn(II) and Hg(II) containing phosphorus ylides. Asian J. Chem.22, 1949–1956 (2010). [Google Scholar]
- 21.Sabounchei, S. J. et al. Structural, theoretical and multinuclear NMR study of mercury (II) and silver (I) complexes with two new ambidentate phosphorus ylides. Polyhedron38, 131–136 (2012). 10.1016/j.poly.2012.02.034 [DOI] [Google Scholar]
- 22.van der Bondi, A. Waals volumes and radii. J. Phys. Chem.68, 441–451 (1964). 10.1021/j100785a001 [DOI] [Google Scholar]
- 23.Morokuma, K. Molecular orbital studies of hydrogen bonds. III. C=O··· H-O hydrogen bond in H2CO···H2O and H2CO···2H2O. J. Phys. Chem.55, 1236–1244 (1971). 10.1063/1.1676210 [DOI] [Google Scholar]
- 24.Ziegler, T., Rauk, A. & Baerends, A. J. Theor. Chim. Acta. (1977). 10.1007/BF02401406 [DOI] [Google Scholar]
- 25.Bayat, M. & Soltani, E. Stabilization of group 14 tetrylene compounds by N-heterocyclic carbene: A theoretical study. Polyhedron123, 39–46 (2017). 10.1016/j.poly.2016.10.053 [DOI] [Google Scholar]
- 26.Bayat, M. & Hatami, M. Nature of the metal–ligand bond in some [(CO)4M←BIIM (R)]{M= Cr, Mo, W; R= H, F, Cl, Br} complexes: A theoretical study. Polyhedron110, 46–54 (2016). 10.1016/j.poly.2016.02.022 [DOI] [Google Scholar]
- 27.Sabounchei, S. et al. A new Pd (II) complex of a sulfur ylide; Synthesis, X-ray characterization, theoretical study and catalytic activity toward the Suzuki-Miyaura reaction. Polyhedron117, 273–282 (2016). 10.1016/j.poly.2016.05.046 [DOI] [Google Scholar]
- 28.Frenking, G. et al. Towards a rigorously defined quantum chemical analysis of the chemical bond in donor–acceptor complexes. Coord. Chem. Rev.238, 55–82 (2003). 10.1016/S0010-8545(02)00285-0 [DOI] [Google Scholar]
- 29.Lein, M. & Frenking, G. in Theory and Applications of Computational Chemistry 291–372 (Elsevier, 2005).
- 30.Bayat, M. & Kamali, S. Computational landscape of the formation and nature of bond in the “1+1” versus “1+2” nano-sized complexes of some adducts of N-heterocyclic carbenes (NHC) with heavy elements of group II (Ca, Sr, Ba) metallocenes. J. Mol. Liq.222, 953–962 (2016). 10.1016/j.molliq.2016.07.097 [DOI] [Google Scholar]
- 31.Aidi, M. et al. Coordination chemistry of some new Mn(II), Cd(II) and Zn(II) macrocyclic Schiff base complexes containing a homopiperazine head unit. Spectral, X-ray crystal structural, theoretical studies and anticancer activity. Inorganica Chim. Acta490, 294–302 (2019). 10.1016/j.ica.2018.12.046 [DOI] [Google Scholar]
- 32.Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. & Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr.42, 339–341 (2009). 10.1107/S0021889808042726 [DOI] [Google Scholar]
- 33.Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. A: Found. Adv71, 3–8 (2015). 10.1107/S2053273314026370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheldrick, G. M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv.71, 3–8 (2015). 10.1107/S2053273314026370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao, Y. & Truhlar, D. G. A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys.10.1063/1.2370993 (2006). 10.1063/1.2370993 [DOI] [PubMed] [Google Scholar]
- 36.Wang, Y., Jin, X., Yu, H. S., Truhlar, D. G. & He, X. Revised M06-L functional for improved accuracy on chemical reaction barrier heights, noncovalent interactions, and solid-state physics. Proc. Natl. Acad. Sci.114, 8487–8492 (2017). 10.1073/pnas.1705670114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc.120, 215–241 (2008). 10.1007/s00214-007-0310-x [DOI] [Google Scholar]
- 38.Wang, Y., Verma, P., Jin, X., Truhlar, D. G. & He, X. Revised M06 density functional for main-group and transition-metal chemistry. Proc. Natl. Acad. Sci.115, 10257–10262 (2018). 10.1073/pnas.1810421115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Austin, A. et al. A density functional with spherical atom dispersion terms. J. Chem. Theory Comput.8, 4989–5007 (2012). 10.1021/ct300778e [DOI] [PubMed] [Google Scholar]
- 40.Autschbach, J., Ziegler, T., van Gisbergen, S. J. & Baerends, E. J. Chiroptical properties from time-dependent density functional theory. I. Circular dichroism spectra of organic molecules. J. Chem. Phys.116, 6930–6940 (2002). 10.1063/1.1436466 [DOI] [Google Scholar]
- 41.Barone, V. et al. Implementation and validation of a multi-purpose virtual spectrometer for large systems in complex environments. Phys. Chem. Chem. Phys.14, 12404–12422 (2012). 10.1039/c2cp41006k [DOI] [PubMed] [Google Scholar]
- 42.Frisch, M. et al. Gaussian 09, Revision a. 02, 200, gaussian. Inc., Wallingford, CT271 (2009).
- 43.Weinhold, F., Landis, C. & Glendening, E. What is NBO analysis and how is it useful?. Int. Rev. Phys. Chem.35, 399–440 (2016). 10.1080/0144235X.2016.1192262 [DOI] [Google Scholar]
- 44.Baerends, E.J. et al. ADF2017, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands. ADF. Available online: http://www.scm.com (accessed on 20 April 2020) (2014).
- 45.Balouiri, M., Sadiki, M. & Ibnsouda, S. K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharma. Anal.6, 71–79 (2016). 10.1016/j.jpha.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that all relevant data are included in the article and/or its Supplementary information files.