Abstract
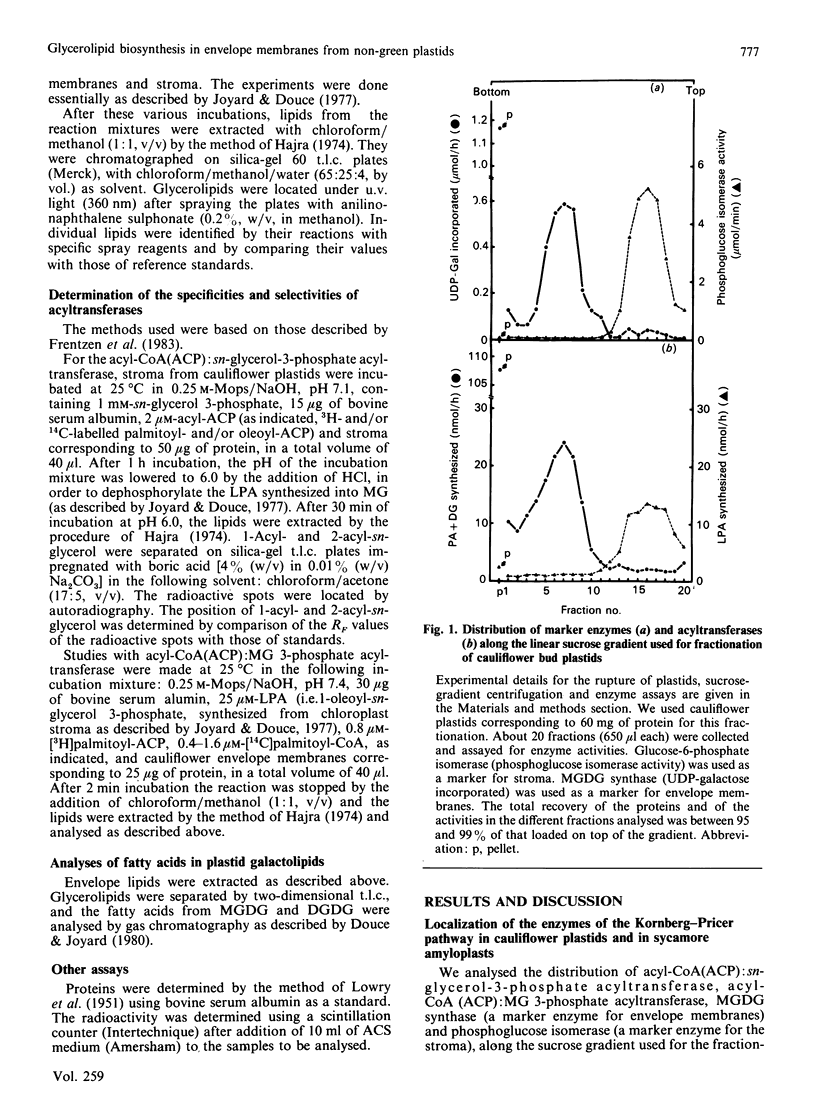
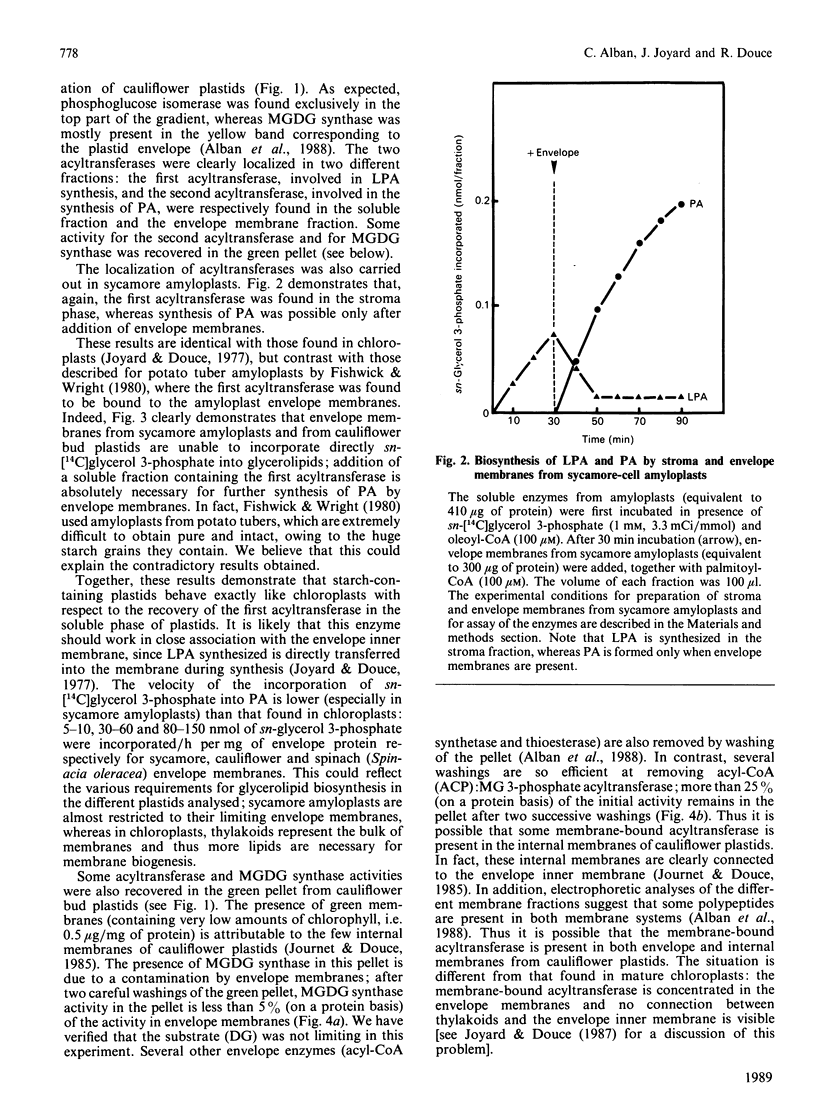
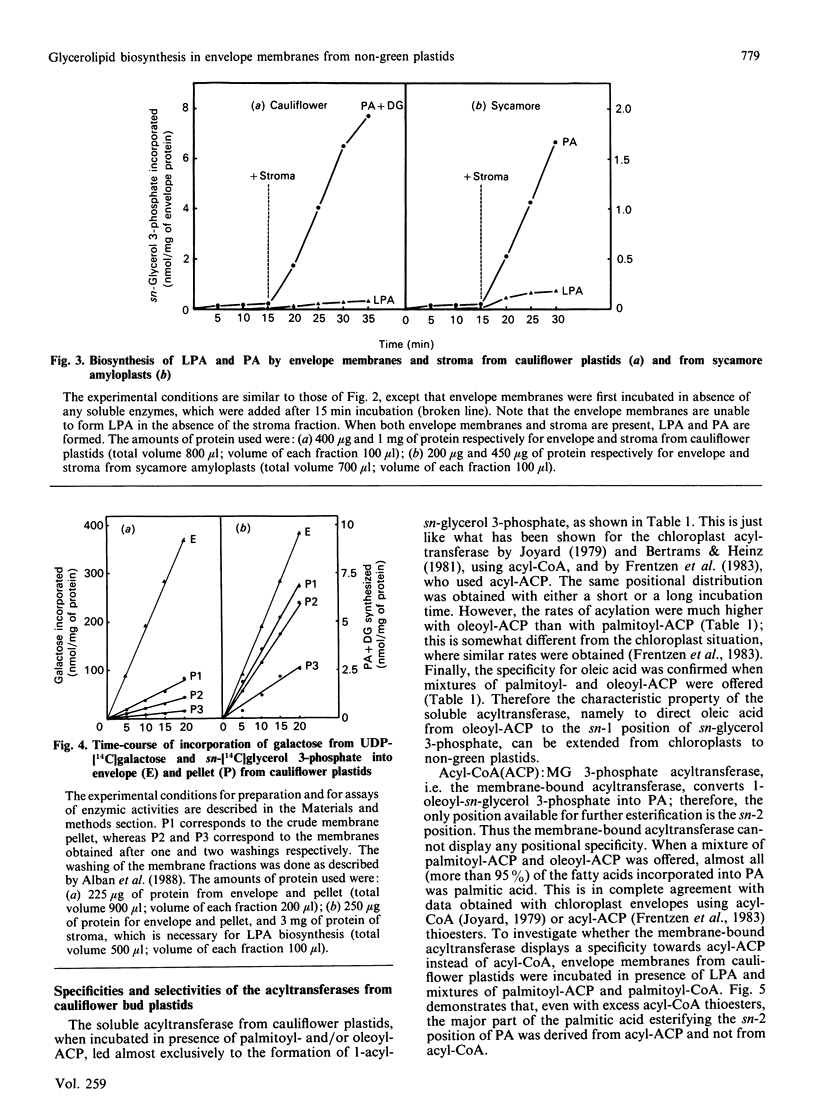
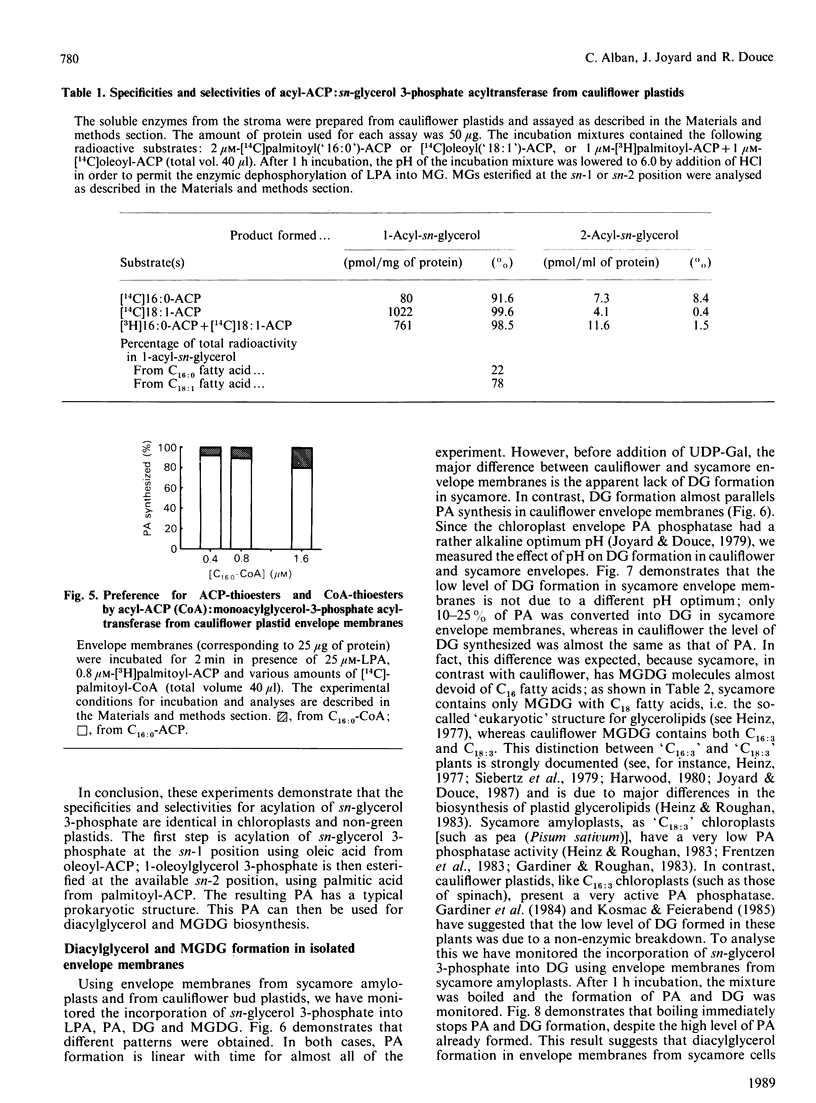
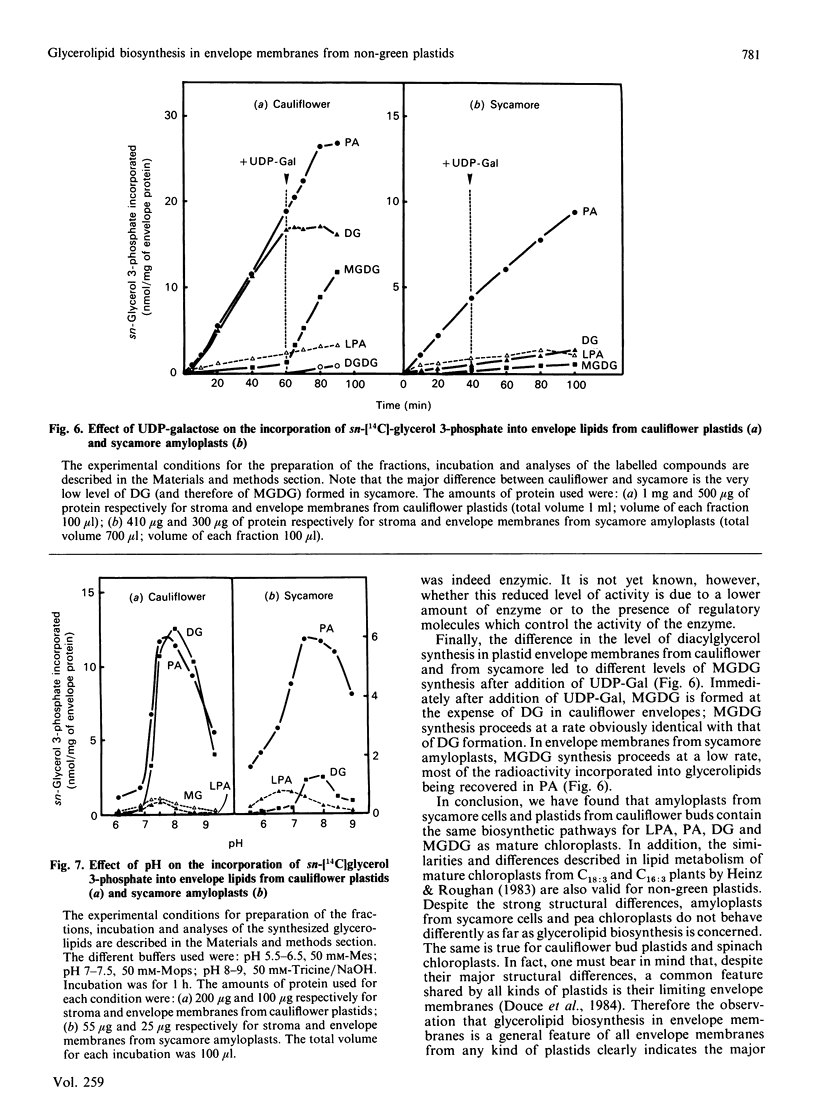
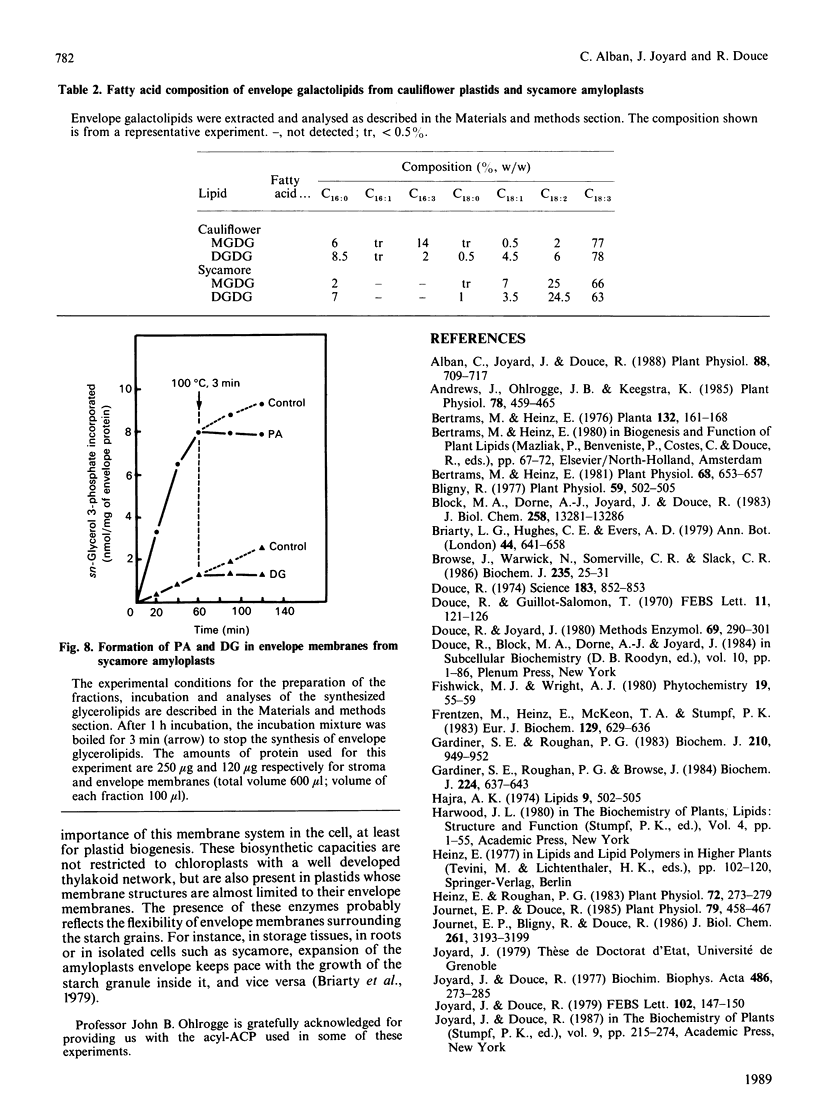
The availability of methods to fractionate non-green plastids and to prepare their limiting envelope membranes [Alban, Joyard & Douce (1988) Plant Physiol. 88, 709-717] allowed a detailed analysis of the biosynthesis of lysophosphatidic acid, phosphatidic acid, diacylglycerol and monogalactosyl-diacylglycerol (MGDG) in two different types of non-green starch-containing plastids: plastids isolated from cauliflower buds and amyloplasts isolated from sycamore cells. An enzyme [acyl-ACP (acyl carrier protein):sn-glycerol 3-phosphate acyltransferase) recovered in the soluble fraction of non-green plastids transfers oleic acid from oleoyl-ACP to the sn-1 position of sn-glycerol 3-phosphate to form lysophosphatidic acid. Then a membrane-bound enzyme (acyl-ACP:monoacyl-sn-glycerol 3-phosphate acyltransferase), localized in the envelope membrane, catalyses the acylation of the available sn-2 position of 1-oleoyl-sn-glycerol 3-phosphate by palmitic acid from palmitoyl-ACP. Therefore both the soluble phase and the envelope membranes are necessary for acylation of sn-glycerol 3-phosphate. The major difference between cauliflower (Brassica oleracea) and sycamore (Acer pseudoplatanus) membranes is the very low level of phosphatidate phosphatase activity in sycamore envelope membrane. Therefore, very little diacylglycerol is available for MGDG synthesis in sycamore, compared with cauliflower. These findings are consistent with the similarities and differences described in lipid metabolism of mature chloroplasts from 'C18:3' and 'C16:3' plants (those with MGDG containing C18:3 and C16:3 fatty acids). Sycamore contains only C18 fatty acids in MGDG, and the envelope membranes from sycamore amyloplasts have a low phosphatidate phosphatase activity and therefore the enzymes of the Kornberg-Pricer pathway have a low efficiency of incorporation of sn-glycerol 3-phosphate into MGDG. By contrast, cauliflower contains MGDG with C16:3 fatty acid, and the incorporation of sn-glycerol 3-phosphate into MGDG by the enzymes associated with envelope membranes is not limited by the phosphatidate phosphatase. These results demonstrate that: (1) non-green plastids employ the same biosynthetic pathway as that previously established for chloroplasts (the formation of glycerolipids is a general property of all plastids, chloroplasts as well as non-green plastids), (2) the envelope membranes are the major structure responsible for the biosynthesis of phosphatidic acid, diacylglycerol and MGDG, and (3) the enzymes of the envelope Kornberg-Pricer pathway have the same properties in non-green starch-containing plastids as in mature chloroplasts from C16:3 and C18:3 plants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alban C., Joyard J., Douce R. Preparation and characterization of envelope membranes from nongreen plastids. Plant Physiol. 1988 Nov;88(3):709–717. doi: 10.1104/pp.88.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J., Ohlrogge J. B., Keegstra K. Final step of phosphatidic Acid synthesis in pea chloroplasts occurs in the inner envelope membrane. Plant Physiol. 1985 Jul;78(3):459–465. doi: 10.1104/pp.78.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrams M., Heinz E. Positional Specificity and Fatty Acid Selectivity of Purified sn-Glycerol 3-Phosphate Acyltransferases from Chloroplasts. Plant Physiol. 1981 Sep;68(3):653–657. doi: 10.1104/pp.68.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligny R. Growth of Suspension-cultured Acer pseudoplatanus L. Cells in Automatic Culture Units of Large Volume. Plant Physiol. 1977 Mar;59(3):502–505. doi: 10.1104/pp.59.3.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M. A., Dorne A. J., Joyard J., Douce R. Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts. II. Biochemical characterization. J Biol Chem. 1983 Nov 10;258(21):13281–13286. [PubMed] [Google Scholar]
- Browse J., Warwick N., Somerville C. R., Slack C. R. Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the '16:3' plant Arabidopsis thaliana. Biochem J. 1986 Apr 1;235(1):25–31. doi: 10.1042/bj2350025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Guillot-Salomon T. Sur l'incorporation de la radioactivite du sn-glycerol-3-phosphate-(14)C dans le monogalactosyldiglyceride des plastes isoles. FEBS Lett. 1970 Nov 18;11(2):121–124. doi: 10.1016/0014-5793(70)80507-5. [DOI] [PubMed] [Google Scholar]
- Douce R. Site of biosynthesis of galactolipids in spinach chloroplasts. Science. 1974 Mar 1;183(4127):852–853. doi: 10.1126/science.183.4127.852. [DOI] [PubMed] [Google Scholar]
- Fordyce P. S., Edington N., Bridges G. C., Wright J. A., Edwards G. B. Use of an ELISA in the differential diagnosis of cauda equina neuritis and other equine neuropathies. Equine Vet J. 1987 Jan;19(1):55–59. doi: 10.1111/j.2042-3306.1987.tb02583.x. [DOI] [PubMed] [Google Scholar]
- Frentzen M., Heinz E., McKeon T. A., Stumpf P. K. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur J Biochem. 1983 Jan 1;129(3):629–636. doi: 10.1111/j.1432-1033.1983.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Gardiner S. E., Roughan P. G., Browse J. Glycerolipid labelling kinetics in isolated intact chloroplasts. Biochem J. 1984 Dec 1;224(2):637–643. doi: 10.1042/bj2240637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. E., Roughan P. G. Relationship between fatty-acyl composition of diacylgalactosylglycerol and turnover of chloroplast phosphatidate. Biochem J. 1983 Mar 15;210(3):949–952. doi: 10.1042/bj2100949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra A. K. On extraction of acyl and alkyl dihydroxyacetone phosphate from incubation mixtures. Lipids. 1974 Aug;9(8):502–505. doi: 10.1007/BF02532495. [DOI] [PubMed] [Google Scholar]
- Heinz E., Roughan P. G. Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants. Plant Physiol. 1983 Jun;72(2):273–279. doi: 10.1104/pp.72.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journet E. P., Bligny R., Douce R. Biochemical changes during sucrose deprivation in higher plant cells. J Biol Chem. 1986 Mar 5;261(7):3193–3199. [PubMed] [Google Scholar]
- Journet E. P., Douce R. Enzymic capacities of purified cauliflower bud plastids for lipid synthesis and carbohydrate metabolism. Plant Physiol. 1985 Oct;79(2):458–467. doi: 10.1104/pp.79.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J., Douce R. Characterization of phosphatidate phosphohydrolase activity associated with chloroplast envelope membranes. FEBS Lett. 1979 Jun 1;102(1):147–150. doi: 10.1016/0014-5793(79)80947-3. [DOI] [PubMed] [Google Scholar]
- Joyard J., Douce R. Site of synthesis of phosphatidic acid and diacyglycerol in spinach chloroplasts. Biochim Biophys Acta. 1977 Feb 23;486(2):273–285. doi: 10.1016/0005-2760(77)90023-6. [DOI] [PubMed] [Google Scholar]
- Kosmac U., Feierabend J. Control of plastidic glycolipid synthesis and its relation to chlorophyll formation. Plant Physiol. 1985 Nov;79(3):646–652. doi: 10.1104/pp.79.3.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Siebertz H. P., Heinz E., Linscheid M., Joyard J., Douce R. Characterization of lipids from chloroplast envelopes. Eur J Biochem. 1979 Nov;101(2):429–438. doi: 10.1111/j.1432-1033.1979.tb19736.x. [DOI] [PubMed] [Google Scholar]
- Simcox P. D., Reid E. E., Canvin D. T., Dennis D. T. Enzymes of the Glycolytic and Pentose Phosphate Pathways in Proplastids from the Developing Endosperm of Ricinus communis L. Plant Physiol. 1977 Jun;59(6):1128–1132. doi: 10.1104/pp.59.6.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]