Abstract
Objective
Long non-coding RNAs (lncRNAs) are closely associated with the pathogenesis of laryngeal squamous cell carcinoma (LSCC). This study aimed to investigate the roles of AC068768.1 in LSCC.
Methods
Exosomes were extracted by ultracentrifugation and identified by transmission electron microscopy (TEM) assay. The expression levels of mRNA and miRNA were determined by real-time quantitative polymerase chain reaction (RT-qPCR). Cellular functions were assesses through immunofluorescence, flow cytometry, colony formation, wound healing and transwell assays. Chromatin immunoprecipitation (ChIP) and luciferase assays were conducted to verify the binding of AC068768.1 by signal transducer and activator of transcription 3 (STAT3). Xenograft assays were performed to confirm the roles of AC068768.1 in LSCC, and hematoxylin-eosin (HE) staining was applied for histological analysis.
Results
LSCC cell-derived exosomes induced M2-like tumor-associated macrophages (TAM2) polarization, which promoted the proliferation, migration, and invasion of LSCCs. Knockdown of exosomal AC068768.1 inhibited M2 polarization and suppressed LSCC aggressiveness both in vitro and in vivo. Moreover, AC068768.1 sponged miR-139-5p, inducing the upregulation of neurogenic locus notch homolog protein 1 (NOTCH1). LSCCs adapted to TAM2 polarization in the tumor microenvironment via AC068768.1-mediated activation of the NOTCH1 pathway. Additionally, NOTCH1 activated STAT3.
Conclusion
The AC068768.1/miR-139-5p/NOTCH1/STAT3 axis promotes the metastasis of LSCC. This finding may provide a novel target for LSCC therapy.
Abbreviation
- ARG1
arginase 1
- ceRNA
competing endogenous RNA
- ChIP
chromatin immunoprecipitation
- DMEM
dulbecco's modified eagle medium
- FBS
fetal bovine serum
- GEPIA
gene expression profiling interactive analysis
- HE
hematoxylin-eosin
- HNSCC
head and neck squamous cell carcinoma
- IF
immunofluorescence
- lncRNA
long non-coding RNA
- LSCC
laryngeal squamous cell carcinoma
- M2
macrophage 2
- NOTCH1
neurogenic locus notch homolog protein 1
- PDX
patient-derived tumor xenograft
- PMA
phorbol 12-myristate 13-acetate
- PVDF
polyvinylidene fluoride;
- RT-qPCR
real-time quantitative polymerase chain reaction
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- STAT3
signal transducer and activator of transcription 3
- TAM
tumor-associated macrophage
- TEM
transmission electron microscopy
- TGF-β:
transforming growth factor β; THP1: tohoku hospital pediatrics-1
- TME
tumor microenvironment
1. Introduction
Laryngeal squamous cell carcinoma (LSCC) is the one of the most common malignant tumors in the head and neck. According to the global cancer statistics in 2020, there were 184615 new cases of laryngeal cancer and 99840 new deaths [1]. Despite significant advances in the treatment of LSCC, including chemotherapy, radiotherapy, and surgery, a meta-analysis concluded that the 5-year overall survival rate for laryngeal cancer remains around 64.2 % with no significant improvement [2,3]. Many patients present with metastasis at the time of diagnosis. Consequently, early detection and treatment of LSCC are vital for improving the survival rates and quality of life, and there is an urgent need to explore therapeutic targets for LSCC [4].
Long non-coding RNAs (lncRNAs) are a member of non-coding RNAs without protein-encoding capability [5]. LncRNAs are involved in regulating various biological processes such as transcription regulation, gene regulation, therapeutic targets, and prognosis [[5], [6], [7], [8]]. However, aberrant levels of lncRNAs are closely associated with the pathogenesis of diseases, including LSCC. For instance, Li et al., reported that ALKBH5-mediated m6A modification of KCNQ1OT1 triggers the development of LSCC via upregulation of HOXA9 [9]. Additionally, lnc-POP1-1 interacts with MCM5 to enhance DNA repair in LSCC, inducing cisplatin resistance [10]. Combined with clinical information, Horozoglu et al., found that higher expression of lncRNA AROD was associated with advanced T stage and shortened overall survival [11]. Some lncRNAs, such as lncRNA SNHG16 and lncRNA NEAT1, promote the proliferation, migration, and invasion growth of LSCC through the Wnt signaling pathway [12,13]. Exosomes are nanoscale vesicles secreted by a variety of living cells, which play a role in intercellular information transmission between cells and regulation of the tumor microenvironment [14]. It has been reported that exosomes secreted by tumor cells can promote the function of tumor-associated macrophages (TAM) through the secretion of lncRNAs, thereby regulating the pathological process of tumors [[15], [16], [17]]. AC068768.1, located on chromosome 12, is a newly discovered lncRNA that targets miR-21-5p to promote the proliferation of renal cancer cells [18]. However, the roles of AC068768.1 in LSCC are still unclear.
Neurogenic locus notch homolog protein 1 (NOTCH1), a member of the NOTCH protein family (including Notch 1, 2, 3, and 4), is located in the cell membrane [19]. NOTCH1 exerts its function by transferring cell-to-cell signals and is closely linked to various tumorigenesis-related signaling pathways, affecting apoptosis, proliferation, chemotherapy sensitivity, immune response, and the number of cancer stem cells. Aberrant expression of NOTCH has been shown to promote the pathogenesis of various cancers. For instance, the activation of NOTCH1 signaling promotes the proliferation of glioblastoma [20], while NOTCH1 deficiency inhibits the metastasis of prostate cancer [21]. NOTCH1 is involved in the migration and proliferation of head and neck squamous cell carcinoma (HNSCC) cell lines, with the expression of the NOTCH1 internal domain being associated with poor survival rates, suggesting its potential as a prognostic marker [22]. In our previous study, NOTCH1 mutation was found to be an independent genetic factor significantly associated with shorter relapse-free survival in early-stage laryngeal cancer, and immune response scores were reduced in recurrent tumors with NOTCH1 mutations [23].
In the present study, we detected the expression of AC068768.1 in exosomes from LSCCs and discovered the exosomal AC068768.1 promotes M2 polarization and metastasis of LSCC via activating NOTCH1 signaling. Therefore, the AC068768.1/miR-139-5p/NOTCH1 axis may serve as a therapeutic target for LSCC.
2. Material and methods
2.1. Cell culture
LSCC cell lines AMC–HN–8 and TU686 cells and HEK-293 T kidney epithelial cell line were obtained from Cell Bank, China Academy of Sciences. The cells were cultured in dulbecco's modified eagle medium (DMEM) medium containing 10 % fetal bovine serum (FBS) in the atmosphere of 5 % CO2 at 37 °C.
2.2. Extraction of exosomes and transmission electron microscopy (TEM) analysis
Cells were centrifuged to remove cell and large molecules. Afterwards, the supernatants were ultracentrifugation at 100, 000×g. Exosomes were collected and quantified by NanoSight NS300 instrument (Malvern Instruments) using nanoparticle tracking analysis software (version2.3). The morphology of the collected exosomes was analyzed by TEM.
2.3. Preparation of macrophage and co-culture with LSCC
THP1 cells were cultured in RPMI-1640 medium containing 10 % FBS, and induced transformation with 100 ng/mL phorbol 12-myristate 13-acetate (PMA, NF-κB activator). The macrophages were then incubated in fresh medium for 48 h. Macrophages were co-cultured with LSCCs to simulate exosome-mediated intercellular communication. LSCC were transfected with Cy3-labeled RNAs.
2.4. Cell transfection
Cell transfection was performed using Lipofectamine 2000 (Invitrogen, USA). Briefly, shAC068768.1 and its negative control were mixed with Lipofectamine 2000 and cultured for 6 h. Afterwards, cells were transfected with the mixture for 48 h. Subsequent experiments used shRNA with more significant transfection efficiency.
2.5. Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using RNA isolation regents and then concentrated using NanoDrop™ 2000 spectrophotometer. Afterwards, cDNA was synthesized using a PrimeScript® RT reagent kit. PCR was performed using a PrimeScript RT-PCR kit on an ABI 7500 Real-Time PCR system. GAPDH served as the loading control. Relative mRNA levels were determined by 2−ΔΔCq method.
2.6. The exosome markers in LSCC were detected bywestern blot
After transfection, cells were collected and lysed. Then total protein was collected and concentrated using a BCA kit. Protein (30 μg) was isolated using 10 % sodium dodecyl sulfate poly acrylamide gel electrophoresis (SDS-PAGE) at 120 v. The protein was transferred onto polyvinylidene fluoride (PVDF) membranes. After blocked with 5 % skimmed milk, the membranes were incubated with primary antibodies and with goat-anti-rabbit IgG H&L (HRP) antibody (ab6721, 1: 5000, Abcam, USA). Finally, the bands were captured by ECL regents and analyzed using ImageJ (V.2.3.0).
2.7. Exosomes derived from LSCC were detected by immunofluorescence (IF) assay
Cells were fixed with 4 % paraformaldehyde and permeabilized with 0.2 % Triton X-100. Afterwards, cells were sealed with 5 % FBS. Then cells were incubated with primary antibodies against CD68 and CD206 and then with secondary antibody. Then cells were counterstained with DAPI. The results were visualized using an immunofluorescence microscope (Zeiss, Germany).
2.8. Detection of cell migration and invasion
After transfection, cells were collected and seeded into a 24-well plate precoated with soft agar. Afterwards, cells were cultured in RPMI-1640 medium containing 10 % FBS for 21 days. The medium was changed every other two days. Subsequently, the cells were captured by a microscope to calculate colony formation.
Cells were seeded in a 24-well plate. Then cells were scratched using a 100-μl plastic pipette tip to make sure the scratches are the same width. After 24 h, cells were captured using a microscope. The migrated cells were analyzed using ImageJ software (Bethesda, USA), and the cell migration of each group was obtained according to the normalization of control cells.
The matrigel invasion assays were conducted using transwell chambers, which were coated with or without the matrigel mix (BD, USA). The homogeneous serum-free single cell suspensions (1 × 105 cells/well for migration and 5 × 105/well for invasion, respectively) were added to the upper chambers and medium with 10 % fetal bovine serum was added into the lower chambers, then incubated for 24 h. The invaded cells were were fixed and stained, 5 visual fields were randomly selected to take photos and count to reflect the invasion of cells.
2.9. The number of macrophages determined by flow cytometry
Transfected cells were harvested, washed and re-suspended with PBS.The cells were fixed and permeabilized, and incubated PE–conjugated antibody (Abcam, USA) detecting CD206-positive or CD206 plus CD11b positive macrophages.
2.10. Binding sites verified by luciferase assay
Full length NOTCH1 and signal transducer and activator of transcription 3 (STAT3) were amplified and inserted into the psiCHECK2 luciferase reporter vector. Then cells were transfected with shSTAT3/STAT3 overexpression plasmids or the negative control and wide type or mutant 3′UTR of TXNIP. After 48 h, the luciferase activity was determined using a dual-luciferase reporter system (Promega).
2.11. Cromatin immunoprecipitation (ChIP) assay
ChIP assay was conducted using a ChIP kit. Cells were fixed in 1 % formaldehyde and resuspended in lysis buffer. Then shearing chromatin was performed by adding with adding enzymatic shearing cocktail and stopped by supplemented with EDTA. After pre-cleared with Protein A/G agarose beads, the supernatants were incubated with antiSTAT3 or antiIgG. The collected chromatin was eluted and proteinase K. ChIP-enriched DNA was determined using PCR.
2.12. Patient-derived tumor xenograft (PDX) experiments
For TU686 cells, 1 × 106 cells per recipient were injected i.v. 1 × 105 cells were treated with FBS or shAC068768.1. Cells were transduced with FBS or, sorted 48h post-transduction and injected i.v. into NSG mice (2 × 105 cells per mouse). Mice were sacrificed 7–8 weeks post-transplantation to assess PDX engraftment. The number of CD206 plus CD11b positive macrophages determined by flow cytometry and histological analysis was performed by using HE staining.
2.13. Statistical analysis
Data analysis was carried on Prism6 software. Data were expressed as mean ± SD. The inter-group difference analysis was performed using student t-test or one-way ANOVA assay. P < 0.05 was considered as statistical significance.
3. Results
3.1. LSCC cell-derived exosomes promote M2 polarization
Cancer cell-derived exosomes facilitate signal transmission and enhance the malignant behaviors of tumor cells. We explored the role of LSCC-derived exosomes in M2 polarization. To investigate the roles of LSCC cell in M2 polarization, LSCCs were co-cultured with PMA-induced tohoku hospital pediatrics-1 (THP1) cells. As shown in Fig. 1A, the diameter of exosomes derived from LSCCs were approximately 100 nm. To determine the effects of LSCCs-derived exosomes on macrophages functions, cells were treated with LSCC-derived exosomes or the exosome formation inhibitor GW4869. We found that LSCC-derived exosome treatment increased DiO positivity in THP1 (Mφ) cells (Fig. 1B). Moreover, LSCC-derived exosome treatment markedly increased the number of CD11b + CD206+ cells (Fig. 1C). However, the protein expression of exosomal makers (such as CD9 and CD81) in LSCCs was decreased with GW4869 treatment (Fig. 1D), suggesting successful inhibition of exosome formation by GW4869 treatment. Furthermore, the increase in the release of M2 markers (ARG1, CD206, transforming growth factor β (TGF-β), IL-10) induced by LSCC treatment was markedly dampened by GW4869 treatment (Fig. 1E). These findings suggested that LSCC-derived exosome promoted M2 polarization of macrophages.
Fig. 1.
LSCC cell derived exosomes promotes M2 polarization to enhance LSCC metastasis. (A) LSCC derived exosomes verified by TEM and NTR assay. (B) The transmission of LSCC cell derived exosomes determined by immunofluorescence. (C) The protein expression of exosome markers in LSCCs after GW4869 treatment determined by Western blot. (D) The number of CD11b + CD206+ macrophages determined by flow cytometry. (E) The release of M2 markers detected by ELISA. **P < 0.01.
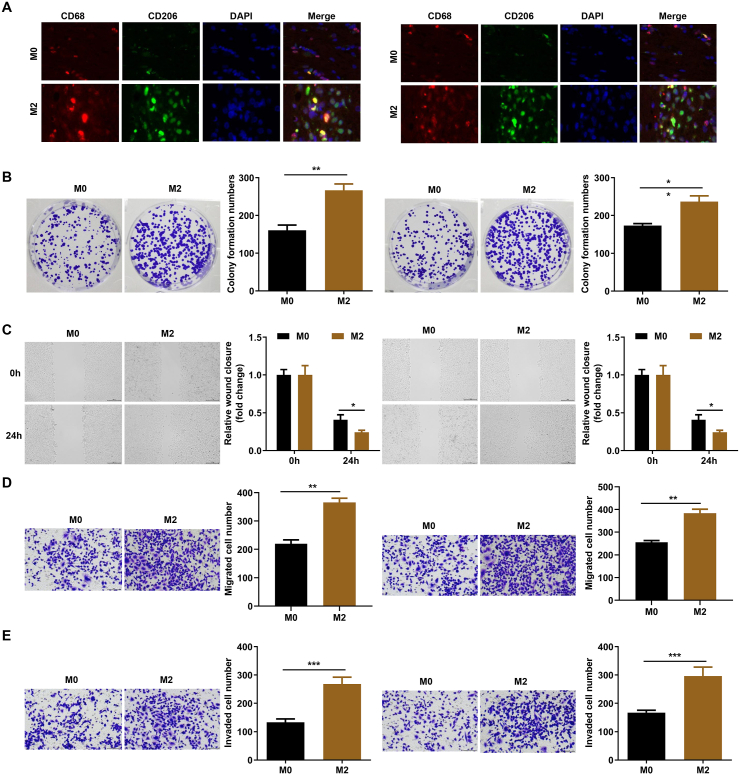
3.2. M2 polarization promotes the proliferation, migration, and invasion of LSCCs
M2 polarization confers to a favorable environment for tumor cells growth and metastasis. The TAM2 co-culture system demonstrated an increase the TAM2-specific marker CD68/CD206 via immunofluorescence assay (Fig. 2A). To verify the effects of M2 polarization of macrophages on LSCC, LSCCs were co-cultured with M0 and M2 macrophages. The results showed a significant increase in colony information in M2 group (Fig. 2B). Moreover, M2 polarization markedly enhanced the migratory ability of LSCCs (Fig. 2C). As shown in Fig. 2D, consistent results from transwell assay also proved that M2 polarization remarkably increased the number of migrated cells. Additionally, M2 polarization promoted the invasion of LSCCs (Fig. 2E), suggesting that M2 polarization enhances the aggressiveness of LSCCs.
Fig. 2.
M2 polarization promotes the proliferation, migration, and invasion of LSCCs. (A) M0 and M2 markers determined by immunofluorescence. (B) The cell proliferation of LSCCs determined by colony formation assay. (C) The migration of LSCC cell detected by wound healing assay. (D) The migration of LSCC cell detected by transwell assay. (E) The invasion of LSCC cell detected by transwell assay. **P < 0.01.
3.3. Exosomal AC068768.1 promotes the M2 polarization
Tumor cell-derived exosomes are rich in lncRNAs, which play key roles in post-translation. Therefore, LSCC derived exosomes may regulate cellular functions of macrophagesLSCCs though lncRNAs. To further verify this, we determined the lncRNAs expression in LSCC derived exosomes. As shown in Fig. 3A, lncRNA expression differed between LSCC and HEK-293T cells, with three lncRNAs was significantly upregulated (Fig. 3B). These differential expressions were also observed between exosomes and LSCCs (Fig. 3C). Among them, AC068768.1 showed remarkable expression (Fig. 3B), and was the focus of this study. The expression of AC068768.1 was markedly decreased in by specific shRNA (Fig. 3D), and exosomes with a low level of AC068768.1 decreased DiO positivity in THP1 (Mφ) cells (Fig. 3E). Furthermore, AC068768.1 deficiency in LSCC-derived exosomes significantly reduced the number of CD11b + CD206+ cells (Fig. 3F) and markedly decreased the release of M2 markers (ARG1, CD206, TGF-β, IL-10) (Fig. 3G). These findings indicate that exosomal AC068768.1 is essential for M2 polarization of macrophages.
Fig. 3.
Exosomal AC068768.1 promotes the M2 polarization. (A) The abnormal expressed lncRNAs in LSCCs. (B) Differentially expressed lncRNAs in LSCCs. (C) The expression of AC068768.1 in LSCCs and exosomes. (D) The transfection efficiency of shAC068768.1 determined by RT-qPCR. (E) The transmission of LSCC cell derived exosomes determined by immunofluorescence. (F) The number of CD11b + CD206+ macrophages after exosomes with lower AC068768.1 treatment determined by flow cytometry. (G) The release of M2 markers determined by ELISA. **P < 0.01.
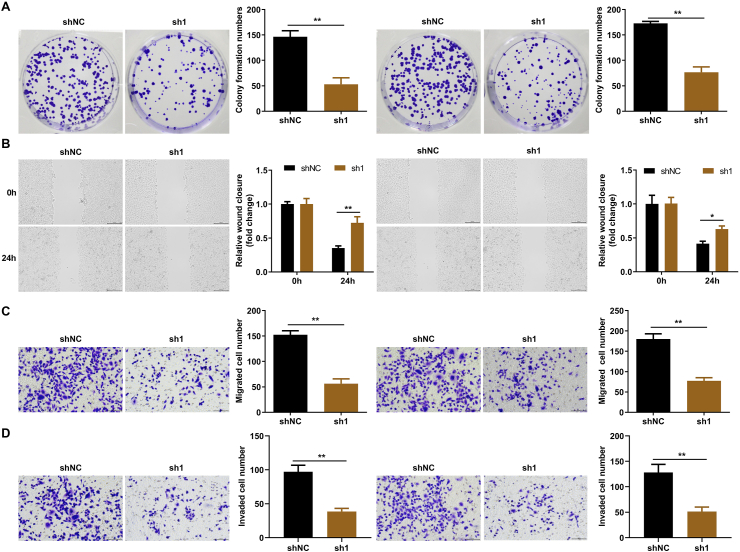
3.4. Exosomal AC068768.1 suppresses the proliferation, migration and invasion of LSCCs
To further investigate the role of AC068768.1 in LSCC, tumor cells were treated with exosomes containing lower levels of AC068768.1. We found that AC068768.1 deficiency markedly decreased colony formation of LSCCs (Fig. 4A) and inhibited their migration and invasive abilities (Fig. 4B–D). These findings suggest that lower exosomal AC068768.1 suppress the malignant behavior of LSCCs.
Fig. 4.
Exosomal AC068768.1 suppresses the proliferation, migration and invasion of LSCCs. (A) The cell proliferation of LSCCs determined by colony formation assay. (B) The migration of LSCC cell detected by wound healing assay. (C) The migration of LSCC cell detected by transwell assay. (D) The invasion of LSCC cell detected by transwell assay. **P < 0.01.
3.5. AC068768.1 sponges miR-139-5p to upregulate NOTCH1
LncRNAs function as competing endogenous RNAs (ceRNAs) to regulate biological processes via the miRNA/mRNA axis. The potential binding sites between AC068768.1 and miR-139-5p, and between miR-139-5p and NOTCH1, were predicted using Starbase3.0 and TargetScan7.2 (Fig. 5A and B). Luciferase assays further verified these binding sites (Fig. 5C and D). Moreover, AC068768.1 knockdown significantly decreased NOTCH1 expression (Fig. 5E), while miR-139-5p inhibitor increased its expression. Data from Starbase showed that NOTCH1 expression was elevated in LSCC patients (Fig. 5F) and that AC068768.1 expression correlated positively with NOTCH1 expression (Fig. 5G), suggesting that AC068768.1-mediated upregulation of NOTCH1 may promote the development of LSCC.
Fig. 5.
AC068768.1 sponges miR-139-5p to upregulate NOTCH1. The potential binding sites between AC068768.1 and miR-139-5p (A) or miR-139-5p and NOTCH1 (B) predicted by Starbase3.0. (C) The binding sites between AC068768.1 and miR-139-5p verified by luciferase assay (C). (D) The binding sites between NOTCH1 and miR-139-5p verified by luciferase assay. (E) NOTCH1 mRNA expression determined by RT-qPCR. (F) NOTCH1 expression analyzed using Starbase3.0. (G) The correlation between AC068768.1 and NOTCH1 expression. **P < 0.01.
3.6. LSCCs adapt to TAM2 polarization in the tumor microenvironment via AC068768.1-mediated NOTCH1 pathway activation
NOTCH1 often functions as an oncogene in various tumors. To confirm the roles of NOTCH1 in LSCC, cells were transfected to overexpress NOTCH1. As shown in Fig. 6A, M2 polarization significantly increased the colony formation of LSCCs, which was abrogated by AC068768.1 knockdown. However, overexpressed NOTCH1 counteracted the effects of shAC068768.1 and markedly increased the colony formation of LSCCs. Moreover, overexpression of NOTCH1 significantly increased the migratory ability of LSCCs (Fig. 6B and C) and markedly promoted their invasion (Fig. 6D).
Fig. 6.
LSCCs adapt to TAM2 polarization in the tumor microenvironment via AC068768.1-mediated NOTCH1 pathway activation. (A) The cell proliferation of LSCCs determined by colony formation assay. (B) The migration of LSCC cell detected by wound healing assay. (C) The migration of LSCC cell detected by transwell assay. (D) The invasion of LSCC cell detected by transwell assay. **P < 0.01.
3.7. AC068768.1-dependent NOTCH1 epigenetically up-regulates STAT3
STAT3 is a key regulator of M2 macrophage polarization. Therefore, we hypothesized that the AC068768.1/NOTCH1 axis may promote M2 macrophage polarization and aggressiveness of LSCC via activation STAT3. Fig. 7A showed that NOTCH1 expression was positively correlated with STAT3 expression. JASPAR (a database of transcription factor binding profiles) showed STAT3 binding motifs (Fig. 7B) and the potential binding sites on the promoter of NOTCH1 (Fig. 7C). These potential binding sites were further verified using by luciferase (Fig. 7D) and ChIP assays (Fig. 7E).
Fig. 7.
AC068768.1-dependent NOTCH1 epigenetically up-regulates STAT3. (A) The correlation between STAT3 and NOTCH1 expression analyzed using gene expression profiling interactive analysis (GEPIA). (B) The binding motif of STAT3. (C) The binding sites of STAT3 on the promoter of NOTCH1 predicted by JASPAR. (D) The binding sites verified by luciferase assay. (E) The binding sites verified by ChIP assay. **P < 0.01.
3.8. AC068768.1 knockdown suppresses lung metastasis of LSCC
To further verify the roles of AC068768.1 in LSCC, in vivo assays were performed. As shown in Fig. 8A, the transfection efficiency of shAC068768.1 was confirmed, and AC068768.1 knockdown markedly decreased CD11b + CD206+ macrophages (Fig. 8B). Moreover, AC068768.1 knockdown suppressed lung metastasis of LSCCs (Fig. 8C) and decreased the expression of NOTCH1 and STAT3 (Fig. 8D).
Fig. 8.
AC068768.1 knockdown suppresses lung metastasis of LSCC. (A) The transfection efficiency of shAC068768.1 determined by RT-qPCR. (B) The number of CD11b + CD206+ macrophages determined by flow cytometry. (C) Histological analysis of lung metastasis detected by HE staining. (D) Nothch1 and STAT3 expression determined by immunohistochemistry. **P < 0.01.
4. Discussion
Most patients with advanced LSCC are limited to total laryngectomy, resulting in the loss of speech and smell. Treatment is crucial for improving survival rates and quality of life, highlighting the urgent need to identify new therapeutic targets for LSCC. In this study, we investigated the role exosomal AC068768.1 derived from LSCC cells in promoting M2 macrophage polarization and metastasis, both in vitro and in vivo. Our findings indicate that AC068768.1 acts as a sponge for miR-139-5p, leading to increased expression of NOTCH1, which subsequently activated STAT3. To our knowledge, this is the first study to explore the functions of AC068768.1 in LSCC, potentially offering a novel therapeutic strategy for LSCC.
Tumor cell–derived exosomes play a significant role in reshaping the tumor microenvironment (TME), establishing favorable scenarios for tumor growth and metastasis. In this study, LSCCs-derived exosomes promote the M2 macrophage polarization, which transforms tumor-associated macrophages (TAMs) and reshapes the TME and pre-metastatic niches. Interestingly, co-culturing LSCCs with M2 macrophages promoted their proliferation and invasion, indicating that M2 macrophage polarization contributes to the aggressiveness of LSCCs, consistent with findings from Wang et al. [24].
LncRNAs present in exosomes are known to regulate various pathological processes in tumors, exhibiting either tumor-suppressive or oncogenic functions [25]. For instance, exosomal lncRNA HOTAIR has been shown to mediate M2 macrophage polarization, enhances the epithelial-mesenchymal transition and metastasis in LSCC. Conversely, exosomes derived from M1 macrophages can inhibit the malignant behaviors of head and neck squamous cell carcinoma (HNSCC) cells and promote tumor cell apoptosis [26]. Therefore, it is crucial to identify the roles of lncRNAs in LSCC. In this study, LSCCs and tumor cell derived exosomes were rich in AC068768.1. Notably, the deficiency of AC068768.1 in LSCCs-derived exosomes suppressed M2 macrophage polarization and inhibited the proliferation, migration, and invasion of LSCCs. This finding suggests that lncRNA AC068768.1 may function as an oncogene and could serve as a therapeutic target for LSCC.
We further found that AC068768.1 sponges miR-139-5p, leading to the upregulation of NOTCH1 expression. MiR-139-5p is recognized as a tumor suppressor in various cancers, such as colorectal cancer, liver cancer, lung cancer and head and neck squamous cell carcinoma, etc. [[27], [28], [29], [30], [31]] Therefore, the interaction between AC068768.1 and miR-139-5p may diminish the anti-tumor effects of miR-139-5p and disrupt its interaction with NOTCH1, providing new sights for the development of LSCC.
NOTCH1 is frequently implicated in promoting tumor cell proliferation, migration, inflammation, and immunosuppression, underscoring its oncogenic function in cancer [32]. In HNSCC, high expression of NOTCH1 is associated with poor prognosis of HNSCC patients. Previous study has reported that NOTCH1 mutation promotes the sensitivity of PI3K/mTOR inhibition therapies [33]. Our findings reveal that NOTCH1 is overexpressed in LSCC, and its overexpression mitigates the effects of AC068768.1 knockdown, promoting LSCCs proliferation, migration and invasion. Therefore, AC068768.1/NOTCH1 axis may represent a promising therapeutic strategy for LSCC.
Macrophages control the tumor microenvironment via secreting cytokines and chemokines to reshape TME [34]. TAM2, an important source of pro-inflammatory cytokines, favors tumor growth and metastasis, and study has reported that STAT3 was involved in TAM2 polarization [35]. STAT3, a key oncogenic transcriptional factor, promotes the tumorigenesis via binding to cytokines or growth factors [36]. As a master mediator of tumor-associated immune tolerance, the activation of STAT3 maintain an immunotolerant TME and immune escape of larynx carcinoma through regulating VEGFR1-TGFβ signaling in TAM2 [37]. Interestingly, inactivation of STAT3 signaling suppresses M2 macrophage polarization and promotes the chemo- and radiotherapy of HNSCC cells [38]. In this study, we observed a positive correlation between STAT3 and NOTCH1 expression. Furthermore, AC068768.1-dependent NOTCH1 epigenetically upregulated STAT3, suggesting that AC068768.1 derived from LSCC cells may promote M2 macrophage polarization and metastasis through the activation of NOTCH1/STAT3 signaling.
Despite the promising results, this study has several limitations. Firstly, we focused on the effects of exosomal AC068768.1 on LSCC through miR139-5p/NOTCH1 pathway, leaving other potential pathways unexplored. Additionally, while cell lines and xenograft models simulate tumor growth and proliferation, they do not fully replicate the complexity of human tumors. Future research should incorporate patient-derived organoids and clinical samples to validate our findings. This target deserves further development and validation in clinical trials.
In summary, our study is the first to elucidate the detailed regulatory mechanisms of exosomal AC068768.1 in LSCC, confirming its role in promoting cell growth and migration through the miR139-5p/NOTCH1/STAT pathway. These findings may provide valuable insights for the development of novel biomarkers and therapeutic strategies for LSCC treatment.
Data availability statement
No data associated in this article has been deposited into a publicly available repository. The data that support the findings of this study are included in article/supp/material/referenced in article.
Funding information
This work was supported by grants from the Jiangsu Province Health Commission Fund, China (No.H2018013).
Ethics statement
The study was approved by the Animal Ethics Committee of The First Affiliated Hospital of Nanjing Medical University (2023-SRFA-062).
Consent for publication
All participants signed an informed consent form, and consented to publication.
CRediT authorship contribution statement
Hai-bin Chen: Writing – original draft, Data curation, Conceptualization. Xiao-yang Gong: Data curation, Conceptualization. Wen-hao Shen: Writing – original draft, Data curation, Conceptualization. Zi-hang Zhu: Writing – original draft, Validation, Data curation. Xi Chen: Validation, Methodology, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Xi Chen reports financial support was provided by First Affiliated Hospital, Nanjing Medical University. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Thanks to Zhaoyi Lu (Department of Otorhinolaryngology, The First Affiliated Hospital, Nanjing Medical University) for suggestions on the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e36358.
Appendix ASupplementary data
The following is the Supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Echanique K.A., Evans L.K., Han A.Y., Chhetri D.K., St John M.A. Cancer of the larynx and hypopharynx. Hematol Oncol Clin North Am. 2021;35:933–947. doi: 10.1016/j.hoc.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Arens C., Schwemmle C., Voigt-Zimmermann S. [Surgical reconstruction in laryngeal carcinoma] HNO. 2020;68:666–677. doi: 10.1007/s00106-020-00916-y. [DOI] [PubMed] [Google Scholar]
- 4.Gong X.-Y., Chen H.-B., Lu Z.-Y., Zhu C., Chen D.-S., Chen X. Prospective role of liquid biopsy for early screening in laryngeal cancer. Invest New Drugs. 2023;41:376–379. doi: 10.1007/s10637-023-01365-4. [DOI] [PubMed] [Google Scholar]
- 5.Ferrer J., Dimitrova N. Transcription regulation by long non-coding RNAs: mechanisms and disease relevance. Nat. Rev. Mol. Cell Biol. 2024;25:396–415. doi: 10.1038/s41580-023-00694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierce J.B., Zhou H., Simion V., Feinberg M.W. Long noncoding RNAs as therapeutic targets. Adv. Exp. Med. Biol. 2022;1363:161–175. doi: 10.1007/978-3-030-92034-0_9. [DOI] [PubMed] [Google Scholar]
- 7.Hong J., Hong A., Tu H., Wan Z., Deng Y., Deng C., Tao B., Yu Y., Zhou L. LncRNA CCAT1 facilitates the proliferation, invasion and migration of human laryngeal squamous cell carcinoma cells via the miR-218-5p/BMI1. PeerJ. 2022;10 doi: 10.7717/peerj.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Y., Li C., Zhang Y.-J., Wu Z.-H. Ferroptosis-Related Long Non-Coding RNA signature predicts the prognosis of Head and neck squamous cell carcinoma. Int. J. Biol. Sci. 2021;17:702–711. doi: 10.7150/ijbs.55552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y., Yan B., Wang X., Li Q., Kan X., Wang J., Sun Y., Wang P., Tian L., Liu M. ALKBH5-mediated m6A modification of lncRNA KCNQ1OT1 triggers the development of LSCC via upregulation of HOXA9. J. Cell Mol. Med. 2022;26:385–398. doi: 10.1111/jcmm.17091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Y., Guo H., Tong T., Xie F., Qin X., Wang X., Chen W., Zhang J. lncRNA lnc-POP1-1 upregulated by VN1R5 promotes cisplatin resistance in head and neck squamous cell carcinoma through interaction with MCM5. Mol. Ther. 2022;30:448–467. doi: 10.1016/j.ymthe.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ban Y., Tan P., Cai J., Li J., Hu M., Zhou Y., Mei Y., Tan Y., Li X., Zeng Z., Xiong W., Li G., Li X., Yi M., Xiang B. LNCAROD is stabilized by m6A methylation and promotes cancer progression via forming a ternary complex with HSPA1A and YBX1 in head and neck squamous cell carcinoma. Mol. Oncol. 2020;14:1282–1296. doi: 10.1002/1878-0261.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang J., Fang F., Gao X., Shi J., Zhao J., Zhao Y. LncRNA NEAT1 promotes proliferation, migration, and invasion of laryngeal squamous cell carcinoma cells through miR-411-3p/FZD3-mediated Wnt signaling pathway. BMC Cancer. 2024;24:904. doi: 10.1186/s12885-024-12661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan L., Gu D., Li P. LncRNA SNHG16 promotes proliferation and migration in laryngeal squamous cell carcinoma via the miR-140-5p/NFAT5/Wnt/β-catenin pathway axis. Pathol. Res. Pract. 2022;229 doi: 10.1016/j.prp.2021.153727. [DOI] [PubMed] [Google Scholar]
- 14.He C., Li L., Wang L., Meng W., Hao Y., Zhu G. Exosome-mediated cellular crosstalk within the tumor microenvironment upon irradiation. Cancer Biol Med. 2021;18:21–33. doi: 10.20892/j.issn.2095-3941.2020.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franzin R., Stasi A., De Palma G., Picerno A., Curci C., Sebastiano S., Campioni M., Cicirelli A., Rizzo A., Di Lorenzo V.F., Pontrelli P., Pertosa G.B., Castellano G., Gesualdo L., Sallustio F. Human adult renal progenitor cells prevent cisplatin-nephrotoxicity by inducing CYP1B1 overexpression and miR-27b-3p down-regulation through extracellular vesicles. Cells. 2023;12:1655. doi: 10.3390/cells12121655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosellini M., Marchetti A., Mollica V., Rizzo A., Santoni M., Massari F. Prognostic and predictive biomarkers for immunotherapy in advanced renal cell carcinoma. Nat. Rev. Urol. 2023;20:133–157. doi: 10.1038/s41585-022-00676-0. [DOI] [PubMed] [Google Scholar]
- 17.Rizzo A., Cusmai A., Gadaleta-Caldarola G., Palmiotti G. Which role for predictors of response to immune checkpoint inhibitors in hepatocellular carcinoma? Expert Rev Gastroenterol Hepatol. 2022;16:333–339. doi: 10.1080/17474124.2022.2064273. [DOI] [PubMed] [Google Scholar]
- 18.Xu Z., Gui D., Fu J., Luo S., Zhao Y., Huang G., Wan J. lncRNA AC068768.1 regulates the cycle and proliferation of renal cancer cells by targeting miR-21-5p. Int. J. Surg. 2021:387–391. [Google Scholar]
- 19.Gharaibeh L., Elmadany N., Alwosaibai K., Alshaer W. Notch1 in cancer therapy: possible clinical implications and challenges. Mol. Pharmacol. 2020;98:559–576. doi: 10.1124/molpharm.120.000006. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Sun Q., Geng R., Liu H., Yuan F., Xu Y., Qi Y., Jiang H., Chen Q., Liu B. Notch intracellular domain regulates glioblastoma proliferation through the Notch1 signaling pathway. Oncol. Lett. 2021;21:303. doi: 10.3892/ol.2021.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rice M.A., Hsu E.-C., Aslan M., Ghoochani A., Su A., Stoyanova T. Loss of Notch1 activity inhibits prostate cancer growth and metastasis and sensitizes prostate cancer cells to antiandrogen therapies. Mol Cancer Ther. 2019;18:1230–1242. doi: 10.1158/1535-7163.MCT-18-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidl B., Siegl M., Boxberg M., Stögbauer F., Jira D., Winter C., Stark L., Pickhard A., Wollenberg B., Wirth M. NOTCH1 intracellular domain and the tumor microenvironment as prognostic markers in HNSCC. Cancers. 2022;14:1080. doi: 10.3390/cancers14041080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong X.-Y., Chen H.-B., Zhang L.-Q., Chen D.-S., Li W., Chen D.-H., Xu J., Zhou H., Zhao L., Song Y.-J., Xiao M.-Z., Deng W.-L., Qi C., Wang X.-R., Chen X. NOTCH1 mutation associates with impaired immune response and decreased relapse-free survival in patients with resected T1-2N0 laryngeal cancer. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.920253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J., Wang N., Zheng Z., Che Y., Suzuki M., Kano S., Lu J., Wang P., Sun Y., Homma A. Exosomal lncRNA HOTAIR induce macrophages to M2 polarization via PI3K/p-AKT/AKT pathway and promote EMT and metastasis in laryngeal squamous cell carcinoma. BMC Cancer. 2022;22:1208. doi: 10.1186/s12885-022-10210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhan A., Soleimani M., Mandal S.S. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77:3965–3981. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang H., Zhou L., Shen N., Ning X., Wu D., Jiang K., Huang X. M1 macrophage-derived exosomes and their key molecule lncRNA HOTTIP suppress head and neck squamous cell carcinoma progression by upregulating the TLR5/NF-κB pathway. Cell Death Dis. 2022;13:183. doi: 10.1038/s41419-022-04640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koma A., Asai S., Minemura C., Oshima S., Kinoshita T., Kikkawa N., Koshizuka K., Moriya S., Kasamatsu A., Hanazawa T., Uzawa K., Seki N. Impact of oncogenic targets by tumor-suppressive miR-139-5p and miR-139-3p regulation in head and neck squamous cell carcinoma. Int. J. Mol. Sci. 2021;22:9947. doi: 10.3390/ijms22189947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Q., Cao Y., Qiu Y., Li C., Liu L., Xu G. Progression of squamous cell carcinoma is regulated by miR-139-5p/CXCR4. Front Biosci (Landmark Ed) 2020;25:1732–1745. doi: 10.2741/4875. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L., Dong Y., Zhu N., Tsoi H., Zhao Z., Wu C.W., Wang K., Zheng S., Ng S.S., Chan F.K., Sung J.J., Yu J. microRNA-139-5p exerts tumor suppressor function by targeting NOTCH1 in colorectal cancer. Mol. Cancer. 2014;13:124. doi: 10.1186/1476-4598-13-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J., Li D.Z., Pang Y., Zhou T., Sun J., Cheng X.Y., Zheng W.V. MicroRNA-139-5p negatively regulates NME1 expression in hepatocellular carcinoma cells. Adv. Clin. Exp. Med. 2022;31:655–670. doi: 10.17219/acem/146579. [DOI] [PubMed] [Google Scholar]
- 31.Sun T., Liu Z. MicroRNA-139-5p suppresses non-small cell lung cancer progression by targeting ATAD2. Pathol. Res. Pract. 2023;249 doi: 10.1016/j.prp.2023.154719. [DOI] [PubMed] [Google Scholar]
- 32.Kawaguchi K., Kaneko S. In: Notch Signaling in Embryology and Cancer. Reichrath J., Reichrath S., editors. Springer International Publishing; Cham: 2021. Notch signaling and liver cancer; pp. 69–80. [DOI] [Google Scholar]
- 33.Sambandam V., Frederick M.J., Shen L., Tong P., Rao X., Peng S., Singh R., Mazumdar T., Huang C., Li Q., Pickering C.R., Myers J.N., Wang J., Johnson F.M. PDK1 mediates NOTCH1-mutated head and neck squamous carcinoma vulnerability to therapeutic PI3K/mTOR inhibition. Clin. Cancer Res. 2019;25:3329–3340. doi: 10.1158/1078-0432.CCR-18-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia Y., Rao L., Yao H., Wang Z., Ning P., Chen X. Engineering macrophages for cancer immunotherapy and drug delivery. Adv Mater. 2020;32 doi: 10.1002/adma.202002054. [DOI] [PubMed] [Google Scholar]
- 35.Wang X., Wang J., Zhao J., Wang H., Chen J., Wu J. HMGA2 facilitates colorectal cancer progression via STAT3-mediated tumor-associated macrophage recruitment. Theranostics. 2022;12:963–975. doi: 10.7150/thno.65411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu Y., Dong Z., Liu K. Unraveling the complexity of STAT3 in cancer: molecular understanding and drug discovery. J. Exp. Clin. Cancer Res. 2024;43:23. doi: 10.1186/s13046-024-02949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du X.-X., He C., Lu X., Guo Y.-L., Chen Z.-H., Cai L.-J. YAP/STAT3 promotes the immune escape of larynx carcinoma by activating VEGFR1-TGFβ signaling to facilitate PD-L1 expression in M2-like TAMs. Exp. Cell Res. 2021;405 doi: 10.1016/j.yexcr.2021.112655. [DOI] [PubMed] [Google Scholar]
- 38.Moreira D., Sampath S., Won H., White S.V., Su Y.-L., Alcantara M., Wang C., Lee P., Maghami E., Massarelli E., Kortylewski M. Myeloid cell-targeted STAT3 inhibition sensitizes head and neck cancers to radiotherapy and T cell-mediated immunity. J. Clin. Invest. 2021;131 doi: 10.1172/JCI137001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data associated in this article has been deposited into a publicly available repository. The data that support the findings of this study are included in article/supp/material/referenced in article.