Abstract
Food security is a pressing issue, emphasizing the necessity for food designs that address the current geopolitical and geoeconomic challenges. This study evaluates the impact of including different percentages (10 %, 20 %, 30 %, and 60 %) of high protein quinoa flour (HPQF) in the development of a new rice-based snack. The aim is to create four snack formulations with a protein content enriched with probiotics, surpassing those currently available in the market. Probiotics Bacillus coagulans were added at a 0.1 % concentration. Once the rice flour and quinoa flour are mixed, they are mixed with the probiotic, to move on to the extrusion process. Following the incorporation of probiotics, the snacks were packaged in a modified atmosphere, and their physicochemical properties, Bacillus coagulans probiotic viability, tolerance to artificial gastroenteric juice (TAGJ), starch digestibility, and sensory acceptance were assessed.
Significant differences were observed in the expansion index, with the 60 % inclusion snack exhibiting the least expansion. Despite having a higher density, this snack reached a porosity index similar to that of the 20 % HPQF snack. Achieving a 17 % protein content in the snacks was possible with a 60 % inclusion rate. Texture was notably affected by the inclusion of HPQF, with snacks having higher inclusion levels showing increased hardness. Probiotic viability evaluation consistently remained above 106 UFC/g of snack, while TAGJ exhibited a viability of 75 %. Although HPQF inclusion led to a decrease in the glycemic index (GI), snacks still maintained a GI above 70 %. Regarding antioxidant properties, snacks with 60 % HPQF inclusion displayed superior results, reaching 35.29, 5.52, and 13.74 μmol of AA/g, measured via ABTS, DPPH, and FRAP methods, respectively. These findings demonstrate a heightened antioxidant capacity compared to other formulations. Our results indicate that the new probiotic snack serves as a rich source of protein and probiotics and is well-received sensorially. However, it is worth noting that it falls within the category of high GI foods, prompting the need for future studies aimed at reducing this parameter.
Keywords: Industrial level, Probiotic snacks, Extrusion process, Food security, Quinoa
Graphical abstract
1. Introduction
Second-generation snacks are those that surpass traditional products, providing innovation and extra nutritional value, including high levels of proteins, fiber, prebiotics, probiotics, vitamins, omega 3, and antioxidants [1]. These snacks predominantly incorporate plant-based ingredients like pea proteins, chickpea flour, or seaweed to offer more sustainable and eco-friendly choices [2]. Typically, the development of these products involves innovative technologies such as 3D printing, ingredient extrusion, or molecular cooking techniques, resulting in intriguing textures and shapes [3].
Developing these new products can enhance production chains through the utilization of ancient raw materials like quinoa. Quinoa, cultivated and consumed for centuries, retains unique genetic traits and is well-adapted to specific environmental conditions. Introducing these innovative foods can play a crucial role in addressing food insecurity, particularly in light of global statistics provided by the Food and Agriculture Organization of the United Nations, as that 843 million people worldwide suffer from hunger, while nearly one billion people have inadequate protein intake. For instance, in Colombia, data from the University Alliance for the Human Right to Adequate Food (ALUDHAA) (2022) reveals that over 71 % of households are classified as food insecure. Moreover, the study highlights that moderate food insecurity affects 26 % of households, with severe food insecurity affecting 14 %. Additionally, some Colombian households lack access to the three basic meals necessary for daily activities such as work, study, and other routine tasks. In response to these challenges, this study suggests structural measures aimed at supporting food production and implementing strategies to safeguard national producers.
New foods made from alternative ingredients are introduced, offering a variety of nutritional benefits such as plant-based snacks [4], seaweed [5], ancestral seeds [6,7], insects [8], and more. These innovative products, known as second-generation snacks, bring a fresh trend of high-quality items to the market. They are affordable, have a long shelf life, are easy to transport, and are rich in protein and other essential nutrients. Additionally, they contain bioactive and probiotic properties. These snacks provide a complementary dietary option for new generations and are appealing choices for all ages, including children, teenagers, and the general population, regardless of the time of day.
In this context, it is important to highlight the significance of products like quinoa, an ancient grain known for its impressive adaptability to various climatic conditions, including environmental stressors. Quinoa seeds are naturally gluten-free and contain abundant amounts of essential nutrients such as unsaturated fatty acids, vitamins, and minerals [9]. This makes them a compelling option for the production of vegan foods, given their high protein content and presence of bioactive compounds [[10], [11], [12], [13]]. Quinoa boasts an impressive protein content of 18 % [14], and its digestibility exceeds 90 % [15] following thermal treatment and high-pressure processing. To ensure the sustainability of these food products, it is essential to develop bioprocesses capable of removing antinutritional factors and extracting bioactive compounds on an industrial scale. This strategic approach aims to streamline large-scale production, ensuring efficient and safe manufacturing processes, and widespread availability in the market for mass consumption.
In addition to ensuring production, quality, preservation, and the nutritional content of macro and micronutrients, innovation in functional foods enhanced with probiotics brings added value to the ancestral grains supply chain. This innovation not only empowers producers but also facilitates the provision of nutritious foods to consumers, which can be easily transported and accessed in any region. The incorporation of probiotic bacteria of the genus Bacillus, known for their spore-forming ability, allows them to withstand challenging conditions, ensuring their survival during various food processing stages, such as high-temperature extrusion processes [16] and low-moisture environments [17]. Additionally, these bacteria exhibit increased resistance to the acidic conditions of gastric and pancreatic juices [18]. Building upon these principles, the objective of this study was to develop a novel snack by incorporating concentrated protein quinoa flour and adding probiotics before the extrusion process. Subsequently, the physicochemical properties of the snack were characterized, and its commercial acceptance was evaluated.
2. Method
2.1. Materials
Bacterial cultures: Freeze-dried probiotic strain of Bacillus coagulans BC30 was obtained from a commercial supplier (GanedenBC30, Kerry USA). The quinoa was sourced from Segalco S.A.S company (Popayán, Colombia). For proximal analysis, the samples were sent to a specialized laboratory for analysis Biotrends Laboratorios SAS, Bogota, Colombia.
2.2. Method
2.2.1. Snack production process
To produce the hyper-protein quinoa flour and whole rice flour snacks, the formulations specified in Table 1 were followed. This process utilized a twin-screw industrial extruder (CY65-II TWN SCREW EXTRUDER, Qingdao, China). The mixtures were wetted to 18 %, probiotic was added at 0.1 % and then subjected to extrusion-cooking at varying temperatures (60 °C, 80 °C, 110 °C) with a screw rotation speed ranging from 251 to 253 rpm. The extrusion nozzle had a diameter of 3 mm. Subsequently, the snacks were dried at 170 °C in a dryer, operating at a speed of 60–65 rpm. They were packaged in a modified atmosphere containing nitrogen, utilizing packaging with multiple layers. The water vapor transmission rate (WVTR) and oxygen transmission rate (OTR) were measured at 0.7 g/m2.day and 1.0 cc/m2.day, respectively. Finally, the snacks were stored at room temperature (20 °C).
Table 1.
Mixtures used in the extrusion process.
| Cereal | Formulation 1 (%) | Formulation 2 (%) | Formulation 3 (%) | Formulation 4 (%) |
|---|---|---|---|---|
| RF | 40 | 70 | 80 | 90 |
| HQHP | 60 | 30 | 20 | 10 |
HQHP: Hyper-protein quinoa flour; RF: rice flour.
2.2.2. Viability of the probiotic Bacillus coagulans during storage
The spore viable counts of B. coagulans was conducted following the methodology as provided by the probiotics’ supplier and [19], with slight modifications. Initially, approximately 1 g of snack was dissolved in 199 mL of sterile saline solution (0.9 % NaCl, w/v). Subsequently, 30 mL of the solution were transferred into a sterile tube and heated at 75 °C for 30 min in a water bath. Afterward, the solution was cooled to approximately 45 °C before pipetting. The mixture was then cultured on GYE agar at 37 °C for 48 h under aerobic conditions.
2.2.3. Assessment of Bacillus coagulans survival in gastric and pancreatic juices
The spore viable counts of the probiotic Bacillus coagulans incorporated into the snack under simulated gastric and enteric conditions was conducted following the protocol outlined in Ref. [17] with some adaptations.
Initially, 1 g of snack from each formulation was dissolved in 9 mL of sterile phosphate-buffered saline solution (PBS) with a pH of 7.4, replicated in sterile 50 mL tubes. Subsequently, 1 mL of this initial dilution was collected for the initial count. The solution was then adjusted to pH 2 using 1 N HCl, and pepsin sourced from porcine stomach mucosa (Sigma-Aldrich) was added to the samples at a concentration of 3 g/L, previously dissolved in PBS adjusted to pH 2. The Falcon tubes were incubated at 37 °C with agitation at 150 rpm (Thermo Scientific MAxQ-4450 Shaker, USA) for 3 h to simulate the gastric phase. Following this incubation period, 1 mL of the solution was cultured on GYE agar at 37 °C for 48 h under aerobic conditions. Next, the pH of the samples was raised to 6.8 using 30 mL of sterile alkaline solution composed of 150 mL of 1 N NaOH, 14 g of PO4H2Na.H2O, and distilled water up to 1 L. Bovine bile (Sigma-Aldrich) and porcine pancreas-derived pancreatin (Sigma-Aldrich) were added to achieve concentrations of 10 g/L and 1 g/L, respectively, during this phase. The three samples were then incubated for an additional 3 h. After this additional incubation period, 1 mL of sample was collected for culturing, following the same procedure as the previous phase. The counts are expressed in log CFU/g of snack. The survival rate at t0 and tf is determined according to Equation (1).
| (1) |
Where, N is the number of CFU (colony forming units).
2.2.4. Starch digestibility and glycemic index calculation
Starch digestibility was determined using the Megazyme kit. The calculation of the glycemic index followed the method outlined by Ref. [20], which involved assessing the area under the curve in the digestibility graphs of the different snack samples compared to white bread, serving as the reference. The glycemic index calculation follows Equation (2):
| (2) |
HI= Hydrolysis index
2.2.5. Bulk density of extrusion and expansion index
Bulk density of extrusion (BDE) was assessed using chia seeds and calculated according to Equation (3):
| (3) |
Here, PE and VE represent the weight and equivalent volume of the extrudate, respectively, while ρs and Ws denote the density and weight of chia seeds. Measurements of extrudate density were conducted fifteen times.
The expansion index (IE) was determined by dividing the radial diameter of the extrudate, measured using a vernier caliper, by the diameter of the extruder die hole (3 mm). Measurements of the expansion index for each extrusion series were repeated ten times, and the mean value was recorded.
2.2.6. Porosity
Porosity was evaluated by analyzing images of the cellular structure of the extruded snacks. The samples were cut transversely and placed within a computer vision system consisting of a chamber with matte black walls and base, illuminated by four 6500 k fluorescent lights (cool white light). These lights were positioned 45 cm away from the base at a 45° angle relative to the sample. Image capture was conducted using a Nikon D5600 camera without zoom flash or filters. Subsequently, the images were processed using ImageJ software, converted to grayscale, and the area of the pores in pixels squared was determined. Finally, the percentage of these pores relative to the cross-sectional area of the sample was calculated.
2.2.7. Texture analysis
The mechanical characteristics of the extruded products were assessed using a texture analyzer (Shimadzu EZ TEST SM, model 500N-168, Japan). The samples were compressed perpendicular to the direction of extrusion until they reached 50 % of their original diameter, employing a 5 mm compression probe at a testing speed of 1.0 mm/s. From the force-deformation curves, the area under the curve (S; N.mm) and the number of peaks (n) exceeding 1.5 N were determined. These parameters were then utilized to calculate the spatial frequency of fractures (Nsr), average crushing force (Fcr), and work of sharpness (Wc), following Equation (4), 5 y 6 [15].
| (4) |
| (5) |
| (6) |
where d = Travel distance of the probe (mm).
2.2.8. Color
The color was analyzed using a Konica Minolta CM-5 Spectrophotometer colorimeter, which was controlled by SpectraMagic NX software. This analysis utilized a D65 illuminant and a 10° observer angle. The resulting measurements included luminosity (L*), green/red chromaticity (a*), and blue/yellow chromaticity (b*). These measurements were then used to calculate chromaticity values (C*), hue angle (h°), total color difference (ΔE), whiteness index (WI), and browning index (BI), according to equation (7) through 11, respectively.
| (7) |
| (8) |
| (9) |
| (10) |
| Equation. (11) |
2.2.9. Viability of the probiotic Bacillus coagulans during storage
The spore viable counts of B. coagulans was performed following the methodology provided by the probiotic's supplier. Initially, about 1 g of snack was dissolved in 199 mL of sterile saline solution (0.9 % NaCl, w/v). Next, 30 mL of this solution were transferred to a sterile tube and heated at 75 °C in a water bath for 30 min. Afterward, the solution was cooled to approximately 45 °C before pipetting. Subsequently, the sample was cultured on GYE agar at 37 °C for 48 h under aerobic conditions.
2.2.10. Extractable phenolic compounds (EPP)
The method for extracting and analyzing EPP followed the procedure outlined in Ref. [21] with minor adjustments. Initially, 2 g ± 0.0500 g of sample, sieved through a 200 μm sieve, were measured. For the first extraction, 8 mL of ethanol/H2O (80/20, v/v with 1 % formic acid) were added and the mixture was agitated on an orbital shaker with chamber (MaxQ 4450, Thermo Scientific, USA) for 15 min. The resulting mixture was then centrifuged for 10 min at 3800 rpm g at 20 °C, and the supernatant was carefully preserved on ice. Subsequently, 8 mL of acetone/H2O (70/30, v/v) were added to the sediment for a second 15-min extraction with agitation. Following centrifugation, the supernatant was combined with the methanolic extract obtained from the first extraction and adjusted to 20 mL with deionized water. This combined extract was stored at −80 °C for subsequent colorimetric analysis using the Folin-Ciocalteu method or assessment of antioxidant capacity. Additionally, the sediment was stored at −80 °C for further analysis to determine hydrolyzable phenolic compounds (HPP) sequentially.
2.2.11. The EPP extracts were used to measure antioxidant properties
2.2.11.1. ABTS
In a test tube, 4 mL of ABTS solution were added and covered with aluminum foil. To start the reaction, 135 μL of either standard solution (for constructing the curve) or EPP extract were added, followed by vortex mixing for 5 s. The blank reagent consisted of 4 mL of acetate buffer and 135 μL of ethanol at 96 %. The zero point was prepared by mixing 4 mL of ABTS solution with 135 μL of ethanol. The test tube was then sealed and allowed to react for 30 min. Afterward, the absorbance was measured at a wavelength of 729.7 nm using a microplate reader (FL TOP ABS L, A - VL0LA0D0 VARIOSKAN LUX Thermo Scientific, USA).
2.2.11.2. DPPH
In a test tube, 3.9 mL of DPPH solution and 100 μL of either standard solution (to construct the curve) or EPP extract were combined to initiate a reaction via vortex mixing for 5 s. Ethanol was used as the blank. The baseline was established by adding 3.9 mL of DPPH solution and 100 μL of ethanol. The sample was then covered and allowed to react for 30 min. Following this, the absorbance was measured at 517 nm using a microplate reader (FL TOP ABS L, A - VL0LA0D0 VARIOSKAN LUX Thermo Scientific, USA).
2.2.11.3. FRAP
A solution containing 2.5 mL of 0.01 M TPTZ, 2.5 mL of 0.02 M FeCl3 and 25 mL of 0.3 M sodium acetate buffer (pH 3.6) was prepared in a test tube. From this solution, 1.8 mL were added. Then, 180 μL of distilled water and 60 μL of the sample solution were added. The mixture was vortexed for 15 s to initiate a reaction, followed by incubation at 37 °C for 30 min. Distilled water was used for the blank reactions (controls). Subsequently, the absorbance was measured at 595 nm using a microplate reader (FL TOP ABS L, A - VL0LA0D0 VARIOSKAN LUX Thermo Scientific, USA).
2.2.11.4. Sensory analysis
We conducted sensory evaluation using the hedonic test methodology with 91 untrained panelists from the University of Cauca, including 52 females and 39 males aged 20–35 years old. Panelists assessed various perception parameters, including overall appearance, color, texture, aroma, flavor, and acidity of the samples. They assigned scores ranging from 5 to 1, where 5 represented extremely good and 1 represented extremely poor. Specifically for acidity, a score of 1 indicated no perception, while 5 indicated high acidity perception. The study received approval from the ethics committee of the University of Cauca, and informed consent was obtained from each participant before their involvement.
2.3. Statistical analysis
We used Minitab v. 18 statistical software for our analyses. We employed a completely randomized single-factor design, focusing on the inclusion of HPQF in the snack formulation as the primary factor. Viability assessment during storage was conducted twice, while in vitro gastric simulation experiments were conducted three times. Results were presented as mean values ± standard deviations. We utilized one-way ANOVA to compare the effects of the four formulations across different tests, with significance set at p < 0.05. Tukey post hoc test was applied for treatment comparisons. Additionally, for sensory analysis, we employed a non-parametric Kruskal-Wallis test, with significance set at p < 0.05. Graphs were created using the OriginPro2018 software.
3. Results
3.1. Measurement of physicochemical properties
3.1.1. Bulk density of extrusion (BDE) is a measurement that indicates the mass or weight of a product divided by its apparent volume, representing the space it occupies [22]
According to statistical analysis, the inclusion of HPQF in snack formulation affects both BDE and the expansion index, as illustrated in Table 2. BDE decreased significantly from 0.1 kg/m3 (F1) to 0.07 kg/m3 (F4) with the lowest inclusion of hyperproteic quinoa flour, while the expansion index increased from 5.92 (F1) to 7.48 (F4). This finding corroborates [22], which suggests that the density of the extruded product and the expansion index are interrelated attributes with an inverse correlation. BDE is linked to snack texture; denser snacks may have a less crispy texture. Formulations with lower quinoa content facilitated the expansion of extrudates at the extruder exit. The starch in formulations F2 to F4 undergoes gelatinization, absorbing water and swelling to form a gel that aids in air retention and creates a matrix for uniform expansion. Conversely, in formulation F1, starch may interact with proteins from HPQF to form a more intricate matrix that influences expansion and texture of the final product [23]. Additionally, as shown in Table 5, amylose content increases with HPQF inclusion. Due to its compact structure and lower water retention capacity [24], amylose may lead to less expansion during extrusion, resulting in snacks with a denser texture and fewer air bubbles.
Table 2.
Proximal Analysis, Bulk density of extrusion (BDE), and Expansion Index (EI).
| Cereal Blend | EI | BDE Kg/m3 |
IP | Protein (%) | Lipids (%) | aw | Fiber | Ashes | Moisture |
|---|---|---|---|---|---|---|---|---|---|
| F 1 | 5.92 ± 0.89c | 0.1 ± 0.00a | 31.77 ± 3.53b | 17.0 | 2.087 | 0.24 | 5.34 | 3.02 | 3.24 |
| F 2 | 6.56 ± 0.59b | 0.09 ± 0.02b | 26.36 ± 5.16c | 15.3 | 1.34 | 0.23 | 3.35 | 2.99 | 3.99 |
| F 3 | 6.91 ± 0.72b | 0.08 ± 0.00c | 30.36 ± 5.47b | 13.20 | 1.27 | 0.23 | 3.55 | 2.13 | 3.46 |
| F 4 | 7.48 ± 0.88a | 0.07 ± 0.02d | 40.16 ± 5.24a | 11.10 | 1.03 | 0.28 | 4.37 | 1.84 | 3.26 |
Values are presented as mean ± SD. For each parameter, different letters indicate significant differences at p < 0.05.
Table 5.
Parameters of antioxidant properties, starch digestibility and amylopectin amylose content of snack formulations.
| Sample | Extractable phenolic compounds (EPP) (mg GAE/100g) |
Hydrolyzable phenolic compounds (HPP) (mg GAE/100g) |
ABTS (μmol de AA/g) | DPPH (μmol de AA/g) | FRAP (μmol de AA/g) | Quantification of amylose- amylopectin (% w/w) | RDS | SDS | TDS | RS | GI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | 36.74 ± 0.06a | 673.43 ± 31.65a | 35.29 ± 2.73a | 5.52 ± 0.63a | 13.74 ± 0.09a | 35.56a - 64.44d | 62.81 ± 0.01d | 4.22 ± 0.01c | 68.38 ± 1.81b | 0.11 ± 0.00 | 71.24 ± 2.30c |
| F2 | 33.00 ± 0.06b | 711.11 ± 30.28a | 29.11 ± 1.76b | 4.82 ± 0.23ab | 7.47 ± 1.42b | 29.73b - 70.20c | 68.24 ± 0.04c | 2.45 ± 0.00d | 71.33 ± 1.24b | 0.086 ± 0.07 | 80.47 ± 1.19b |
| F3 | 21.06 ± 0.00c | 729.89 ± 19.52a | 25.63 ± 0.98b | 4.32 ± 0.15b | 7.09 ± 1.04b | 28.25b–71.75b | 69.67 ± 0.01b | 5.16 ± 0.04b | 81.26 ± 1.45a | 0.095 ± 0.01 | 81.20 ± 2.10b |
| F4 | 10.08 ± 0.01d | 636.31 ± 40.17ab | 17.56 ± 0.33c | 4.37 ± 0.25b | 5.05 ± 1.32b | 26.80b–73.20a | 72.61 ± 0.02a | 6.24 ± 0.02a | 80.95 ± 1.78a | 0.064 ± 0.00 | 90.41 ± 1.78a |
Values are presented as mean ± SD (n = 3). For each parameter, different letters indicate significant differences at p < 0.05.
As shown in Fig. 1, snacks containing higher amounts of quinoa show reduced expansion levels. This is because of their elevated protein and fiber content, where the fiber, in particular, breaks cell walls and inhibits the formation of air bubbles, thereby limiting expansion to its maximum potential [25]. Similar investigations into extrudates with protein levels surpassing 30 % [26] also note snacks characterized by high density, greater hardness, and diminished crispiness.
Fig. 1.
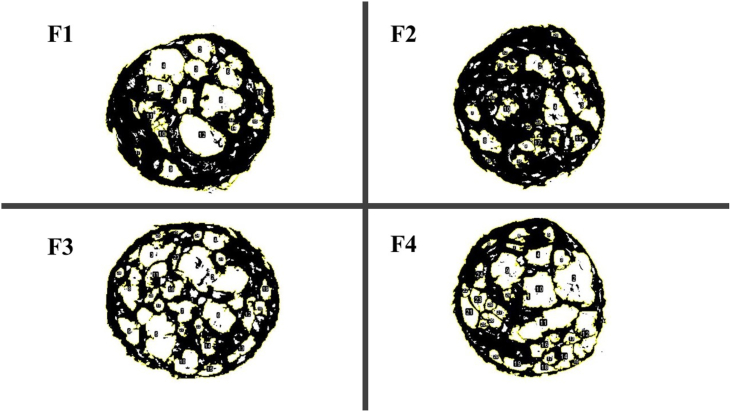
Image showing snacks containing hyperproteic quinoa and probiotics.
3.2. Expansion index
The expansion index refers to how much a food can increase in volume when exposed to high temperatures or pressures during processing. In extrudates with high protein content, expansion can be influenced by a range of factors, such as the formulation of raw materials, extrusion process parameters, and operating conditions [27].
The relationship between the composition of high-protein quinoa flour (HPQF) and its behavior during extrusion is a importing aspect of snack quality. The expansion index, a critical factor in snack quality, inversely correlates with the protein and fiber content of HPQF. This is due to the structural properties of quinoa's primary proteins, 11S globulin and 2S albumin, which are interconnected by disulfide bridges, lending a certain rigidity to the protein network [28] Consequently, higher levels of HPQF in snacks can lead to less expansion during the extrusion process. However, the low presence of prolamins in quinoa, ranging from 0.5 % to 7 % of the total protein content, is a beneficial characteristic, particularly for individuals with celiac disease, as it avoids the gluten found in many other grains. This makes quinoa-based snacks not only a healthier option but also inclusive for those with specific dietary restrictions [29].
In the context of high-protein quinoa flour (HPQF) and its use in extrusion to produce snacks, fiber plays a significant role in influencing the physical properties of the final product. Fiber, particularly insoluble fiber, is known to impact the expansion index of extruded products. The presence of fiber in HPQF can interfere with the expansion process during extrusion, leading to a denser and less expanded product. This is because fiber can absorb water and increase the viscosity of the dough, which restricts the dough's ability to expand and form a light, airy structure. Additionally, fiber can also affect the textural properties of the snacks, contributing to a harder and more compact bite. Despite these effects on expansion and texture, the inclusion of fiber-rich ingredients like quinoa is beneficial due to their nutritional profile, offering a healthier alternative to traditional snacks that are often low in fiber and other essential nutrients [30].
3.3. Porosity index
The porosity of an extruded food product made from plants describes how much the product expands during extrusion. Fig. 1 shows the formation of air cells during extrusion, resulting in expanded snacks with varied sizes and numbers of pores, creating a porous, sponge-like structure. This occurs because of the formation of many small steam bubbles due to the rapid release of pressure after leaving the extruder nozzle.
As shown in the table, various levels of HPQF inclusion significantly affect the porosity index. However, there were no significant differences between 20 % and 60 % inclusion. As illustrated in Fig. 1, the number of cells formed with 60 % inclusion is similar to that with 20 % inclusion, indicating less expansion with higher HPQF inclusion. Nevertheless, the porosity of snacks with higher quinoa inclusion is 31.77, similar to that of snacks with 20 % inclusion. This may be because quinoa starch granules, when exposed to high temperatures, maintain their integrity better than starch granules from other plant sources [31].
3.4. Color parameters
Color change is a key indicator of how extrusion cooking affects extrudates, with L* being particularly effective for assessing browning kinetics [32]. Statistical analysis demonstrates that different concentrations of HPQF significantly influence color (p < 0.05). Extrusion can alter the structure and composition of natural pigments in foods, potentially leading to color changes. High temperatures and pressure during extrusion may degrade or eliminate certain pigments, resulting in darker hues due to Maillard product formation, which improves the visual appeal of the final products.
Table 3 presents the color parameters of extrudates supplemented with quinoa.
Table 3.
Assessment of the color attributes of the snacks.
| Parameter | F 1 | F 2 | F 3 | F 4 | |
|---|---|---|---|---|---|
| Color Parameters | L* | 61.50 ± 1.146c | 67.22 ± 1.137b | 66.11 ± 1.653b | 69.05 ± 1.284a |
| a* | 5.63 ± 0.876a | 4.00 ± 0.272b | 4.19 ± 0.419b | 3.58 ± 0.458c | |
| b* | 17.70 ± 0.915b | 18.16 ± 0.934a | 18.22 ± 1.425a | 18.20 ± 0.398a | |
| C* | 4.83 ± 0.234a | 4.70 ± 0.095b | 4.73 ± 0.148b | 4.67 ± 0.046b | |
| h° | 1.26 ± 1.009b | 1.35 ± 0.014a | 1.35 ± 0.019a | 1.37 ± 0.021a | |
| ΔE | – | 5.97 ± 1.278b | 4.86 ± 1.355c | 7.85 ± 1.09a | |
| WI | 57.24 ± 1.023d | 62.30 ± 0.622b | 61.29 ± 1.017c | 63.91 ± 1.047a | |
| BI | 40.11 ± 0.877a | 35.29 ± 1.649b | 36.30 ± 2.799b | 33.45 ± 1.450c | |
Values are presented as mean ± SD (n = 5). For each parameter, different letters indicate significant differences at p < 0.05.
Comparing them to formulations with 60 % quinoa inclusion, extrudates F2, F3, and F4 show significantly higher values of L* (lightness) and b* (yellowness), and lower values of a* (redness). Furthermore, Fig. 2 illustrates that with increasing HPQF inclusion, the browning index rises from 40.11 to 33.45. The increased brightness in formulation 4 may be attributed to the higher rice flour content, which not only enhances the formation of air bubbles, leading to a greater number of cells in the snack [33], but also contributes to a lighter color. Moreover, during the typical Maillard reaction initiated by the interaction between the carbonyl group of reducing sugar and the amino group of protein, formulations with higher HQHF content may contain a greater number of amino groups. These groups can undergo various chain reactions such as condensation, dehydrogenation, rearrangement, and isomerization with amino groups, eventually forming brown polymers or copolymers containing nitrogen, collectively known as melanoidins, which impart a brown color [34].
Fig. 2.
Digital image of the snacks with probiotics of the 4 formulations.
In formulations containing higher levels of quinoa, the combination of intense dry heat and elevated shear forces during extrusion can trigger starch dextrinization. This chemical process breaks down starch into dextrins, potentially causing a partial shift in the color of extruded products from yellow to brown.
3.5. Texture
The texture of extruded foods with high protein content can be affected by numerous factors, such as the composition of the formulation, processing conditions, and the moisture level of the final product [29]. Our previous studies [23] indicate that extruded foods with high protein content from quinoa may have a less crispy and tougher texture. This is attributed to the nature of proteins, which tend to create a more elastic and less brittle structure compared to carbohydrates or fats.
Fig. 3 provides information on the texture properties of the extrudates. The hardness and crushing force of the snacks were evaluated. It was observed that addition of 60 % HQHP in F1 increased hardness and work of rupture almost 3 times compared to F4 (10 % HQHP). On the other hand, sample F4 (low HQHP inclusion value) recorded 0.4N*mm of Fcr, this value is 3 times lower than sample F1. In general, the F3 and F4 samples required less work (Wc) and were crispier compared to the high HQHP samples. These changes in the texture of the snacks were also observed by Ref. [30] when they added lentil flour (High protein) to corn flour to produce corn-lentil extrudates. This effect was observed in the inclusion of white cheese as a protein source in snacks [35]. The F3 and F4 also showed the highest degree of expansion, consistent with the findings of [25], who concluded that texture properties are strongly influenced by the extent of expansion. Essentially, extrudates with higher expansion indices were less hard and more fracturable [36]. In a study on the mechanical and microstructural properties of extrudates made from high-amylose corn starch and soy protein concentrate, it was observed that hardness increased due to decreased expansion and protein agglomeration caused by high temperature and shear.
Fig. 3.
Texture Parameters (n = 3). Hardness, crispness, and crunchiness values with different letters indicate significant differences (p < 0.05).
3.6. Viability of the probiotic Bacillus coagulans and tolerance to artificial gastroenteric juice (TAGJ)
According to Table 4, all four formulations of snacks containing Bacillus coagulans maintain viability above 6 log CFU/g, which meets the permitted limit for probiotic foods [37]. Counts range between 6.48 log CFU/g and 6.92 log CFU/g. It is possible that the spore-forming probiotic strain does not affect its viability and remains stable during the analysis period. Studies conducted by Ref. [16] indicate that probiotics of the Bacillus genus are known for their resilience to various adverse conditions, such as exposure to heat and pressure generated during extrusion. This resilience is partly due to their ability to form spores, a dormant form that protects them from unfavorable environmental conditions. Although the resistance of Bacillus probiotics to extrusion may depend on several factors, these microorganisms have generally proven to be more resistant compared to some other probiotic strains, and their application in foods may have a better effect when added to food matrices. According to Ref. [38], the efficacy of Bacillus probiotic microorganisms consumed in tablets differs from that obtained with the consumption of probiotic foods, as probiotics, along with the food matrix, may have a synergistic effect. Furthermore, in terms of viability, the food matrix provides nutrients, favoring their stability and germination in the gastrointestinal tract [38].
Table 4.
Tolerance of Bacillus coagulans to simulated gastric juice.
| Hours |
||||
|---|---|---|---|---|
| 0 | 3 | 6 | Survival (%) | |
| F1 | 6.49 ± 0.02 | 5.01 ± 0.00 | 4.92 ± 0.02 | 77.10 ± 1.02a |
| F2 | 6.64 ± 0.06 | 4.98 ± 0.03 | 4.73 ± 0.12 | 71.13 ± 1.04b |
| F3 | 6.48 ± 0.01 | 4.82 ± 0.02 | 4.97 ± 0.10 | 76.09 ± 0.98a |
| F4 | 6.92 ± 0.09 | 5.06 ± 0.00 | 4.95 ± 0.08 | 71.17 ± 0.14b |
Table 4 presents the survival rates of four snack formulations containing Bacillus coagulans when subjected to artificial gastrointestinal juice tolerance.
The viability of B. coagulans in the food under simulated in vitro gastrointestinal conditions was significantly influenced by the different formulations, despite the consistent amount of added probiotic. Between 0 and 3 h of testing, reductions ranged from 1.86 log CFU/g for F4 to 1.48 log CFU/g for F1. During the period from 3 to 6 h, a decrease of 0.11 log CFU/g for F4 and 0.09 log CFU/g for F1 was observed. Notably, the most significant decline in viability occurred during the gastric phase, indicating high sensitivity to simulated gastric juice containing HCL and pepsin. B. coagulans, a lactic acid-producing probiotic, remains in a spore state under stress conditions such as low water activity (0.23–0.28). This means that metabolic activity is dormant until favorable conditions for germination occur, such as in the digestive system where humidity, temperature, and pH can promote growth. Probiotics like Bacillus coagulans, which produce spores, are marketed in lyophilized powder and microcapsule forms to safeguard them during various food manufacturing processes. This helps to control oxidation, ensure controlled release, mask odors, flavors, or colors, and protect cells as they traverse the digestive system. However, as observed in prior studies [17], they demonstrate increased sensitivity to stomach acidity.
Table 4 indicates that the survival rates of the probiotic in the snacks are approximately 77 %, a figure comparable to those reported in Ref. [39]. Probiotic microorganisms undergo germination in the intestine after passing through the gastric phase. During germination, they produce various metabolites that offer several benefits to the host. These advantages may include improved digestive health, alleviation of symptoms associated with digestive disorders like irritable bowel syndrome, inflammatory bowel disease, and constipation, bolstering of the immune system, and enhancement of mental well-being by reducing anxiety, uplifting mood, and easing symptoms of depression.
3.7. Determination of phenolic compounds and antioxidant capacity evaluation
The antioxidant capacity of extruded foods can vary due to several factors, including the ingredients used, the extrusion process, and processing conditions. However, in general, the extrusion process can affect the antioxidant capacity of foods. Our findings, as shown in the table, indicate that the addition of HPQF in the snack formulation significantly impacts the content of phenolic compounds and antioxidant capacity (p < 0.05). Specifically, our results reveal that approximately 5 % of the total analyzed phenolic compounds are free soluble phenolic compounds, with the remaining 95 % being bound phenolic compounds. This aligns with previous research [40], which has found higher proportions of conjugated phenolic compounds, likely due to the predominant conjugation of these compounds with various molecules in plants. The release of conjugated or bound compounds in extruded foods may occur due to the breakdown of cell wall structure and partial hydrolysis of polysaccharides from fibers, attributed to the high temperature and pressure of the extrusion process, as observed in earlier studies [15]. Similar findings attribute this observation to factors such as grain types, the nature and location of phenolic compounds in grains, and the severity and duration of thermal treatment. To achieve a more accurate estimation of phenols and antioxidant capacities in cereal products, they suggested accounting for both free and bound phenolic components during analysis [41].
The main phenolic compounds of quinoa include vanillic acid, ferulic acid. The antioxidant activities of phenolic compounds have been recognized for decades, and research and development on the use of natural substances or food ingredients containing phenolic antioxidants will remain of great interest to the food industry. The biological activities of phenolic compounds have become well known in recent years. The most important biological activity of phenolic compounds is likely their numerous observed inhibitory effects on mutagenesis and carcinogenesis [42].
Extrusion processes can lead to the formation of new or enhanced antioxidant compounds, such as Maillard products, which contribute to the overall antioxidant capacity of extruded foods. The antioxidant capacity of foods is not solely determined by individual compounds but also by the interaction and synergy among different antioxidants. Therefore, the impact of extrusion on antioxidant capacity is complex and varies depending on each food's characteristics and the specific extrusion method used. Conducting targeted studies is essential to comprehensively understand how different foods and extrusion methods influence antioxidant capacity.
3.7.1. In vitro glycemic index
Research across various food types demonstrates a strong correlation between clinically measured in vivo glycemic index (GI) values and in vitro starch digestion [20]. In this study, we employed an in vitro starch hydrolysis method to mimic the digestion process of carbohydrates in snacks derived from HPQF.
The starch digestibility curve for snacks containing varying proportions of HPQF exhibited a notable decrease across all levels of inclusion (Fig. 4). Concurrently, the glycemic index (GI) exhibited reductions of 11 %, 12 %, and 26 % with increasing HPQF incorporation levels (Table 5). In a separate investigation [43], observed that the inclusion of quinoa flour led to a reduction in the in vivo GI of milk samples. Nonetheless, despite a declining trend associated with HPQF addition, the GI of all four formulations surpassed 70 %, categorizing the snacks as high glycemic index foods, according to the low (GI ≤ 55), medium (GI: 56–69), and high (GI ≥ 70) classifications. Understanding the starch digestibility behavior necessitates considering its distribution within the food matrix. For instance, starches derived from cereals, rice, potatoes, and all green plants consist of repetitive glucose units arranged in straight-chain (amylose) and branched (amylopectin) polysaccharides [44]. The estimated GI significantly decreased upon HPQF incorporation, attributed to the amylose-to-amylopectin ratio inherent in quinoa. The variation in glycemic indices among snacks with higher HPQF inclusion levels can be partially elucidated by this amylose-to-amylopectin ratio.
Fig. 4.
Starch hydrolysis curve in snack formulation.
According to Ref. [31], quinoa starch demonstrates higher enzymatic susceptibility compared to other sources such as barley, corn, millet, wheat, and sorghum. This characteristic is attributed to the elevated specific surface area of quinoa starch. Furthermore, the enzymatic susceptibility of quinoa starch exhibited a negative correlation with the amylose content, suggesting that amylose may mitigate enzyme susceptibility during the initial stages of hydrolysis. As shown in the table, formulations with higher amylose content exhibited reduced hydrolysis rates. The absorption rate, and consequently the glycemic index, of these starches is contingent upon the ratio between amylose and amylopectin.
3.8. Sensorial
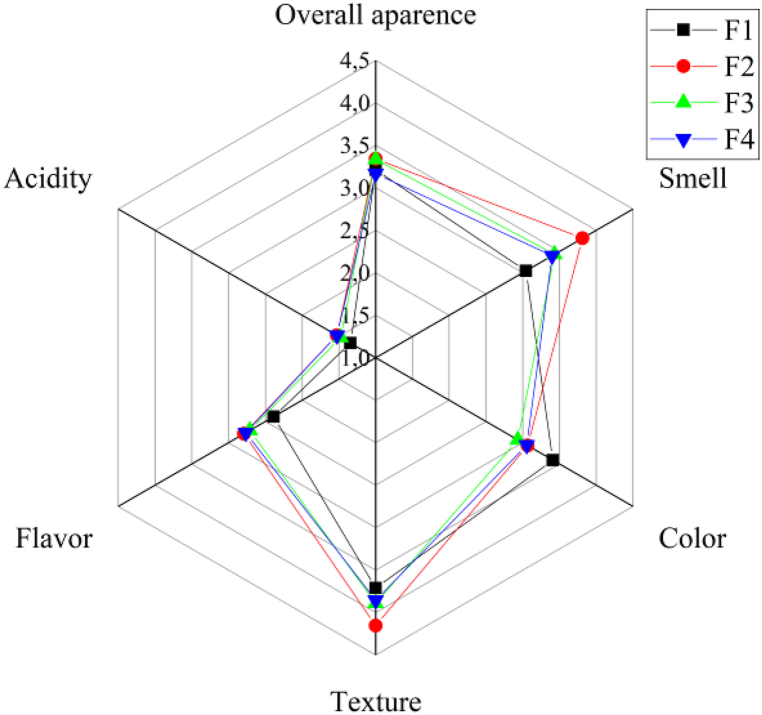
According to the results of the sensory analysis shown in Fig. 5, the snacks are rated between 3 and 3.5. The addition of probiotic has no effect on the sensory perception, i.e. the panelists do not perceive changes in the acidity of the snacks, a parameter that could evidence the generation of lactic acid by the probiotic, this is because as we explained before, Bacillus coagulans in the presence of low aw does not initiate metabolic processes, therefore it is in latency or in spore form, without generating flavors in the food.
Fig. 5.
Sensory perception of panelists.
As for the general aspect, it is not affected by the HPQF inclusions p > 0.05, the color has an influence by the addition of quinoa flour, this is because the proportion of quinoa and rice in the formulations generates different colors that are perceived by the panelists, a result corroborated by the color delta that has a value higher than 4.5 confirming that the different snacks present changes in color that reach to be noticed by the human eye.
The color of the F1 is the one with the highest rating, i.e., the panelists prefer a browner shade in the snacks. Texture and aroma are also affected by the inclusion of quinoa, according to the panelists the texture of the snacks of the formulations with lower HPQF content, have a higher rating, as well as the aroma. Possibly 60 % of HPQF may have an aroma and texture that are not characteristic of products such as snacks, as mentioned before the texture of F1 is harder and less crunchy, an aspect that is perceived by the panelists. On the contrary, flavor was not affected by HPQF p < 0.05, however, the highest rating is for samples F2, F3 and F4.
4. Conclusions
The different formulations showed a statistically significant effect on the physical characteristics of expansion index, porosity, bulk density, texture and color. The crispiness of the extruded foods changed with the addition of HPQF. It was found that the higher the amount of quinoa flour the lower the crispiness, an aspect that can be improved by optimizing the formulation, the processing conditions, possibly the use of enzymes and the control of the moisture of the final product. This can help ensure that extruded high-protein foods are attractive and palatable for consumption.
In vitro studies of probiotic resistance to gastric juices provide initial information on the ability of probiotics to survive stomach conditions. However, further clinical studies are required to confirm the effectiveness of probiotics in the human digestive tract. The results of the study showed that quinoa probiotic snacks made with the addition of B. coagulans have a final concentration of 106UFC/g.
Survival during the simulated intestinal tract conditions in the food matrix presented a percentage of 75 %. Starch digestibility in the different formulations resulted in snacks with high GI, although the addition of HPQF decreases this parameter, it is necessary to improve the formulation with additives rich in fiber and prebiotics that allow slowing down the action of digestive enzymes.
The addition of HPQF improved the antioxidant properties and bound phenolic compounds, corroborating that extrusion is an assertive process for the release of these compounds, which are more bound to other structures within the food matrix. The 60 % inclusion had the highest rating for color, and the lowest for texture, aroma and flavor. The F2 with 30 % HPQF had an acceptable score for all the parameters evaluated, which suggests that in future studies this level of inclusion plus other additives in the mixture can result in a snack with a protein percentage higher than 15 % and good textural properties, expansion and, above all, sensory acceptance.
CRediT authorship contribution statement
Karen Sofia Muñoz Pabon: Writing – review & editing, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Diego Fernando Roa Acosta: Supervision. Jesús Eduardo Bravo: Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work has been fully financed by the Colombian General Reimbursement System, the University of Cauca and SEGALCO S.A.S., within the framework of the SGR BPIN 2020000100052 Project.
References
- 1.Delić J., Ikonić P., Jokanović M., Peulić T., Ikonić B., Banjac V., et al. Sustainable snack products: impact of protein- and fiber-rich ingredients addition on nutritive, textural, physical, pasting and color properties of extrudates. Innov Food Sci Emerg Technol. 2023;87(June) [Google Scholar]
- 2.Félix-Medina J.V., Montes-Ávila J., Reyes-Moreno C., Perales-Sánchez J.X.K., Gómez-Favela M.A., Aguilar-Palazuelos E., et al. Second-generation snacks with high nutritional and antioxidant value produced by an optimized extrusion process from corn/common bean flours mixtures. Lwt [Internet] 2020;124(October 2019) doi: 10.1016/j.lwt.2020.109172. [DOI] [Google Scholar]
- 3.Melgar-Lalanne G., Hernández-Álvarez A.J., Salinas-Castro A. Edible insects processing: traditional and innovative technologies. Compr. Rev. Food Sci. Food Saf. 2019;18:1166–1191. doi: 10.1111/1541-4337.12463. [DOI] [PubMed] [Google Scholar]
- 4.On-Nom N., Promdang P., Inthachat W., Kanoongon P., Sahasakul Y., Chupeerach C., et al. Wolffia globosa-based nutritious snack formulation with high protein and dietary fiber contents. Foods. 2023;12(14) doi: 10.3390/foods12142647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva PC da, Toledo T., Brião V., Bertolin T.E., Costa J.A.V. Development of extruded snacks enriched by bioactive peptides from microalga Spirulina sp. LEB 18. Food Biosci. 2021;42 November 2020. [Google Scholar]
- 6.Jiapong S., Ruttarattanamongkol K. Development of direct expanded high protein snack products fortified with sacha inchi seed meal. J. Microbiol. Biotechnol. Food Sci. 2021;10(4):680–684. [Google Scholar]
- 7.Barraza-Jáuregui G., Valderrama-Amasifuen F., Arteaga H., Flores A., Obregón J. Snacks A base de maíz morado, quinua Y kiwicha. Características Físicas Y Sensoriales. 2021:1–7. [Google Scholar]
- 8.Roncolini A., Milanović V., Aquilanti L., Cardinali F., Garofalo C., Sabbatini R., et al. Lesser mealworm (Alphitobius diaperinus) powder as a novel baking ingredient for manufacturing high-protein, mineral-dense snacks. Food Res. Int. 2020;131 doi: 10.1016/j.foodres.2020.109031. September 2019. [DOI] [PubMed] [Google Scholar]
- 9.Reguera M., Poza-Viejo L., Redondo-Nieto M., Matías J., Cruz V., Granado-Rodríguez S., et al. vols. 1–17. Authorea Prepr; 2022. https://www.authorea.com/users/562338/articles/609681-shotgun-proteomics-of-quinoa-seeds-reveals-chitinases-enrichment-under-rainfed-conditions?commit=971c48c0ab32826a3265360a5d2f793eeea3eb97 (Shotgun Proteomics of Quinoa Seeds Reveals Chitinases Enrichment under Rainfed Conditions). [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S., Zhu F. Formulation and quality attributes of quinoa food products. Food Bioprocess Technol. 2016;9(1):49–68. [Google Scholar]
- 11.Ramos Diaz J.M., Kirjoranta S., Tenitz S., Penttilä P.A., Serimaa R., Lampi A.M., et al. Use of amaranth, quinoa and kañiwa in extruded corn-based snacks. J Cereal Sci [Internet] 2013;58(1):59–67. doi: 10.1016/j.jcs.2013.04.003. [DOI] [Google Scholar]
- 12.Väkeväinen K., Ludena-Urquizo F., Korkala E., Lapveteläinen A., Peräniemi S., von Wright A., et al. Potential of quinoa in the development of fermented spoonable vegan products. Lwt [Internet] 2020;120(June 2019) doi: 10.1016/j.lwt.2019.108912. [DOI] [Google Scholar]
- 13.Mhd Rodzi N.A.R., Lee L.K. Sacha Inchi (Plukenetia Volubilis L.): recent insight on phytochemistry, pharmacology, organoleptic, safety and toxicity perspectives. Heliyon. 2022;8(9) doi: 10.1016/j.heliyon.2022.e10572. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez D., Celi R., Toledo L., Aparecida V., Queiroz V., Ribeiro M., et al. Lwt - food Science and Technology Mixed sorghum and quinoa flour improves protein quality and increases antioxidant capacity in vivo. LWT - Food Sci Technol [Internet] 2020;129(May) doi: 10.1016/j.lwt.2020.109597. [DOI] [Google Scholar]
- 15.Muñoz-Pabon K.S., Roa-Acosta D.F., Bravo-Gómez, Jesús Eduardo Ortiz-Gómez V. 2022. Quinoa Snack Production at an Industrial Level: Effect of Extrusion and Baking on Digestibility, Bioactive, Rheological, and Physical Properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almada-Érix C.N., Almada C.N., Souza Pedrosa G.T., dos Santos P., Schmiele M., Clerici M.T.P.S., et al. Quantifying the impact of eight unit operations on the survival of eight Bacillus strains with claimed probiotic properties. Food Res. Int. 2021;142 doi: 10.1016/j.foodres.2021.110191. October 2020. [DOI] [PubMed] [Google Scholar]
- 17.Muñoz Pabon KS., Hoyos Concha J.L., Solanilla Duque J.F. Quinoa extruded snacks with probiotics: physicochemical and sensory properties. Front. Sustain. Food Syst. 2022;6 [Google Scholar]
- 18.Soares M.B., Martinez R.C.R., Pereira E.P.R., Balthazar C.F., Cruz A.G., Ranadheera C.S., et al. The resistance of Bacillus, Bifidobacterium, and Lactobacillus strains with claimed probiotic properties in different food matrices exposed to simulated gastrointestinal tract conditions. Food Res Int [Internet] 2019;125(February) doi: 10.1016/j.foodres.2019.108542. [DOI] [PubMed] [Google Scholar]
- 19.Majeed M., Nagabhushanam K., Natarajan S., Sivakumar A., Eshuis-de Ruiter T., Booij-Veurink J., et al. Evaluation of genetic and phenotypic consistency of Bacillus coagulans MTCC 5856: a commercial probiotic strain. World J. Microbiol. Biotechnol. 2016;32(4):1–12. doi: 10.1007/s11274-016-2027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goñi I., Garcia-Alonso A., Saura-Calixto F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997;17(3):427–437. [Google Scholar]
- 21.Pico J., Xu K., Guo M., Mohamedshah Z., Ferruzzi M.G., Martinez M.M. Manufacturing the ultimate green banana flour: impact of drying and extrusion on phenolic profile and starch bioaccessibility. Food Chem [Internet] 2019;297(February) doi: 10.1016/j.foodchem.2019.124990. [DOI] [PubMed] [Google Scholar]
- 22.Tas A.A., Shah A.U. The replacement of cereals by legumes in extruded snack foods: science, technology and challenges. Trends Food Sci Technol [Internet] 2021;116(April):701–711. doi: 10.1016/j.tifs.2021.08.016. [DOI] [Google Scholar]
- 23.Muñoz K.S., Parra A.S., Roa D.F., Hoyos J.L., Bravo J.E. Physical and paste properties comparison of four snacks produced by high protein quinoa flour extrusion cooking. 2022;6(March):1–10. [Google Scholar]
- 24.Jenkins P.J., Donald A.M. The influence of amylose on starch granule structure. Int. J. Biol. Macromol. 1995;17(6):315–321. doi: 10.1016/0141-8130(96)81838-1. [DOI] [PubMed] [Google Scholar]
- 25.Jozinović A., Šubarić D., Ačkar D., Babić J., Miličević B. Influence of spelt flour addition on properties of extruded products based on corn grits. J. Food Eng. 2016;172:31–37. [Google Scholar]
- 26.Luo S., Koksel F. Application of physical blowing agents in extrusion cooking of protein enriched snacks: effects on product expansion, microstructure, and texture. Trends Food Sci Technol [Internet] 2023;133(November 2022):49–64. doi: 10.1016/j.tifs.2023.01.012. [DOI] [Google Scholar]
- 27.Paula A.M., Conti-Silva A.C. Texture profile and correlation between sensory and instrumental analyses on extruded snacks. J Food Eng [Internet] 2014;121(1):9–14. doi: 10.1016/j.jfoodeng.2013.08.007. [DOI] [Google Scholar]
- 28.Dakhili S., Abdolalizadeh L., Hosseini S.M., Shojaee-Aliabadi S., Mirmoghtadaie L. Quinoa protein: composition, structure and functional properties. Food Chem [Internet] 2019;299(January) doi: 10.1016/j.foodchem.2019.125161. [DOI] [PubMed] [Google Scholar]
- 29.Acosta D.F.R., Gómez J.E.B., Duque J.F.S., Galindez J.Z.Z., Cruz J.A.M. Antioxidant potential of extruded snacks enriched with hyper-protein quinoa flour and vegetable extracts. Food Sci Technol. 2022;42 [Google Scholar]
- 30.Lazou A., Krokida M., Zogzas N., Karathanos V. Lentil-based snacks: structural and textural evaluation. Procedia Food Sci [Internet] 2011;1:1593–1600. doi: 10.1016/j.profoo.2011.09.236. [DOI] [Google Scholar]
- 31.Li G., Zhu F. Quinoa starch: structure, properties, and applications. Carbohydr. Polym. 2018;181(September 2017):851–861. doi: 10.1016/j.carbpol.2017.11.067. [DOI] [PubMed] [Google Scholar]
- 32.Alam M.S., Kaur J., Khaira H., Gupta K. Extrusion and extruded products: changes in quality attributes as affected by extrusion process parameters: a review. Crit. Rev. Food Sci. Nutr. 2016;56(3):445–473. doi: 10.1080/10408398.2013.779568. [DOI] [PubMed] [Google Scholar]
- 33.Li X., Masatcioglu M.T., Koksel F. Physical and functional properties of wheat flour extrudates produced by nitrogen injection assisted extrusion cooking. J Cereal Sci [Internet] 2019;89(March) doi: 10.1016/j.jcs.2019.102811. [DOI] [PubMed] [Google Scholar]
- 34.Jia W., Guo A., Zhang R., Shi L. Mechanism of natural antioxidants regulating advanced glycosylation end products of Maillard reaction. Food Chem [Internet] 2023;404(PA) doi: 10.1016/j.foodchem.2022.134541. [DOI] [PubMed] [Google Scholar]
- 35.Köprüalan Ö., Elmas F., Bodruk A., Arıkaya Ş., Koç M., Koca N., et al. Impact of pre-drying on the textural, chemical, color, and sensory properties of explosive puffing dried white cheese snacks. Lwt. 2022;154 (June 2021. [Google Scholar]
- 36.Zhu L.J., Shukri R., De Mesa-Stonestreet N.J., Alavi S., Dogan H., Shi Y.C. Mechanical and microstructural properties of soy protein - high amylose corn starch extrudates in relation to physiochemical changes of starch during extrusion. J Food Eng [Internet] 2010;100(2):232–238. doi: 10.1016/j.jfoodeng.2010.04.004. [DOI] [Google Scholar]
- 37.Konuray G., Erginkaya Z. Quality evaluation of probiotic pasta produced with Bacillus coagulans GBI-30. Innov Food Sci Emerg Technol [Internet] 2020;66(July) doi: 10.1016/j.ifset.2020.102489. [DOI] [Google Scholar]
- 38.Soares M.B., Almada C.N., Pereira E.P.R., Ferreira B.M., Balthazar C.F., Khorshidian N., et al. Review - sporeforming probiotic bacteria: characteristics, health benefits, and technological aspects for their applications in foods and beverages. Trends Food Sci. Technol. 2023;138(April):453–469. [Google Scholar]
- 39.Konuray Altun G., Erginkaya Z. Identification and characterization of Bacillus coagulans strains for probiotic activity and safety. Lwt. 2021;151(July) [Google Scholar]
- 40.Antognoni F., Potente G., Biondi S., Mandrioli R., Marincich L., Ruiz K. Free and conjugated phenolic profiles and antioxidant activity and environment. Plants. 2021;10:1046. doi: 10.3390/plants10061046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kadiri O. A review on the status of the phenolic compounds and antioxidant capacity of the flour: effects of cereal processing. Int J Food Prop [Internet] 2017;20(1):S798–S809. doi: 10.1080/10942912.2017.1315130. [DOI] [Google Scholar]
- 42.Tuberoso C.I.G., Orrù C.D. Phenolic compounds in food. Prog Food Chem. 2008;(4):1–45. [Google Scholar]
- 43.Pineli L.L.O., Botelho R.B.A., Zandonadi R.P., Solorzano J.L., de Oliveira G.T., Reis C.E.G., et al. Low glycemic index and increased protein content in a novel quinoa milk. Lwt [Internet] 2015;63(2):1261–1267. doi: 10.1016/j.lwt.2015.03.094. [DOI] [Google Scholar]
- 44.Frost G., Dornhorst A. Glycemic index. Encycl Hum Nutr. 2012;2–4:393–398. [Google Scholar]