Abstract
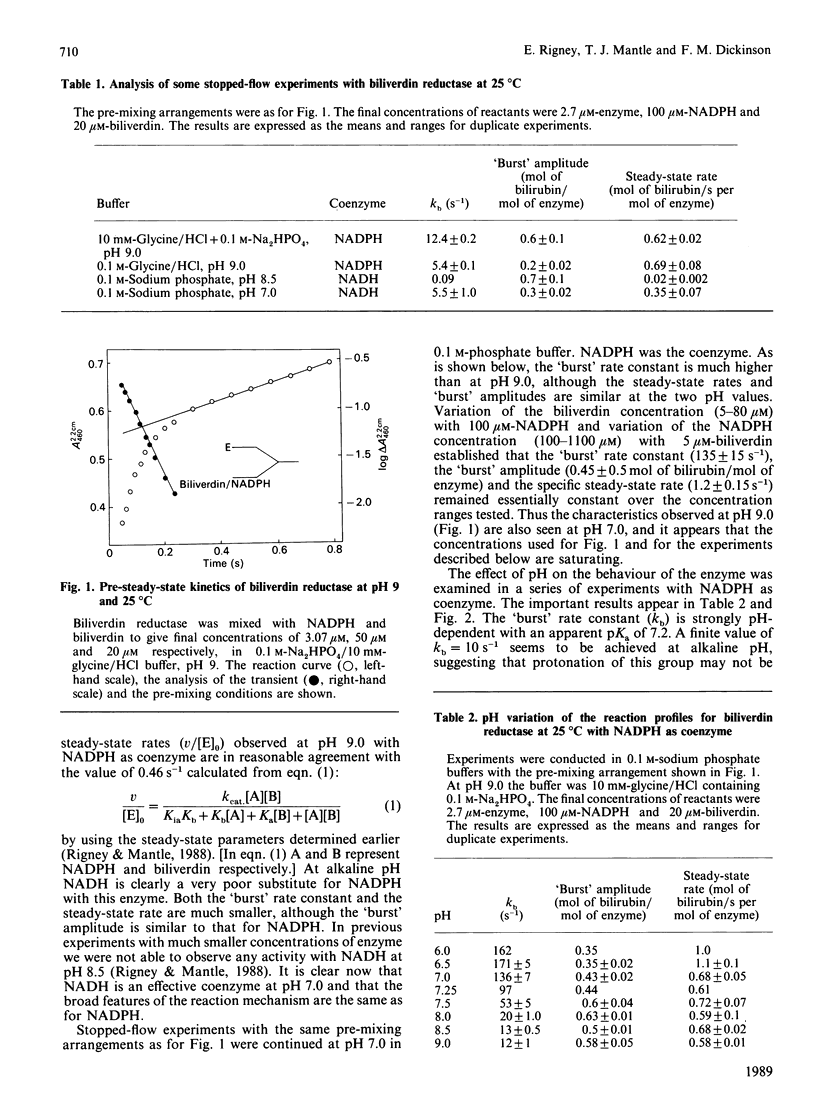
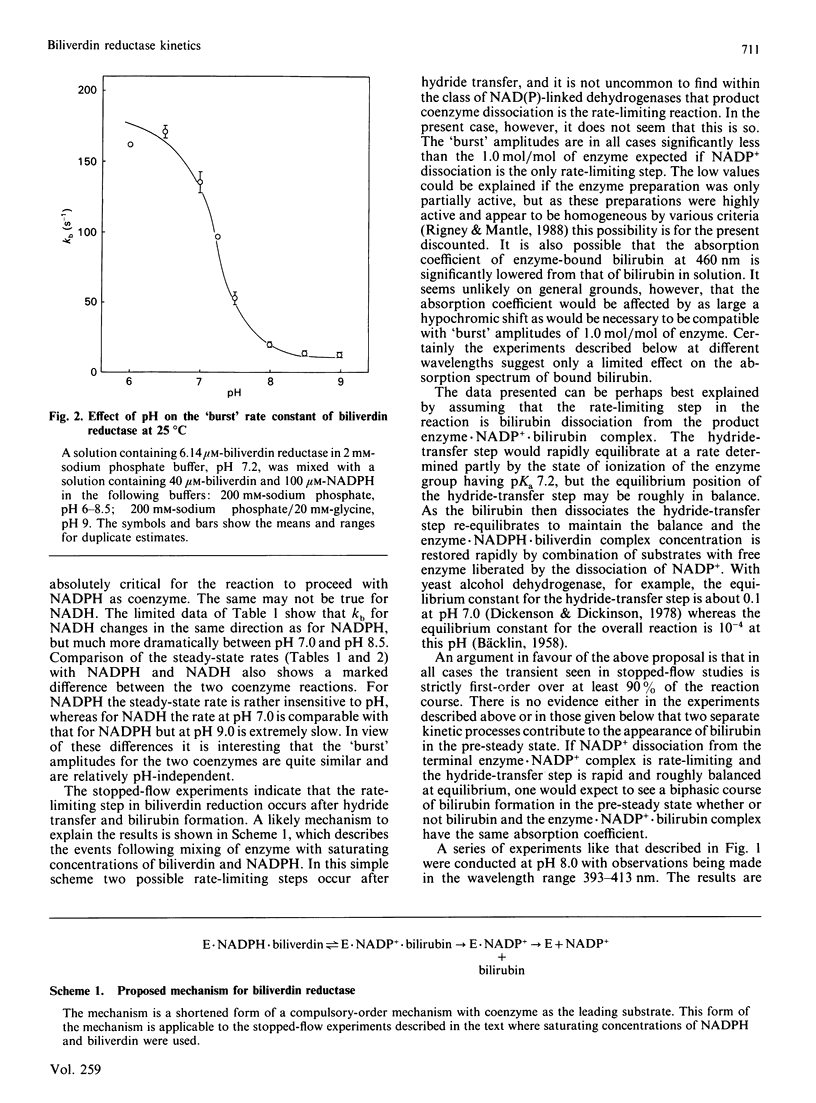
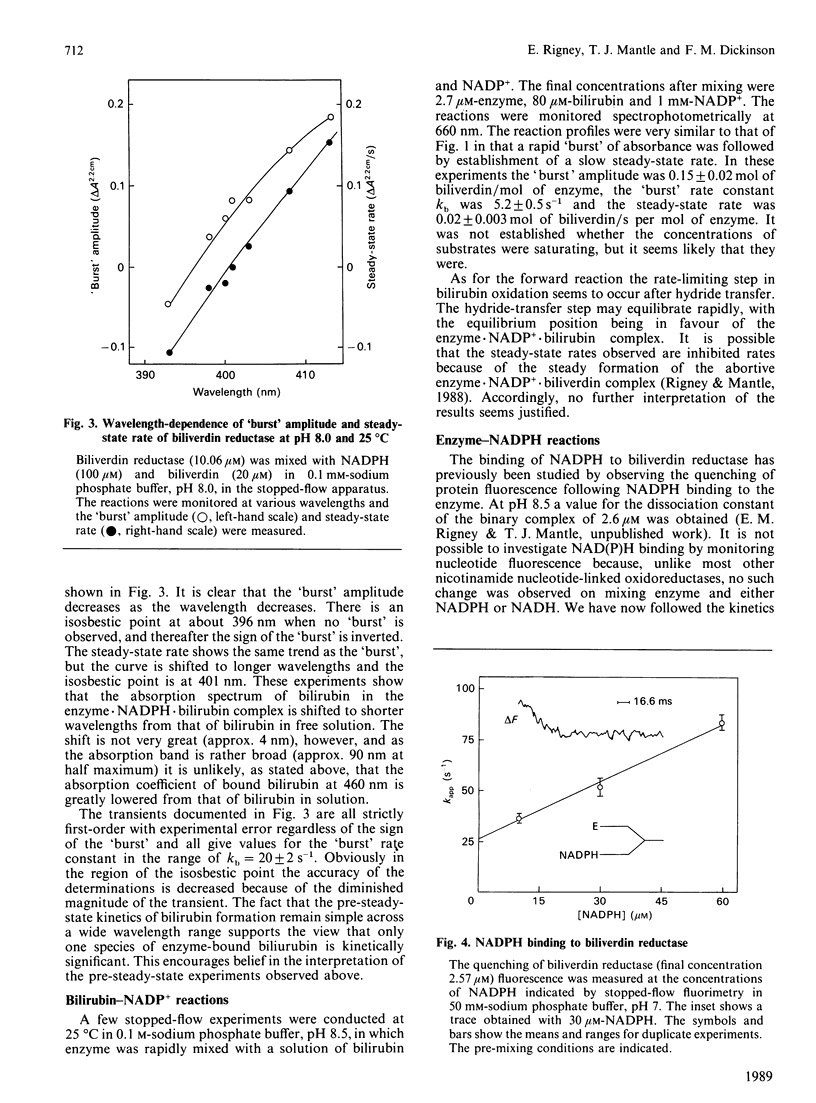
When the production of bilirubin by biliverdin reductase was monitored at 460 nm by stopped-flow spectrophotometry a 'burst' was observed with a first-order rate constant at pH 8 of 20 s-1. The steady-state rate was established on completion of the 'burst'. When the reaction was monitored at 401 nm there was no observed steady-state rate, but a diminished pre-steady-state 'burst' reaction was still seen with a rate constant of 22 s-1. We argue that the rate-limiting reaction is the dissociation of bilirubin from an enzyme.NADP+.bilirubin complex. With NADPH as the cofactor the hydride-transfer step was shown to exhibit pH-dependence associated with an ionizing group with a pK of 7.2. The kinetics of NADPH binding to the enzyme at pH 7.0 were measured by monitoring the quenching of protein fluorescence on binding the coenzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dickenson C. J., Dickinson F. M. Inhibition by ethanol, acetaldehyde and trifluoroethanol of reactions catalysed by yeast and horse liver alcohol dehydrogenases. Biochem J. 1978 Jun 1;171(3):613–627. doi: 10.1042/bj1710613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M., Dickenson C. J. Estimation of rate and dissociation constants involving ternary complexes in reactions catalysed by yeast alcohol dehydrogenase. Biochem J. 1978 Jun 1;171(3):629–637. doi: 10.1042/bj1710629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevery J., Van Damme B., Michiels R., De Groote J., Heirwegh K. P. Bilirubin conjugates in bile of man and rat in the normal state and in liver disease. J Clin Invest. 1972 Sep;51(9):2482–2492. doi: 10.1172/JCI107062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutty R. K., Maines M. D. Purification and characterization of biliverdin reductase from rat liver. J Biol Chem. 1981 Apr 25;256(8):3956–3962. [PubMed] [Google Scholar]
- Noguchi M., Yoshida T., Kikuchi G. Purification and properties of biliverdin reductases from pig spleen and rat liver. J Biochem. 1979 Oct;86(4):833–848. doi: 10.1093/oxfordjournals.jbchem.a132615. [DOI] [PubMed] [Google Scholar]
- Phillips O., Mantle T. J. Some kinetic and physical properties of biliverdin reductase. Biochem Soc Trans. 1981 Aug;9(4):275–278. doi: 10.1042/bst0090275. [DOI] [PubMed] [Google Scholar]
- Rigney E. M., Phillips O., Mantle T. J. Some physical and immunological properties of ox kidney biliverdin reductase. Biochem J. 1988 Oct 15;255(2):431–435. doi: 10.1042/bj2550431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigney E., Mantle T. J. The reaction mechanism of bovine kidney biliverdin reductase. Biochim Biophys Acta. 1988 Nov 23;957(2):237–242. doi: 10.1016/0167-4838(88)90278-6. [DOI] [PubMed] [Google Scholar]
- Stocker R., Ames B. N. Potential role of conjugated bilirubin and copper in the metabolism of lipid peroxides in bile. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8130–8134. doi: 10.1073/pnas.84.22.8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Glazer A. N., Ames B. N. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987 Feb 27;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]


