Abstract
Salmonellosis is still one of the most reported zoonoses worldwide and poultry meat is a major source, as chickens are often persistent carriers of Salmonella. Medium-chain fatty acids (MCFA) are known for their strong antimicrobial activity. MCFAs used today in the animal feed industry, however, mainly originate from the palm oil industry, which is notorious for its negative impact on the climate. We investigated the effect of a specific blend of palm-free MCFAs (ranging from C6 to C9) on Salmonella Enteritidis (SE) colonization in broiler chickens and in vitro SE characteristics. Fifty Ross 308 broiler chickens were randomly divided in 2 treatment groups. Chickens received either un-supplemented feed or feed supplemented with 300 ppm MCFAs from D0 onwards. On D7, all chickens were orally inoculated with 1600 CFU of SE. Cloacal swabs (D11) and samples of liver and caeca (D12) of all animals were collected and SE was enumerated. Percentage of SE-positive caecum samples was significantly (P = 0.044) reduced in birds receiving MCFAs compared to those receiving unsupplemented feed (36% vs. 64%). In vitro work performed with the same SE strain showed that preincubating the Salmonella bacteria with MCFAs at a sub-minimal inhibitory concentration significantly (p < 0.05) reduced bacterial adhesion to and invasion in Caco-2 cells, which may explain the observed reduction in intestinal SE colonization in the in vivo trial. Together, these results show that the tested eco-friendly MCFA blend could be a promising tool in the control of Salmonella in broilers.
Key words: medium-chain fatty acid, Salmonella Enteritidis, broiler chicken, colonization
INTRODUCTION
Salmonellosis is still one of the most reported zoonoses in the European Union and poultry products such as meat and eggs pose a great threat. Chickens are often persistent carriers of Salmonella, which can lead to carcass contamination in the processing plant (Zeng et al., 2021). In order to efficiently reduce human salmonellosis, the potential transmission of Salmonella from poultry products to humans should be minimized.
Medium-chain fatty acids (MCFA) are saturated, unbranched acids with carbon chain lengths ranging from C6:0 to C12:0, recognized for their potent antibacterial properties against common poultry pathogens (Szabo et al., 2023). Currently, the predominant methods for producing MCFAs involve petrochemical processes or the use of plant and animal oils (Ahn et al., 2023). Notably, the MCFAs utilized in the animal feed industry predominantly originate from the hydrolysis of palm kernel oil. However, concerns are emerging regarding the role of palm plantations in activities such as deforestation, biodiversity loss, and climate change. Recently, more sustainable MCFA sources are becoming available via microbial fermentation utilizing palm-free carbon substrates, such as food waste and biological oils (Ahn et al., 2023). Such eco-friendly MCFA sources offer the opportunity to provide odd-numbered MCFAs as well, which are less readily available from natural sources (Ahn et al., 2023), potentially rendering them more resistant to microbial metabolism within the gastrointestinal tract and thus exhibiting greater antimicrobial activity, as previously hypothesized by De Smet et al. (2016).
The antibacterial effect of MCFA against Salmonella has been described before (Van Immerseel et al., 2004), with specifically C6 and C8 showing pronounced effectiveness against Salmonella. Next to a direct antibacterial effect, some MCFA (such as C6 and C8, but not C10) can also reduce the virulence of Salmonella by downregulating hilA, thereby reducing its invasion potential in gut epithelial cells. As such, gut colonization and translocation to organs of this bacterium may be reduced, at concentrations much lower than those needed to kill Salmonella or inhibit its growth.
The primary objective of this study was to assess the potential of a low dose of a palm-free blend of even and odd-numbered MCFAs in promoting broiler chicken resilience against Salmonella colonization. Additionally, we assessed whether a reduction in colonization could be attributed to reduced Salmonella adhesion and invasion in vitro. By concurrently investigating both the in vivo and in vitro effects, this research provides an initial assessment of the effectiveness of the tested MCFA as a potential strategy for mitigating Salmonella colonization in poultry production.
MATERIALS AND METHODS
Adhesion and Invasion Test
Chemicals and Bacterial strain. The MCFA blend (even and odd-numbered, C6 to C9) was supplied by Nutrition Sciences NV, Drongen, Belgium, comprising 80% active MCFAs and 20% emulsifier. An emulsifier-only control (100%) was used. Salmonella Enteritidis strain U.09.76 SENalr was originally isolated from a commercial farm in Belgium.
Minimum inhibitory concentrations. Serial 2-fold dilutions of MCFAs ranged from 32% to 0.004% (v/v) in Mueller Hinton (MH) broth at pH 6. Controls included 2% emulsifier in MH broth at pH 6. Bacteria were grown for 16 h in 5 ml Luria-Bertani broth (LB) at 37°C and used to obtain the final inoculum of approximately 108 cfu/ml in MH broth at pH 6 of which 100 µl was added to all wells. Growth was assessed after 20 h incubation at 37°C.
Culture of Caco-2 cell line. Caco-2 cells were cultured in cell medium (87% Dulbecco's modified Eagle's medium nutrient mix (DMEM), 10% fetal calf serum, 1% nonessential amino acid, 1% kanamycin (10 000 µg/ml), 1% penicillin (10 000 U/ml)-streptomycin (10,000 µg/ml), in a humidified incubator at a constant temperature of 37°C in a 5% CO2 enriched atmosphere. For viability, adhesion and invasion assays, Caco-2 cells were seeded in 96 or 24-well plates at a density of approximately 2×104 or 105 cells per well, respectively. The cells were allowed to grow for 24 h to reach confluency, which was checked by light microscopy. During the infection experiments, cell medium without kanamycin and penicillin–streptomycin was used.
Viability assay. Cells were exposed to Caco-2 cell medium (Raaymakers et al.. (2017) pH 7 or pH 6 with sub-MIC concentrations of MCFAs (0.04%), emulsifier (0.01%) or sodium deoxycholate (0.2%) for 4 h in 96-well plates. Viability was assessed using neutral red uptake (Repetto et al., 2008).
Adhesion and invasion assays. Highly invasive Salmonella cultures were prepared in LB broth with emulsifier (0.01%) or sub-MIC MCFA (0.04%) at pH 6, incubated for 4 h, then used to infect Caco-2 cells in 24-well plates at a multiplicity of infection (MOI) of 10:1. Adhesion and invasion capacities were determined as previously described (Verbrugghe et al., 2011).
Animal Trial
This study was approved by the Ethical Commission (EC) of Poulpharm under trial code P22335-A2. Housing was according to the EU directive 2010/63/EU and EU Guideline L197 “for the accommodation and care of animals used for experimental and other scientific purposes” and ETS123 (appendix A), implemented in Belgian law on 29th May 2013 “KB betreffende de bescherming van proefdieren”. Housing units were placed in such a way that light and environmental conditions were similar for all units.
Experimental design, diet and animal husbandry. Fifty day-old Ross 308 chickens of mixed sex were randomly divided over 2 nonphysically separated pens, 1 pen per treatment group, both situated in a HEPA-filtered floor pen room, with > 5 meter distance between the pens (D0). Chickens received either unsuplemented (CTRL) feed, or feed supplemented with MCFAs at 300 ppm (MCFA) from D0 until the end of the experiment (D12). All groups received ad libitum feed and water.
Preparation of Inoculum. The U.09.76 SENalr stock was plated on a Columbia Sheep blood (COLSB) plate and incubated at 37°C for 24 h. Thereafter, colonies were identified as Salmonella spp. via matrix assisted laser desorption/ionization time-of-flight mass spectrometer as confirmation. In parallel, 5 SE colonies were inoculated into 10 mL of brain heart infusion broth and put in a shaking incubator at 37°C for 24 h (= preliminary inoculum). Afterwards, an enumeration was done by making a dilution series (1:10). The remaining broth was kept at 4°C to avoid further growth. The dilutions were plated on COLSB plates (20 µL in triplicate per dilution step) and incubated at 37°C for 24 h. Then, the number of colonies were quantified to obtain a titration of the inoculum. Finally, the dose of the preliminary Salmonella inoculum was adjusted to obtain a final dose of 1.00×103 CFU. A back titration was performed which determined to be 1.60×103 CFU/ml.
Salmonella Inoculation. On D0, 1 pooled cloacal swab of 5 birds per pen was collected to confirm the Salmonella-free status of the chicks. Also 3 feed samples, a water sample and an environmental swab were collected. To determine the Salmonella status, first a pre-enrichment in buffered peptone water (BPW) was performed, followed by selective enrichment in Rappaport Vassiliadis Soya (RVS) broth (water, feed and environmental samples) or Muller-Kauffmann Tetrathionate-Novobiocin (MKttn) broth (cloacal swabs). Inoculated RVS and MKttn broth were incubated for 24 h at 41.5°C, respectively 37°C. On D7, all chickens were orally inoculated with 1.0 mL of SE strain U.09.76 SENalr at 1.60×103 CFU/dose. Cloacal swabs were collected from each chicken, 4 d after inoculation (D11). Five d after inoculation, all chicks were euthanized (as described in the approved EC dossier) and liver and caecum of alle animals were collected for the analysis of SE colonization (D12). All samples were transferred on ice and arrived within 2 h after the collection in the lab for further analysis.
Monitoring of Salmonella Excretion. Presence of SE strain U.09.76 was assumed in case growth was observed on the selective agar plates described below. Briefly, swab samples were enriched in 1 mL BPW. An aliquot of 100µl BPW was directly plated onto Brilliant Green agar supplemented with 10 µg/mL novobiocine and 200 µg/mL nalidixic acid (BGA Nov/Nal). These plates were incubated at 37°C overnight and suspect Salmonella-positive colonies were counted. The remaining BPW was aerobically incubated overnight at 37°C to perform the detection.
For the detection, an aliquot of 100 µL was transferred into 10 mL of MKttn broth and aerobically incubated overnight at 37°C. This broth was streaked with a 10 µL inoculation loop onto BGA Nov/Nal for re-isolation of SE U.09.76 upon incubation at 37°C overnight.
Organ Colonization. Each organ was transferred into an ultraturrax tube, followed by a 10-fold dilution with BPW and mixed for 1 minute by the ultraturrax mixer at maximal speed. An 100 µL aliquot was directly plated onto BGA Nov/Nal and aerobically incubated at 37°C overnight for subsequent colony counting. The remainder of the BPW was incubated aerobically overnight at 37°C, transferred into MKttn broth, incubated and plated for colony counting as described before.
Samples that were negative after direct plating but positive after enrichment were given an arbitrary CFU/swab or CFU/g value based on the limit of detection of the enumeration method: 100 CFU/g for organ tissues and 10 CFU/swab.
Statistics
Data analyses in vitro tests. In vitro tests were conducted in triplicate with 3 independent repeats. Cell viability was assessed as explained in Verbrugghe et al. (2011). Adhesion and invasion rates were calculated as the proportion of adhered or invaded Salmonella bacteria to the initially applied inoculum. All statistical analyses and graph design were conducted in R (v4.2.3) (R core team, 2018). To evaluate the effect of different treatments (MCFA, control, emulsifier control) on cell viability, adhesion, and invasion rates, linear mixed models (LMM) were used, incorporating biological replicate as a random effect. Tukey's all-pair comparisons with Bonferroni-corrected p-values were performed using the glht function from the multcomp package (Hothorn et al., 2008).
Data analyses in vivo trial. Data were analyzed using SAS Studio (version 3.81). Detection of the SE strain in cloacal swabs, liver and caecum samples was analyzed by a 1-sided Fisher exact test, analyzing the association between Salmonella status and feed type (CTRL vs. MCFA).
The SE enumeration data (CFU/unit) of positive cases were analyzed between groups by a linear regression model with treatment group as fixed effect. Residual plots were checked to evaluate model fit. Values were back-transformed for representation. Statistical significance was assessed at P ≤ 0.05, while 0.05 ≤ P ≤ 0.1 was considered a trend.
RESULTS AND DISCUSSION
Salmonella Positivity in Cecal Samples Was Reduced in the MCFA Group
Most research done with MCFA focusses on even-numbered MCFAs, while less is known about the effect of odd-numbered MCFAs (De Smet et al., 2016). With a preliminary broiler challenge model we aimed at examining the potential of a low dosage of a palm-free MCFA blend (C6–C9), with the inclusion of odd-numbered MCFAs. A trend (P = 0.08) towards a higher percentage of Salmonella positive swabs in the MCFA-treated group (68%) was observed compared to the control group (44%). However, a tendency was seen towards lower Salmonella counts in the MCFA group compared to the control group (56 ± 105 vs. 385 ± 889 CFU/swab, respectively; P = 0.10). In the MCFA group, the percentage of positive caecum samples was significantly lower compared to the CTRL group (36% vs. 64%; P = 0.044). However, the estimated mean contents in the positive cases (including samples positive after enrichment), only showed a trend (P = 0.08) towards lower Salmonella loads in the MCFA group (122 ± 67 CFU/g) compared to the CTRL group (1,950 ± 6,901 CFU/g). No significant difference was observed (P = 0.14) in the proportion of positive liver samples between the 2 groups (12% vs. 28% in the MCFA, respectively CTRL group. Similarly, no significant difference was detected (P = 0.31) in the estimated mean contents in liver tissue between the treated (100 ± 0 CFU/g) and control (686 ± 1,084 CFU/g) birds. The results are shown in Table 1.
Table 1.
Effect of MCFA blend on Salmonella enteritidis recovery in swabs (D11) and organ samples (D12).
| Swab recovery |
Caecal recovery |
Liver recovery |
||||
|---|---|---|---|---|---|---|
| Experimental group | +/tot. (%) | cfu/swab | +/tot. (%) | cfu/g | +/tot. (%) | cfu/g |
| Control | 11/25 (44) | 385 ± 889 | 16/25 (64) | 1950 ± 6901 | 7/25 (28) | 686 ± 1,084 |
| MCFA | 17/25 (68) | 56 ± 105 | 9/25 (36) | 122 ± 67 | 3/25 (12) | 100 ± 0 |
| P-value | 0.08 | 0.10 | 0.044 | 0.08 | 0.14 | 0.31 |
The model demonstrated to be moderately successful. Though statistically significant effects were obtained, also (borderline) trends were observed which may be partially attributed to the moderate infection level observed in the control group or the lack of pen repeats. Nonetheless, even at a low concentration (300 ppm), the preliminary in vivo trial showed a significant reduction in Salmonella positivity in caecum samples for the MCFA group compared to the control group, but without a reduction in bacterial load. These findings corroborate prior investigations demonstrating reduced Salmonella burden in poultry using MCFA in feed, albeit at higher concentrations than employed in the current study. For example, Van Immerseel et al. (2004) showed reduced Salmonella numbers in caeca of layers fed a diet supplemented with 3,000 ppm caproic acid, 3 d after inoculation. This may suggest that the incorporation of odd-numbered MCFAs in our blend, along with the potential synergistic effects of combining different MCFAs, might be a promising new control tool for Salmonella in poultry, even though this should be further validated.
MCFA Blend Reduces Salmonella Adhesion and Invasion Rates in Caco-2 Cell Culture
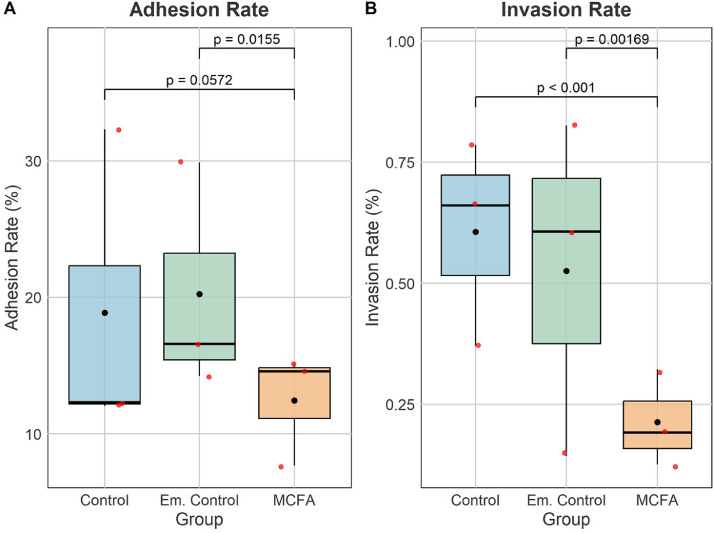
We investigated the impact of pretreatment with a sub-inhibitory concentration of 0.04% (chosen based on the minimal inhibitory concentration of 0.25% (data not shown) and similar to the proposed in-feed concentration [300 ppm]) of the MCFA blend on the ability of Salmonella Enteritidis U.09.76 SENalr to adhere to and invade a caco-2 culture. The adhesion rate (Figure 1A) was significantly lower when Salmonella bacteria were grown in LB medium containing MCFAs compared to bacteria grown in LB medium supplemented with the emulsifier (Padj = 0.016), giving mean adhesion rate values of 12.4 ± 4.15% and 20.2% ± 8.44%, respectively. In addition, the invasion rate (Figure 1B) of Salmonella bacteria grown in MCFA-supplemented LB medium was approximately 3 times lower (0.21 ± 0.10%) compared to the invasion rate of bacteria grown in unsupplemented LB medium supplemented with emulsifier (0.53 ± 0.35%) with Padj < 0.002.
Figure 1.
Cell-association efficiencies of Salmonella enteritidis after treatment with a MCFA blend. Salmonella bacteria to/in Caco-2 cells after being grown to logarithmic cultures for 4 h in the presence of MCFAs (0.04%), emulsifier (0.01% ; Em. Control) at pH 6. Control bacteria were included which were grown in unsupplemented LB medium (Control), at pH 6. Three technical replicates were performed per biological replicate, with 3 biological replicates in total. Figures represent the mean, median and variation (standard deviation) of the biological replicates. Mean is shown as a black circle, median as a vertical line inside the box, the first and third quartiles as the lower and upper edges of the box, respectively, and the minimum and maximum values as whiskers. Red circles represent biological replicates. The significance of difference between the distributions in shown with adjusted P-values obtained via Tukey's multiple comparisons corrections.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Ahn J.H., Jung K.H., Lim E.S., Kim S.M., Han S.O., Um Y. Recent advances in microbial production of medium chain fatty acid from renewable carbon resources: A comprehensive review. Bioresour. Technol. 2023;381 doi: 10.1016/j.biortech.2023.129147. [DOI] [PubMed] [Google Scholar]
- De Smet S., Michiels J., Ovyn A., Dierick N., Laget M., Cools A., Lauwaerts A. Gut antibacterial effects of C7 and C9 carboxylic acids in the diet of piglets. J. Anim. Sci. 2016;94:54–57. [Google Scholar]
- Hothorn T., Brezt F., Westfall P. Simultaneous inference in general parametric models. Biom. J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- Raaymakers C., Verbrugghe E., Hernot S., Hellebuyck T., Betti C., Peleman C., Claeys M., Bert W., Caveliers V., Ballet S., Martel A., Pasmans F., Roelants K. Antimicrobial peptides in frog poisons constitute a molecular toxin delivery system against predators. Nat. Commun. 2017;8:1495. doi: 10.1038/s41467-017-01710-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetto G., del Peso A., Zurita J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008;3:1125–1131. doi: 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- Szabo R.T., Kovács-Weber M., Zimborán A., Kovács L., Erdélyi M. Effects of short- and medium-chain fatty acids on production, meat quality, and microbial attributes-a review. Molecules. 2023;28:4956. doi: 10.3390/molecules28134956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Immerseel F., De Buck J., Boyen F., Bohez L., Pasmans F., Volf J., Sevcik M., Rychlik I., Haesebrouck F., Ducatelle R. Medium-chain fatty acids decrease colonization and invasion through hilA suppression shortly after infection of chickens with Salmonella enterica serovar Enteritidis. Appl. Environ. Microbiol. 2004;70:3582–3587. doi: 10.1128/AEM.70.6.3582-3587.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugghe E., Boyen F., Van Parys A., Van Deun K., Croubels S., Thompson A., Shearer N., Leyman B., Haesebrouck F., Pasmans F. Stress induced Salmonella typhimurium recrudescence in pigs coincides with cortisol induced increased intracellular proliferation in macrophages. Vet. Res. 2011;42:118. doi: 10.1186/1297-9716-42-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H., De Reu K., Gabriël S., Mattheus W., De Zutter L., Rasschaert G. Salmonella prevalence and persistence in industrialized poultry slaughterhouses. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]