Abstract
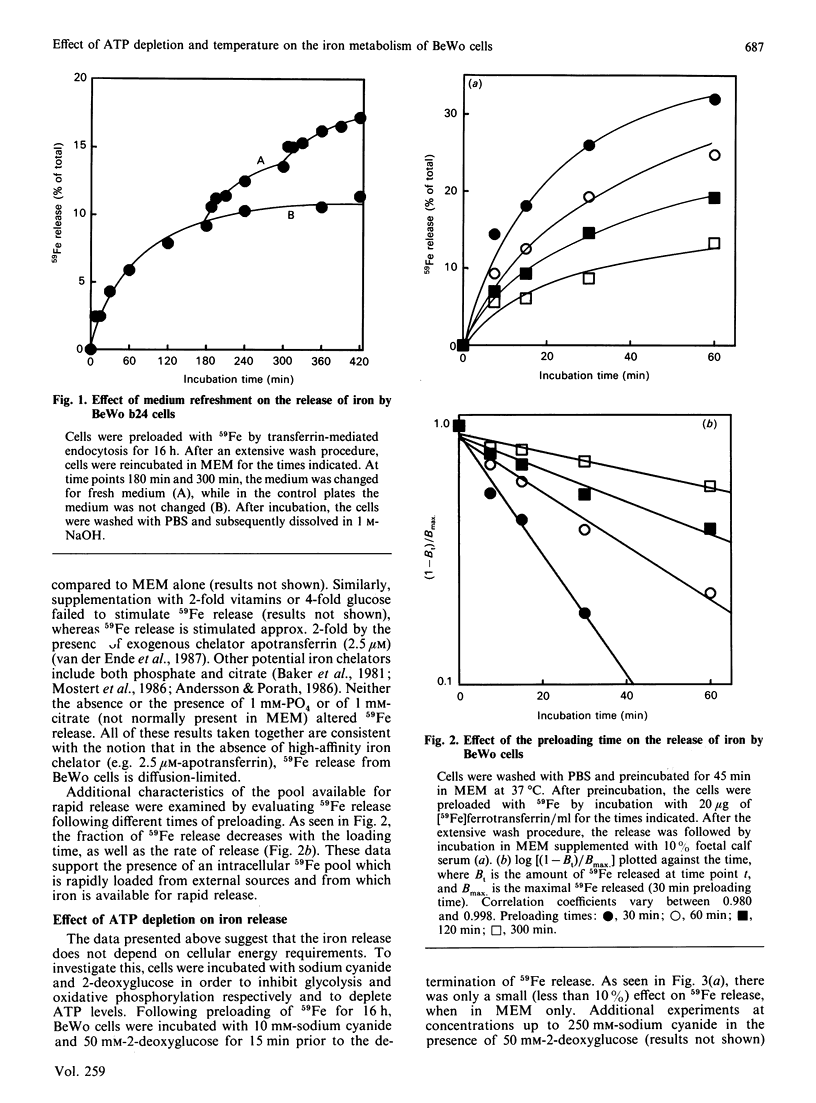
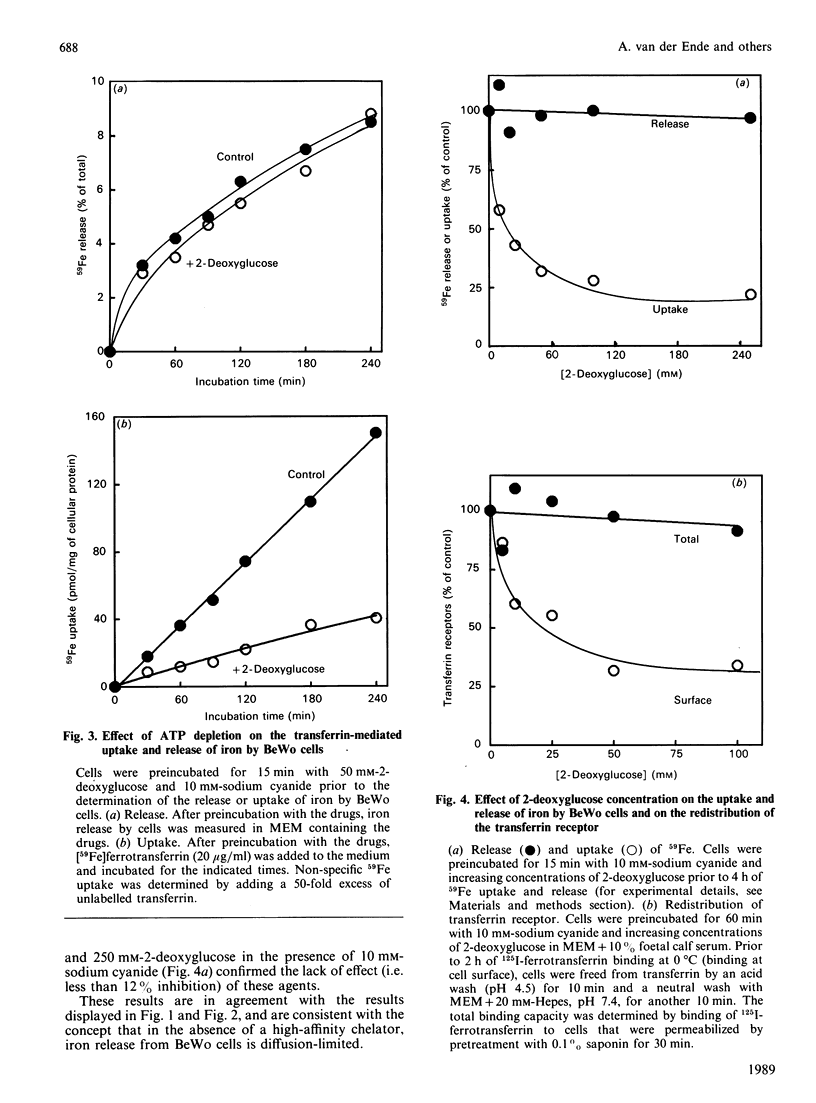
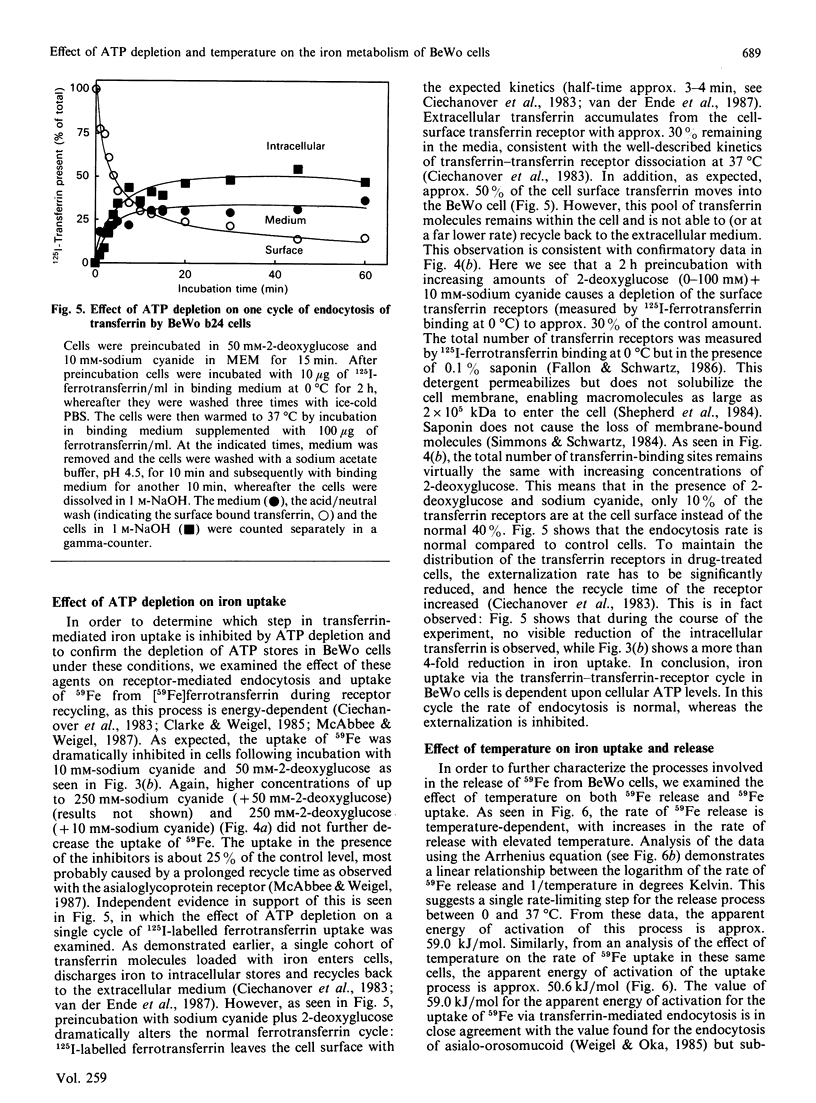
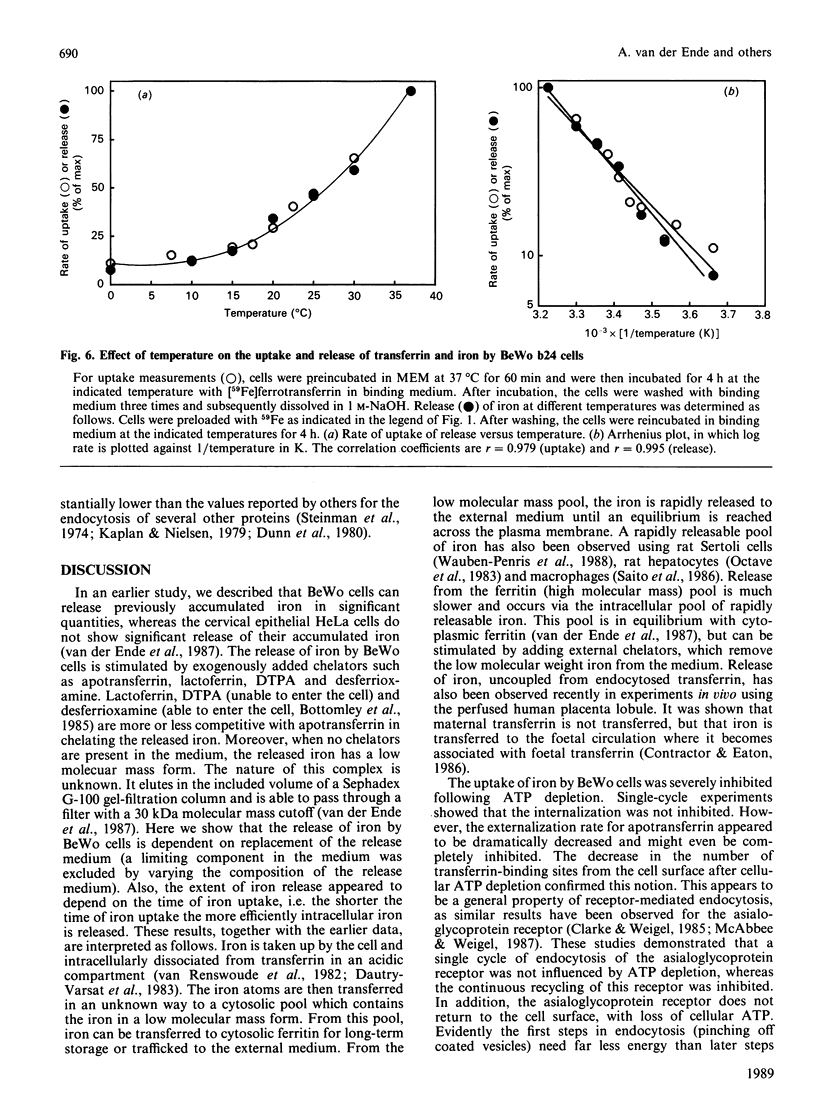
We have recently described the transferrin-mediated uptake and release of iron by BeWo cells [van der Ende, du Maine, Simmons, Schwartz & Strous (1987) J. Biol. Chem. 262, 8910-8916]. We now extend our studies of the mechanisms responsible for uptake and release of iron by these cells. Following preloading, 59Fe release was maximal (about 12%) after about 4 h. Replacement of the extracellular medium with an equal volume of fresh medium either prior to or following the time at which equilibrium was reached further stimulated 59Fe release. Both the rate and maximum amount of iron release decreased if longer loading times were used. Preincubation of BeWo cells for 15 min with 10 mM-sodium cyanide and 50 mM-2-deoxyglucose prior to the determination of 59Fe release did not alter the amount released into medium (which did not contain a high-affinity iron chelator). However, under these conditions, the uptake of 59Fe was dramatically inhibited as a result of prolongation of the transferrin-transferrin-receptor complex recycling time. These results demonstrate that the release of iron from BeWo cells is independent of cellular ATP levels, whereas iron uptake is ATP-dependent. Rates of both 59Fe release and 59Fe uptake were temperature-dependent. Analysis of these data via an Arrhenius plot suggests a single rate-limiting step for the release and uptake processes between 0 and 37 degrees C. The apparent energies of activation of these processes are very similar (approx. 59.0 kJ/mol for iron release and 50.6 kJ/mol for iron uptake), which raises the possibility that the release and uptake of iron share a common thermodynamically rate-limiting step. Possible mechanisms involved in iron release out of the cell and out of the endosome are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Andersson L., Porath J. Isolation of phosphoproteins by immobilized metal (Fe3+) affinity chromatography. Anal Biochem. 1986 Apr;154(1):250–254. doi: 10.1016/0003-2697(86)90523-3. [DOI] [PubMed] [Google Scholar]
- Baker E., Vicary F. R., Huehns E. R. Iron release from isolated hepatocytes. Br J Haematol. 1981 Apr;47(4):493–504. doi: 10.1111/j.1365-2141.1981.tb02678.x. [DOI] [PubMed] [Google Scholar]
- Bakkeren D. L., de Jeu-Jaspars C. M., van der Heul C., van Eijk H. G. Analysis of iron-binding components in the low molecular weight fraction of rat reticulocyte cytosol. Int J Biochem. 1985;17(8):925–930. doi: 10.1016/0020-711x(85)90177-6. [DOI] [PubMed] [Google Scholar]
- Bottomley S. S., Wolfe L. C., Bridges K. R. Iron metabolism in K562 erythroleukemic cells. J Biol Chem. 1985 Jun 10;260(11):6811–6815. [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A. L., Dautry-Varsat A., Lodish H. F. Kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. J Biol Chem. 1983 Aug 25;258(16):9681–9689. [PubMed] [Google Scholar]
- Clarke B. L., Weigel P. H. Recycling of the asialoglycoprotein receptor in isolated rat hepatocytes. ATP depletion blocks receptor recycling but not a single round of endocytosis. J Biol Chem. 1985 Jan 10;260(1):128–133. [PubMed] [Google Scholar]
- Contractor S. F., Eaton B. M. Role of transferrin in iron transport between maternal and fetal circulations of a perfused lobule of human placenta. Cell Biochem Funct. 1986 Jan;4(1):69–74. doi: 10.1002/cbf.290040111. [DOI] [PubMed] [Google Scholar]
- Crichton R. R., Charloteaux-Wauters M. Iron transport and storage. Eur J Biochem. 1987 May 4;164(3):485–506. doi: 10.1111/j.1432-1033.1987.tb11155.x. [DOI] [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. A., Hubbard A. L., Aronson N. N., Jr Low temperature selectively inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused rat liver. J Biol Chem. 1980 Jun 25;255(12):5971–5978. [PubMed] [Google Scholar]
- Fallon R. J., Schwartz A. L. Regulation by phorbol esters of asialoglycoprotein and transferrin receptor distribution and ligand affinity in a hepatoma cell line. J Biol Chem. 1986 Nov 15;261(32):15081–15089. [PubMed] [Google Scholar]
- Faulk W. P., Galbraith G. M. Trophoblast transferrin and transferrin receptors in the host--parasite relationship of human pregnancy. Proc R Soc Lond B Biol Sci. 1979 Mar 26;204(1154):83–97. doi: 10.1098/rspb.1979.0014. [DOI] [PubMed] [Google Scholar]
- Forgac M., Cantley L., Wiedenmann B., Altstiel L., Branton D. Clathrin-coated vesicles contain an ATP-dependent proton pump. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1300–1303. doi: 10.1073/pnas.80.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. J., Skehan P. Morphological differentiation of human choriocarcinoma cells induced by methotrexate. Cancer Res. 1979 Jun;39(6 Pt 1):1960–1967. [PubMed] [Google Scholar]
- GITLIN D., KUMATE J., URRUSTI J., MORALES C. THE SELECTIVITY OF THE HUMAN PLACENTA IN THE TRANSFER OF PLASMA PROTEINS FROM MOTHER TO FETUS. J Clin Invest. 1964 Oct;43:1938–1951. doi: 10.1172/JCI105068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A. Low molecular weight intracellular iron transport compounds. Blood. 1977 Sep;50(3):433–439. [PubMed] [Google Scholar]
- Kaplan J., Nielsen M. L. Analysis of macrophage surface receptors. II. Internalization of alpha-macroglobulin . trypsin complexes by rabbit alveolar macrophages. J Biol Chem. 1979 Aug 10;254(15):7329–7335. [PubMed] [Google Scholar]
- Klausner R. D., Van Renswoude J., Ashwell G., Kempf C., Schechter A. N., Dean A., Bridges K. R. Receptor-mediated endocytosis of transferrin in K562 cells. J Biol Chem. 1983 Apr 25;258(8):4715–4724. [PubMed] [Google Scholar]
- Kojima N., Bates G. W. The formation of Fe3+-transferrin-CO3(2-) via the binding and oxidation of Fe2+. J Biol Chem. 1981 Dec 10;256(23):12034–12039. [PubMed] [Google Scholar]
- McAbee D. D., Weigel P. H. ATP depletion causes a reversible redistribution and inactivation of a subpopulation of galactosyl receptors in isolated rat hepatocytes. J Biol Chem. 1987 Feb 15;262(5):1942–1945. [PubMed] [Google Scholar]
- Moore M. S., Mahaffey D. T., Brodsky F. M., Anderson R. G. Assembly of clathrin-coated pits onto purified plasma membranes. Science. 1987 May 1;236(4801):558–563. doi: 10.1126/science.2883727. [DOI] [PubMed] [Google Scholar]
- Mostert L. J., de Jong G., Koster J. F., van Eijk H. G. Iron mobilization from isolated hepatocytes. Int J Biochem. 1986;18(11):1061–1064. doi: 10.1016/0020-711x(86)90254-5. [DOI] [PubMed] [Google Scholar]
- Octave J. N., Schneider Y. J., Crichton R. R., Trouet A. Iron mobilization from cultured hepatocytes: effect of desferrioxamine B. Biochem Pharmacol. 1983 Nov 15;32(22):3413–3418. doi: 10.1016/0006-2952(83)90370-2. [DOI] [PubMed] [Google Scholar]
- Pattillo R. A., Gey G. O. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 1968 Jul;28(7):1231–1236. [PubMed] [Google Scholar]
- Saito K., Nishisato T., Grasso J. A., Aisen P. Interaction of transferrin with iron-loaded rat peritoneal macrophages. Br J Haematol. 1986 Feb;62(2):275–286. doi: 10.1111/j.1365-2141.1986.tb02930.x. [DOI] [PubMed] [Google Scholar]
- Schmid S. L., Braell W. A., Schlossman D. M., Rothman J. E. A role for clathrin light chains in the recognition of clathrin cages by 'uncoating ATPase'. Nature. 1984 Sep 20;311(5983):228–231. doi: 10.1038/311228a0. [DOI] [PubMed] [Google Scholar]
- Shepherd V. L., Freeze H. H., Miller A. L., Stahl P. D. Identification of mannose 6-phosphate receptors in rabbit alveolar macrophages. J Biol Chem. 1984 Feb 25;259(4):2257–2261. [PubMed] [Google Scholar]
- Simmons C. F., Jr, Schwartz A. L. Cellular pathways of galactose-terminal ligand movement in a cloned human hepatoma cell line. Mol Pharmacol. 1984 Nov;26(3):509–519. [PubMed] [Google Scholar]
- Speeg K. V., Jr, Azizkhan J. C., Stromberg K. The stimulation by methotrexate of human chorionic gonadotropin and placental alkaline phosphatase in cultured choriocarcinoma cells. Cancer Res. 1976 Dec;36(12):4570–4576. [PubMed] [Google Scholar]
- Steinman R. M., Silver J. M., Cohn Z. A. Pinocytosis in fibroblasts. Quantitative studies in vitro. J Cell Biol. 1974 Dec;63(3):949–969. doi: 10.1083/jcb.63.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone D. K., Xie X. S., Racker E. An ATP-driven proton pump in clathrin-coated vesicles. J Biol Chem. 1983 Apr 10;258(7):4059–4062. [PubMed] [Google Scholar]
- Sun I. L., Navas P., Crane F. L., Morré D. J., Löw H. NADH diferric transferrin reductase in liver plasma membrane. J Biol Chem. 1987 Nov 25;262(33):15915–15921. [PubMed] [Google Scholar]
- Thorstensen K., Romslo I. Uptake of iron from transferrin by isolated rat hepatocytes. A redox-mediated plasma membrane process? J Biol Chem. 1988 Jun 25;263(18):8844–8850. [PubMed] [Google Scholar]
- Wauben-Penris P. J., Veldscholte J., van der Ende A., van der Donk H. A. The release of iron by Sertoli cells in culture. Biol Reprod. 1988 Jun;38(5):1105–1113. doi: 10.1095/biolreprod38.5.1105. [DOI] [PubMed] [Google Scholar]
- Weigel P. H., Oka J. A. The surface content of asialoglycoprotein receptors on isolated hepatocytes is reversibly modulated by changes in temperature. J Biol Chem. 1983 Apr 25;258(8):5089–5094. [PubMed] [Google Scholar]
- Yamashiro D. J., Fluss S. R., Maxfield F. R. Acidification of endocytic vesicles by an ATP-dependent proton pump. J Cell Biol. 1983 Sep;97(3):929–934. doi: 10.1083/jcb.97.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. P., Aisen P. Transferrin receptors and the uptake and release of iron by isolated hepatocytes. Hepatology. 1981 Mar-Apr;1(2):114–119. doi: 10.1002/hep.1840010205. [DOI] [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ende A., du Maine A., Simmons C. F., Schwartz A. L., Strous G. J. Iron metabolism in BeWo chorion carcinoma cells. Transferrin-mediated uptake and release of iron. J Biol Chem. 1987 Jun 25;262(18):8910–8916. [PubMed] [Google Scholar]
- van der Heul C., Kroos M. J., van Eijk H. G. Binding sites of iron transferrin on rat reticulocytes. Inhibition by specific antibodies. Biochim Biophys Acta. 1978 Aug 17;511(3):430–441. doi: 10.1016/0005-2736(78)90279-1. [DOI] [PubMed] [Google Scholar]


