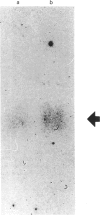
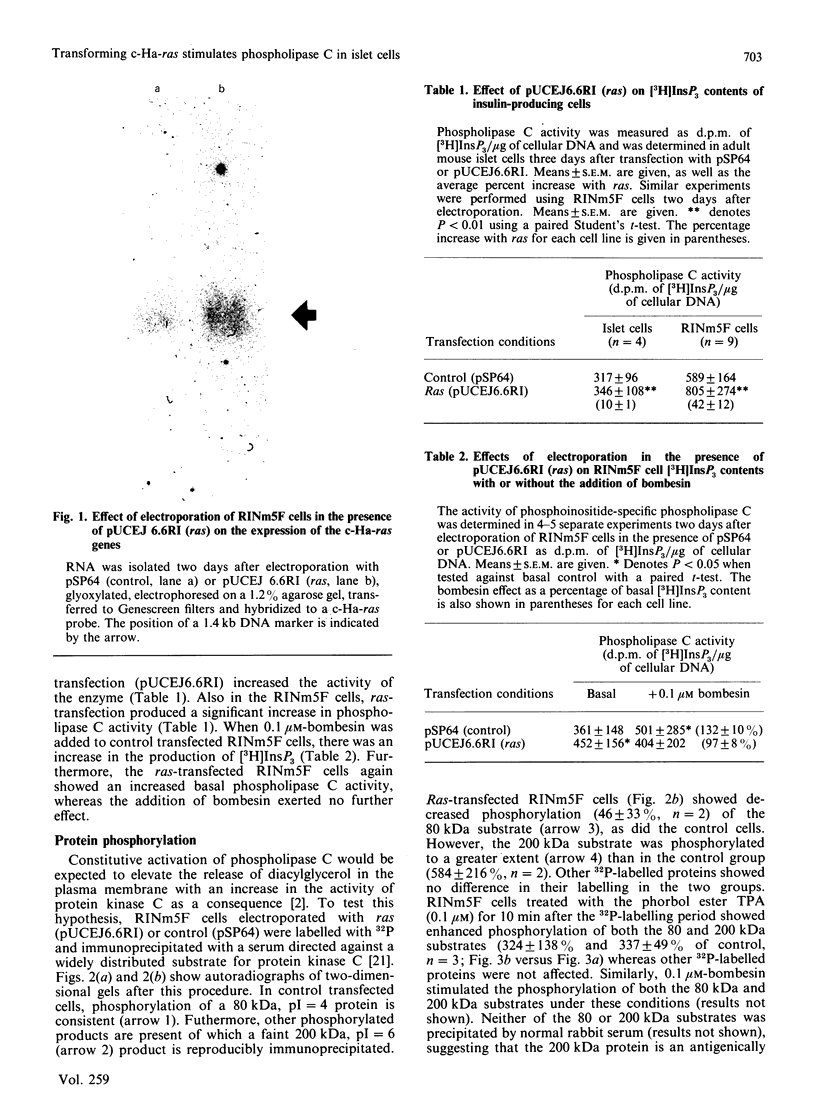
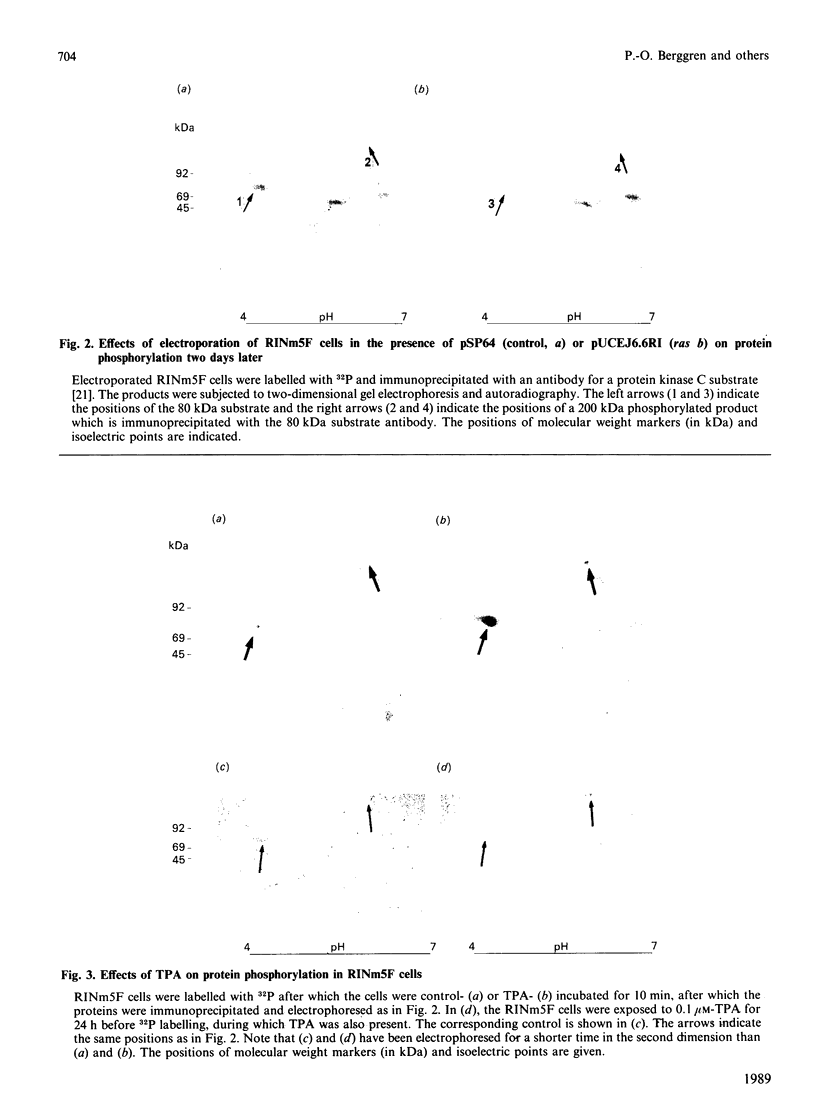
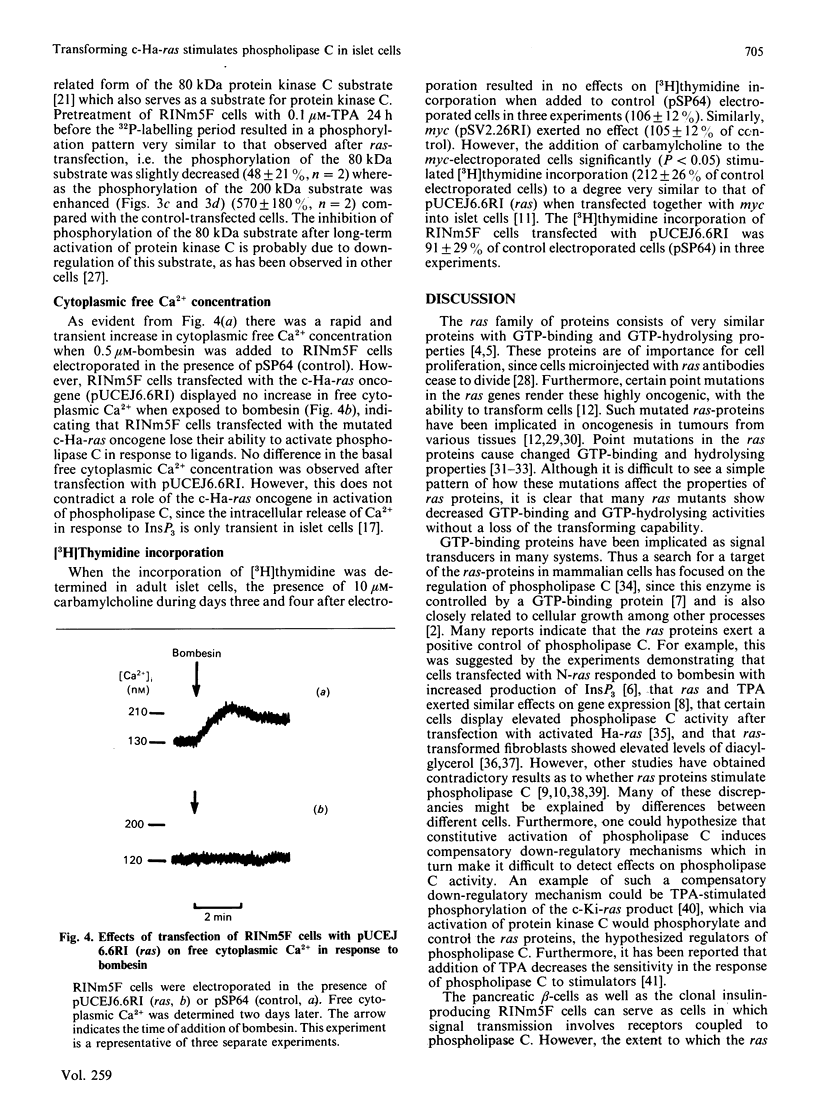
Abstract
Pancreatic islet beta-cells and insulin-producing RINm5F cells were electroporated in the presence of the c-Ha-ras oncogene, to assess the possible involvement of the encoded product in coupling extracellular receptors to phospholipase C. After two days the c-Ha-ras-transfected cells increased their expression of c-Ha-ras mRNA. These cells were also found to contain more [3H]InsP3, suggesting an increased basal (non-ligand-activated) phospholipase C activity. In addition, the transfected cells were unable to respond to ligand (bombesin) activation of phospholipase C. The ras-transfected insulin-producing cells showed enhanced phosphorylation of a 200 kDa substrate crossreacting with an antibody to an 80 kDa protein kinase C substrate. The phorbol ester 12-O-tetradecanoyl 13-acetate and bombesin also induced phosphorylation of the 200 kDa substrate. All of these changes occurred without changes in the rates of [3H]thymidine incorporation. The results suggest that the mutated c-Ha-ras oncogene directly or indirectly stimulates the basal phospholipase C activity of these cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert K. A., Walaas S. I., Wang J. K., Greengard P. Widespread occurrence of "87 kDa," a major specific substrate for protein kinase C. Proc Natl Acad Sci U S A. 1986 May;83(9):2822–2826. doi: 10.1073/pnas.83.9.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson A. Isolated mouse pancreatic islets in culture: effects of serum and different culture media on the insulin production of the islets. Diabetologia. 1978 Jun;14(6):397–404. doi: 10.1007/BF01228134. [DOI] [PubMed] [Google Scholar]
- Ballester R., Furth M. E., Rosen O. M. Phorbol ester- and protein kinase C-mediated phosphorylation of the cellular Kirsten ras gene product. J Biol Chem. 1987 Feb 25;262(6):2688–2695. [PubMed] [Google Scholar]
- Benjamin C. W., Tarpley W. G., Gorman R. R. The lack of PDGE-stimulated PGE2 release from ras-transformed NIH-3T3 cells results from reduced phospholipase C but not phospholipase A2 activity. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1254–1259. doi: 10.1016/0006-291x(87)91572-5. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M., Hamon M. H., Laurie M. S., Corps A. N. Protein kinase C-mediated negative-feedback inhibition of unstimulated and bombesin-stimulated polyphosphoinositide hydrolysis in Swiss-mouse 3T3 cells. Biochem J. 1987 Aug 1;245(3):631–639. doi: 10.1042/bj2450631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Der C. J., Pan B. T., Cooper G. M. rasH mutants deficient in GTP binding. Mol Cell Biol. 1986 Sep;6(9):3291–3294. doi: 10.1128/mcb.6.9.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T., Der C. J., Cooper G. M. Activation of ras genes in human tumors does not affect localization, modification, or nucleotide binding properties of p21. Cell. 1984 May;37(1):151–158. doi: 10.1016/0092-8674(84)90310-6. [DOI] [PubMed] [Google Scholar]
- Forrester K., Almoguera C., Han K., Grizzle W. E., Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. 1987 May 28-Jun 3Nature. 327(6120):298–303. doi: 10.1038/327298a0. [DOI] [PubMed] [Google Scholar]
- Gibbs J. B., Sigal I. S., Poe M., Scolnick E. M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Marshall C. J., McKay I. A., Gardner S., Houslay M. D., Hall A., Wakelam M. J. Mutant but not normal p21 ras elevates inositol phospholipid breakdown in two different cell systems. Oncogene. 1988 Aug;3(2):187–193. [PubMed] [Google Scholar]
- Hanley M. R., Jackson T. The ras gene. Transformer and transducer. Nature. 1987 Aug 20;328(6132):668–669. doi: 10.1038/328668a0. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Hellman B. The significance of calcium for glucose stimulation of insulin release. Endocrinology. 1975 Aug;97(2):392–398. doi: 10.1210/endo-97-2-392. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Moore J. P., Taylor M. V., Metcalfe J. C. Free cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes. J Biol Chem. 1983 Apr 25;258(8):4876–4882. [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. Potassium ions and the secretion of insulin by islets of Langerhans incubated in vitro. Biochem J. 1968 Jun;108(1):17–24. doi: 10.1042/bj1080017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Lacal J. C., Moscat J., Aaronson S. A. Novel source of 1,2-diacylglycerol elevated in cells transformed by Ha-ras oncogene. Nature. 1987 Nov 19;330(6145):269–272. doi: 10.1038/330269a0. [DOI] [PubMed] [Google Scholar]
- Lernmark A., Nathans A., Steiner D. F. Preparation and characterization of plasma membrane-enriched fractions from rat pancreatic islets. J Cell Biol. 1976 Nov;71(2):606–623. doi: 10.1083/jcb.71.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lernmark A. The preparation of, and studies on, free cell suspensions from mouse pancreatic islets. Diabetologia. 1974 Oct;10(5):431–438. doi: 10.1007/BF01221634. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Montague W., Morgan N. G., Rumford G. M., Prince C. A. Effect of glucose on polyphosphoinositide metabolism in isolated rat islets of Langerhans. Biochem J. 1985 Apr 15;227(2):483–489. doi: 10.1042/bj2270483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Newmark P. Oncogenes and cell growth. Nature. 1987 May 14;327(6118):101–101. doi: 10.1038/327101a0. [DOI] [PubMed] [Google Scholar]
- Nilsson T., Arkhammar P., Hallberg A., Hellman B., Berggren P. O. Characterization of the inositol 1,4,5-trisphosphate-induced Ca2+ release in pancreatic beta-cells. Biochem J. 1987 Dec 1;248(2):329–336. doi: 10.1042/bj2480329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oie H. K., Gazdar A. F., Minna J. D., Weir G. C., Baylin S. B. Clonal analysis of insulin and somatostatin secretion and L-dopa decarboxylase expression by a rat islet cell tumor. Endocrinology. 1983 Mar;112(3):1070–1075. doi: 10.1210/endo-112-3-1070. [DOI] [PubMed] [Google Scholar]
- Parries G., Hoebel R., Racker E. Opposing effects of a ras oncogene on growth factor-stimulated phosphoinositide hydrolysis: desensitization to platelet-derived growth factor and enhanced sensitivity to bradykinin. Proc Natl Acad Sci U S A. 1987 May;84(9):2648–2652. doi: 10.1073/pnas.84.9.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdew G. H., Schaup H. W., Selivonchick D. P. The use of a zwitterionic detergent in two-dimensional gel electrophoresis of trout liver microsomes. Anal Biochem. 1983 Dec;135(2):453–455. doi: 10.1016/0003-2697(83)90711-x. [DOI] [PubMed] [Google Scholar]
- Seuwen K., Lagarde A., Pouysségur J. Deregulation of hamster fibroblast proliferation by mutated ras oncogenes is not mediated by constitutive activation of phosphoinositide-specific phospholipase C. EMBO J. 1988 Jan;7(1):161–168. doi: 10.1002/j.1460-2075.1988.tb02796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar S., Notario V., Martin-Zanca D., Barbacid M. Induction of mammary carcinomas in rats by nitroso-methylurea involves malignant activation of H-ras-1 locus by single point mutations. Nature. 1983 Dec 15;306(5944):658–661. doi: 10.1038/306658a0. [DOI] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]
- Walter M., Clark S. G., Levinson A. D. The oncogenic activation of human p21ras by a novel mechanism. Science. 1986 Aug 8;233(4764):649–652. doi: 10.1126/science.3487832. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Imler J. L., Perez-Mutul J., Wasylyk B. The c-Ha-ras oncogene and a tumor promoter activate the polyoma virus enhancer. Cell. 1987 Feb 13;48(3):525–534. doi: 10.1016/0092-8674(87)90203-0. [DOI] [PubMed] [Google Scholar]
- Welsh M., Welsh N., Nilsson T., Arkhammar P., Pepinsky R. B., Steiner D. F., Berggren P. O. Stimulation of pancreatic islet beta-cell replication by oncogenes. Proc Natl Acad Sci U S A. 1988 Jan;85(1):116–120. doi: 10.1073/pnas.85.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfman A., Macara I. G. Elevated levels of diacylglycerol and decreased phorbol ester sensitivity in ras-transformed fibroblasts. Nature. 1987 Jan 22;325(6102):359–361. doi: 10.1038/325359a0. [DOI] [PubMed] [Google Scholar]
- Wolfman A., Wingrove T. G., Blackshear P. J., Macara I. G. Down-regulation of protein kinase C and of an endogenous 80-kDa substrate in transformed fibroblasts. J Biol Chem. 1987 Dec 5;262(34):16546–16552. [PubMed] [Google Scholar]