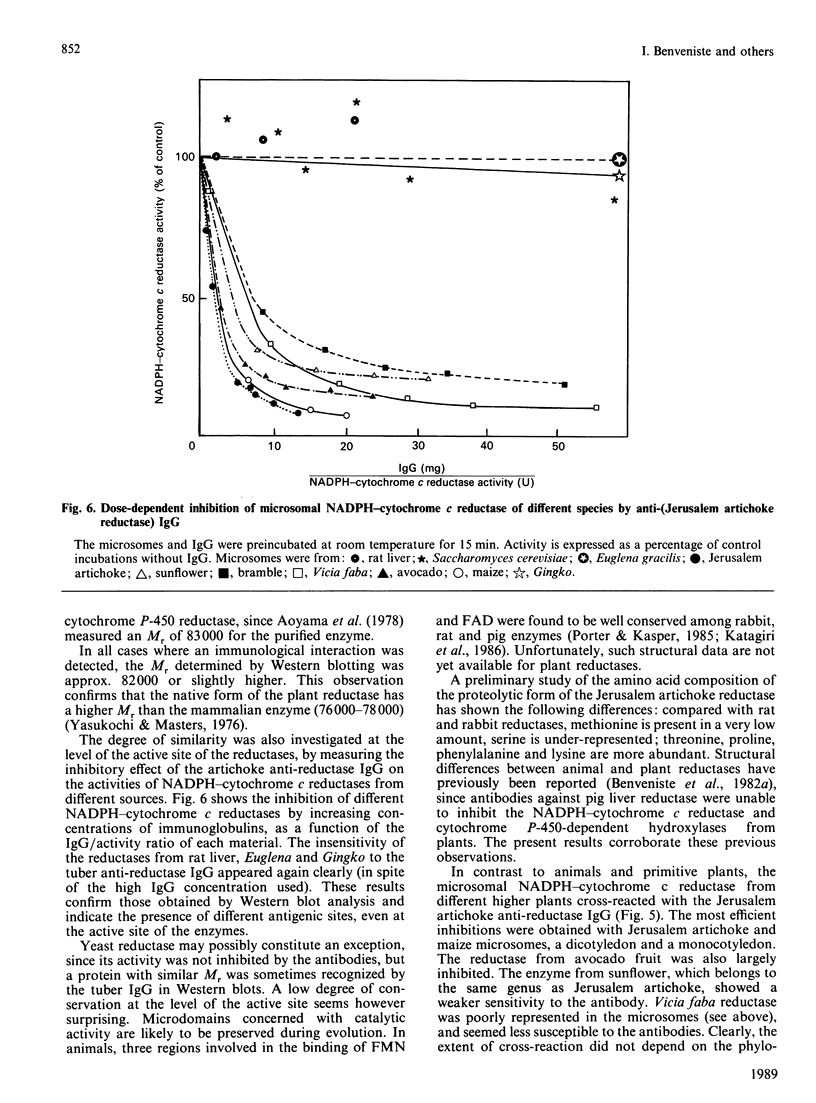
Abstract
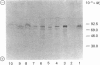
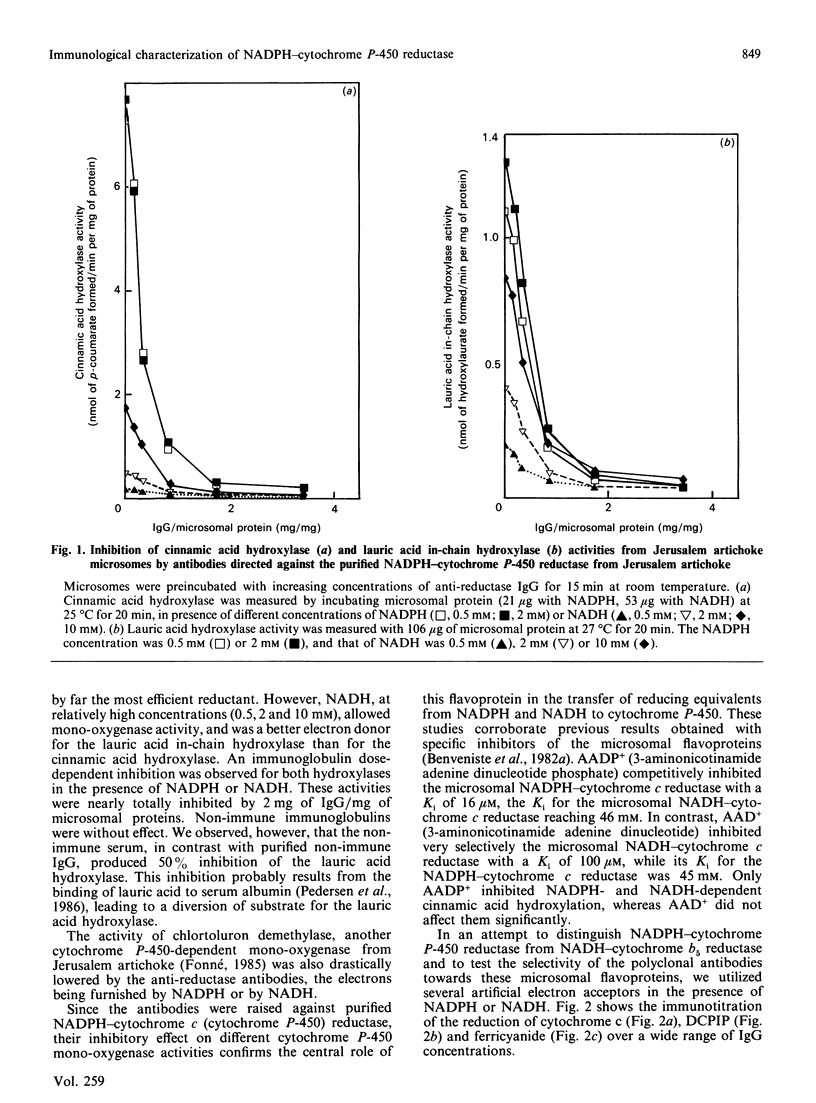
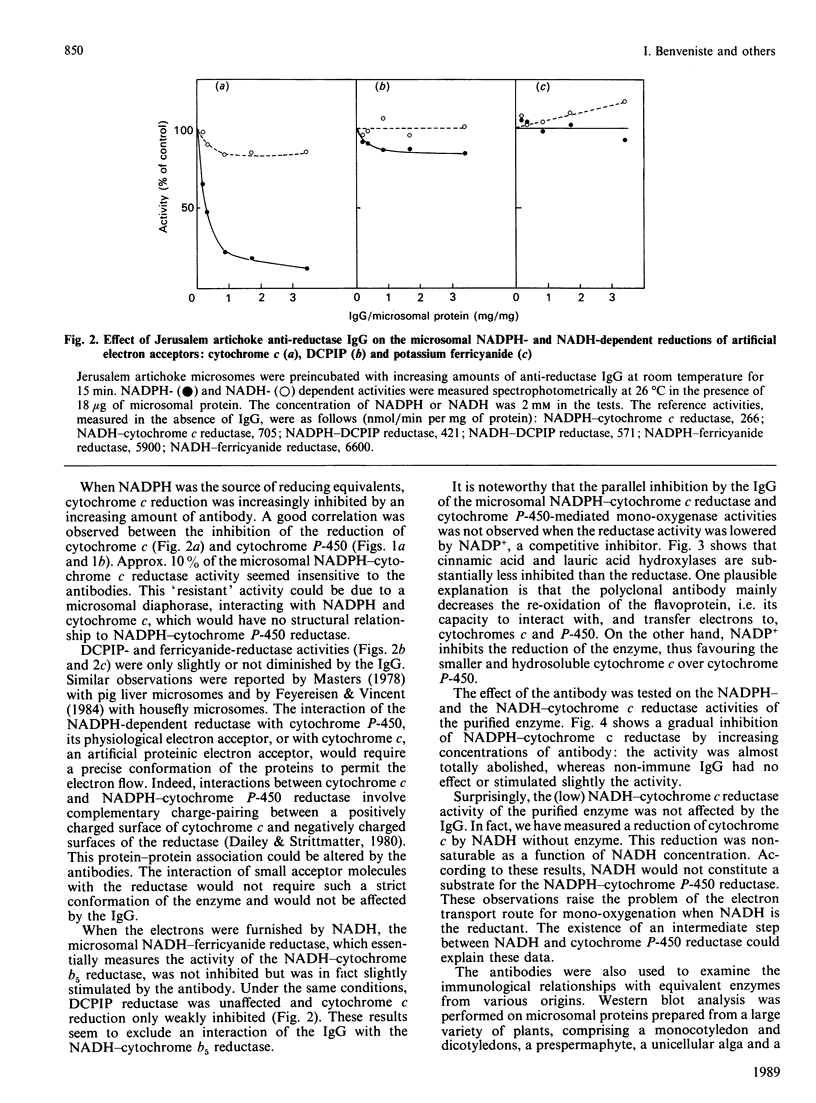
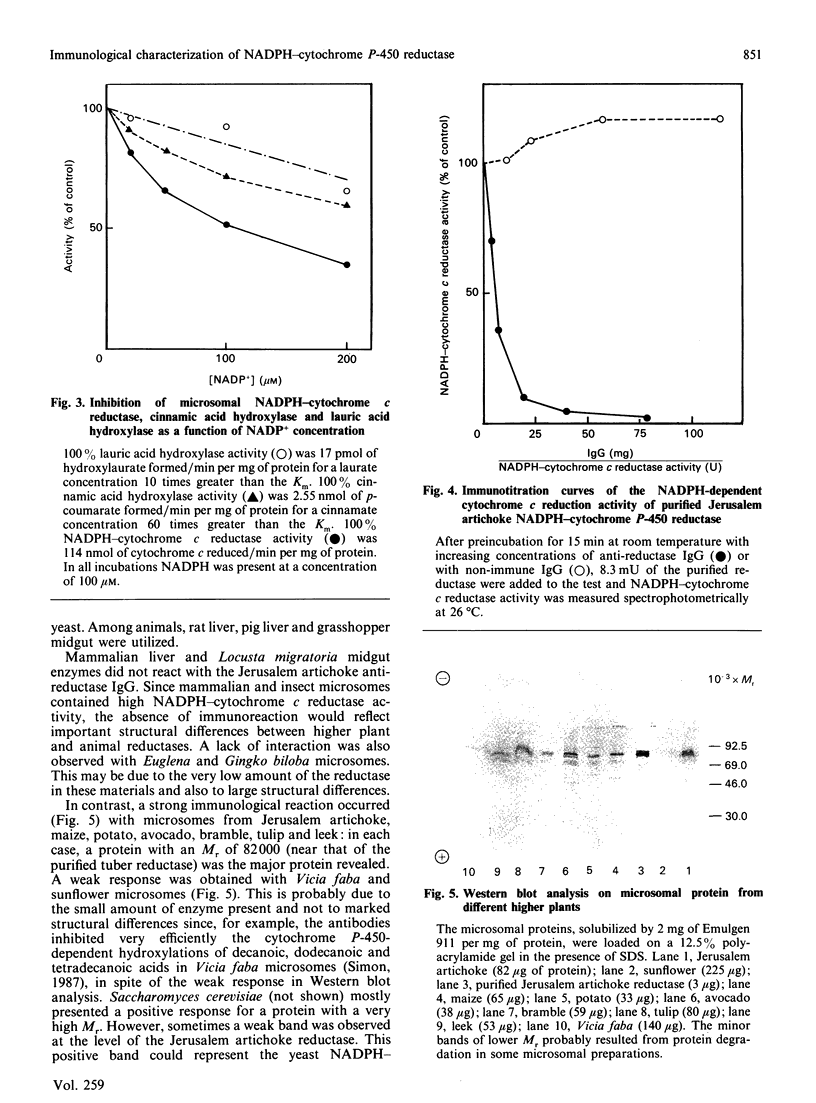
Polyclonal antibodies were prepared against NADPH-cytochrome P-450 reductase purified from Jerusalem artichoke. These antibodies inhibited efficiently the NADPH-cytochrome c reductase activity of the purified enzyme, as well as of Jerusalem artichoke microsomes. Likewise, microsomal NADPH-dependent cytochrome P-450 mono-oxygenases (cinnamate and laurate hydroxylases) were efficiently inhibited. The antibodies were only slightly inhibitory toward microsomal NADH-cytochrome c reductase activity, but lowered NADH-dependent cytochrome P-450 mono-oxygenase activities. The Jerusalem artichoke NADPH-cytochrome P-450 reductase is characterized by its high Mr (82,000) as compared with the enzyme from animals (76,000-78,000). Western blot analysis revealed cross-reactivity of the Jerusalem artichoke reductase antibodies with microsomes from plants belonging to different families (monocotyledons and dicotyledons). All of the proteins recognized by the antibodies had an Mr of approx. 82,000. No cross-reaction was observed with microsomes from rat liver or Locusta migratoria midgut. The cross-reactivity generally paralleled well the inhibition of reductase activity: the enzyme from most higher plants tested was inhibited by the antibodies; whereas Gingko biloba, Euglena gracilis, yeast, rat liver and insect midgut activities were insensitive to the antibodies. These results point to structural differences, particularly at the active site, between the reductases from higher plants and the enzymes from phylogenetically distant plants and from animals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama Y., Yoshida Y., Kubota S., Kumaoka H., Furumichi A. NADPH-cytochrome P-450 reductase of yeast microsomes. Arch Biochem Biophys. 1978 Jan 30;185(2):362–369. doi: 10.1016/0003-9861(78)90178-9. [DOI] [PubMed] [Google Scholar]
- Benveniste I., Gabriac B., Durst F. Purification and characterization of the NADPH-cytochrome P-450 (cytochrome c) reductase from higher-plant microsomal fraction. Biochem J. 1986 Apr 15;235(2):365–373. doi: 10.1042/bj2350365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste I., Salaün J. P., Simon A., Reichhart D., Durst F. Cytochrome P-450-Dependent omega-Hydroxylation of Lauric Acid by Microsomes from Pea Seedlings. Plant Physiol. 1982 Jul;70(1):122–126. doi: 10.1104/pp.70.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey H. A., Strittmatter P. Characterization of the interaction of amphipathic cytochrome b5 with stearyl coenzyme A desaturase and NADPH:cytochrome P-450 reductase. J Biol Chem. 1980 Jun 10;255(11):5184–5189. [PubMed] [Google Scholar]
- Fujita M., Oba K., Uritani I. Properties of a Mixed Function Oxygenase Catalyzing Ipomeamarone 15-Hydroxylation in Microsomes from Cut-Injured and Ceratocystis fimbriata-Infected Sweet Potato Root Tissues. Plant Physiol. 1982 Aug;70(2):573–578. doi: 10.1104/pp.70.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann M. L., Heller W., Grisebach H. Induction and characterization of a microsomal flavonoid 3'-hydroxylase from parsley cell cultures. Eur J Biochem. 1983 Aug 15;134(3):547–554. doi: 10.1111/j.1432-1033.1983.tb07601.x. [DOI] [PubMed] [Google Scholar]
- Hardie G., van Regenmortel M. H. Isolation of specific antibody under conditions of low ionic strength. J Immunol Methods. 1977;15(4):305–314. doi: 10.1016/0022-1759(77)90092-8. [DOI] [PubMed] [Google Scholar]
- Hasson E. P., West C. A. Properties of the System for the Mixed Function Oxidation of Kaurene and Kaurene Derivatives in Microsomes of the Immature Seed of Marah macrocarpus: Electron Transfer Components. Plant Physiol. 1976 Oct;58(4):479–484. doi: 10.1104/pp.58.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri M., Murakami H., Yabusaki Y., Sugiyama T., Okamoto M., Yamano T., Ohkawa H. Molecular cloning and sequence analysis of full-length cDNA for rabbit liver NADPH-cytochrome P-450 reductase mRNA. J Biochem. 1986 Oct;100(4):945–954. doi: 10.1093/oxfordjournals.jbchem.a121807. [DOI] [PubMed] [Google Scholar]
- Kochs G., Grisebach H. Enzymic synthesis of isoflavones. Eur J Biochem. 1986 Mar 3;155(2):311–318. doi: 10.1111/j.1432-1033.1986.tb09492.x. [DOI] [PubMed] [Google Scholar]
- Madyastha K. M., Meehan T. D., Coscia C. J. Characterization of a cytochrome P-450 dependent monoterpene hydroxylase from the higher plant Vinca rosea. Biochemistry. 1976 Mar 9;15(5):1097–1102. doi: 10.1021/bi00650a023. [DOI] [PubMed] [Google Scholar]
- Masters B. S. The preparation and use of antibodies as diagnostic biochemical probes. Methods Enzymol. 1978;52:240–251. doi: 10.1016/s0076-6879(78)52027-2. [DOI] [PubMed] [Google Scholar]
- Pedersen A. O., Hust B., Andersen S., Nielsen F., Brodersen R. Laurate binding to human serum albumin. Multiple binding equilibria investigated by a dialysis exchange method. Eur J Biochem. 1986 Feb 3;154(3):545–552. doi: 10.1111/j.1432-1033.1986.tb09433.x. [DOI] [PubMed] [Google Scholar]
- Porter T. D., Kasper C. B. Coding nucleotide sequence of rat NADPH-cytochrome P-450 oxidoreductase cDNA and identification of flavin-binding domains. Proc Natl Acad Sci U S A. 1985 Feb;82(4):973–977. doi: 10.1073/pnas.82.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts J. R., Weklych R., Conn E. E., Rowell J. The 4-hydroxylation of cinnamic acid by sorghum microsomes and the requirement for cytochrome P-450. J Biol Chem. 1974 Aug 25;249(16):5019–5026. [PubMed] [Google Scholar]
- Rahier A., Taton M. The 14 alpha-demethylation of obtusifoliol by a cytochrome P-450 monooxygenase from higher plants' microsomes. Biochem Biophys Res Commun. 1986 Nov 14;140(3):1064–1072. doi: 10.1016/0006-291x(86)90743-6. [DOI] [PubMed] [Google Scholar]
- Salaün J. P., Benveniste I., Reichhart D., Durst F. A microsomal (cytochrome P-450)-linked lauric-acid-monooxygenase from aged Jerusalem-artichoke-tuber tissues. Eur J Biochem. 1978 Sep 15;90(1):155–159. doi: 10.1111/j.1432-1033.1978.tb12586.x. [DOI] [PubMed] [Google Scholar]
- Soliday C. L., Kolattukudy P. E. Biosynthesis of Cutin omega-Hydroxylation of Fatty Acids by a Microsomal Preparation from Germinating Vicia faba. Plant Physiol. 1977 Jun;59(6):1116–1121. doi: 10.1104/pp.59.6.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukochi Y., Masters B. S. Some properties of a detergent-solubilized NADPH-cytochrome c(cytochrome P-450) reductase purified by biospecific affinity chromatography. J Biol Chem. 1976 Sep 10;251(17):5337–5344. [PubMed] [Google Scholar]