Abstract
Phosphatidylinositol-specific phospholipase C (PI-PLC) produced by Bacillus thuringiensis has been used as a probe for the distribution of phosphatidylinositol in hepatocyte membranes. Approx. 50% of this phospholipid was hydrolysed in microsomal vesicles (endoplasmic reticulum) with no significant hydrolysis of the remaining membrane phospholipids. Latency of mannose-6-phosphatase was retained during treatment indicating that the vesicles remained impermeable. Stripping of the ribosomes did not increase hydrolysis of phosphatidylinositol; however, when the vesicles were opened using dilute sodium carbonate, hydrolysis increased to greater than 90%. Hydrolysis of phosphatidylinositol of Golgi membranes was 35% and of plasma membranes was 50%. After treatment with PI-PLC, radiolabelled secretory proteins were retained in Golgi membranes and trapped lactate dehydrogenase was retained in plasma-membrane preparations indicating that the vesicles remained closed. Hydrolysis of phosphatidylinositol increased to greater than 90% when the membranes were opened by treatment with dilute sodium carbonate. These observations indicate that PI-PLC of Bacillus thuringiensis is a suitable probe for the distribution of phosphatidylinositol in membranes, and that in liver membranes this phospholipid occurs on each side of the bilayer, a topography consistent with its diverse roles.
Full text
PDF
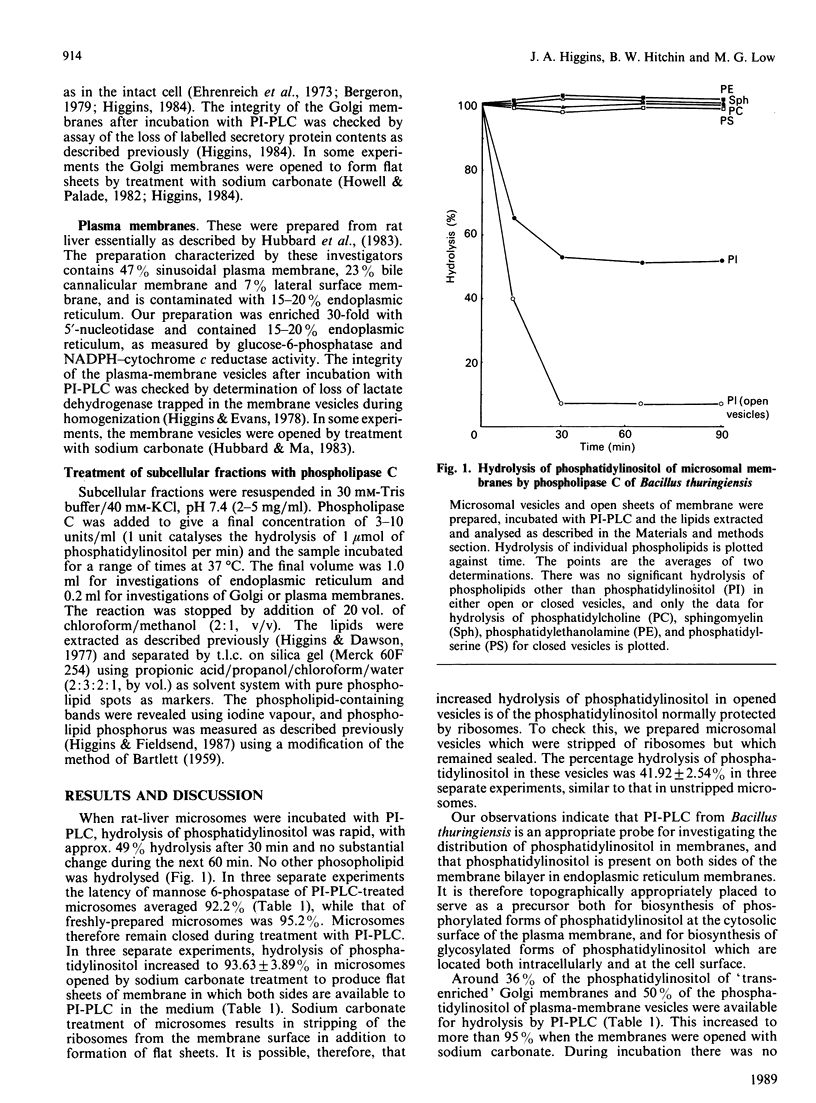
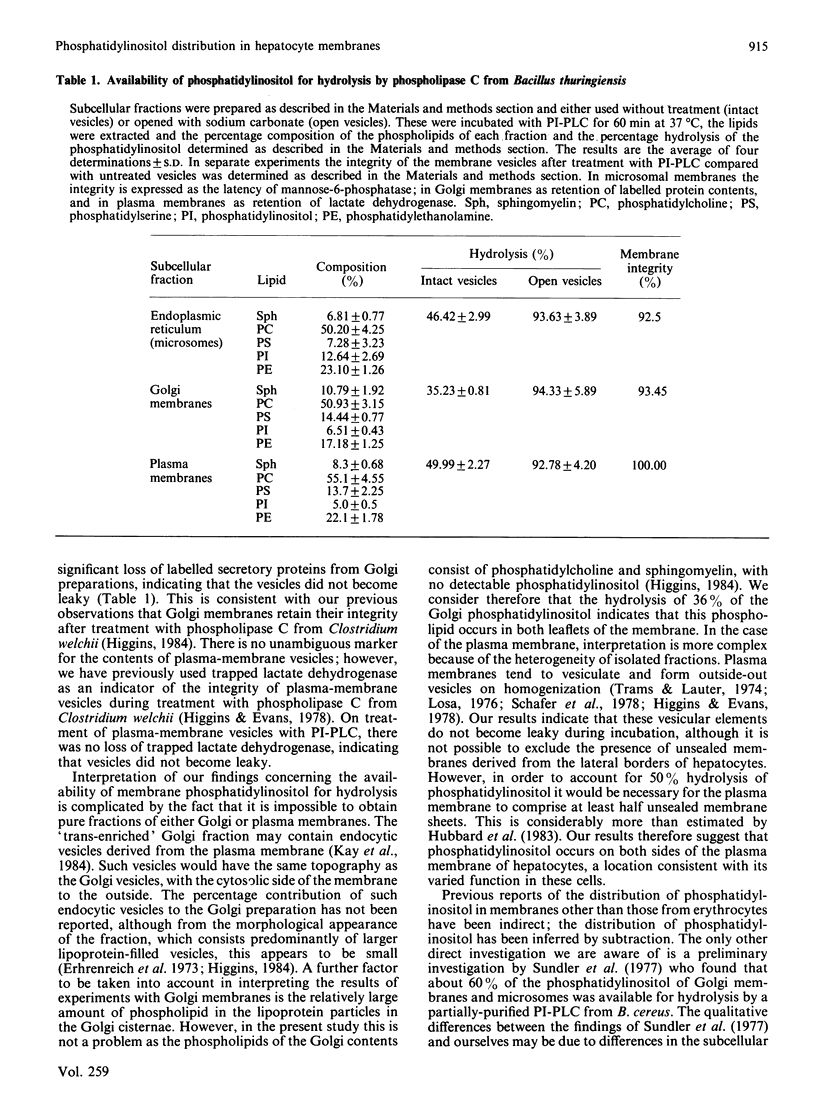

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bergeron J. J. Golgi fractions from livers of control and ethanol-intoxicated rats. Enzymic and morphologic properties following rapid isolation. Biochim Biophys Acta. 1979 Aug 23;555(3):493–503. doi: 10.1016/0005-2736(79)90402-4. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Ehrenreich J. H., Bergeron J. J., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. I. Isolation procedure and morphological characterization. J Cell Biol. 1973 Oct;59(1):45–72. doi: 10.1083/jcb.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase W., Schäfer A., Murer H., Kinne R. Studies on the orientation of brush-border membrane vesicles. Biochem J. 1978 Apr 15;172(1):57–62. doi: 10.1042/bj1720057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. A. Biogenesis of endoplasmic reticulum phosphatidylcholine. Translocation of intermediates across the membrane bilayer during methylation of phosphatidylethanolamine. Biochim Biophys Acta. 1981 Jan 8;640(1):1–15. doi: 10.1016/0005-2736(81)90527-7. [DOI] [PubMed] [Google Scholar]
- Higgins J. A., Dawson R. M. Asymmetry of the phospholipid bilayer of rat liver endoplasmic reticulum. Biochim Biophys Acta. 1977 Nov 1;470(3):342–356. doi: 10.1016/0005-2736(77)90126-2. [DOI] [PubMed] [Google Scholar]
- Higgins J. A., Evans W. H. Transverse organization of phospholipids across the bilayer of plasma-membrane subfractions of rat hepatocytes. Biochem J. 1978 Aug 15;174(2):563–567. doi: 10.1042/bj1740563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. A., Fieldsend J. K. Phosphatidylcholine synthesis for incorporation into membranes or for secretion as plasma lipoproteins by Golgi membranes of rat liver. J Lipid Res. 1987 Mar;28(3):268–278. [PubMed] [Google Scholar]
- Higgins J. A., Hutson J. L. The roles of Golgi and endoplasmic reticulum in the synthesis and assembly of lipoprotein lipids in rat hepatocytes. J Lipid Res. 1984 Dec 1;25(12):1295–1305. [PubMed] [Google Scholar]
- Higgins J. A. The transverse distribution of phospholipids in the membranes of Golgi subfractions of rat hepatocytes. Biochem J. 1984 Apr 1;219(1):261–272. doi: 10.1042/bj2190261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell K. E., Palade G. E. Hepatic Golgi fractions resolved into membrane and content subfractions. J Cell Biol. 1982 Mar;92(3):822–832. doi: 10.1083/jcb.92.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Ma A. Isolation of rat hepatocyte plasma membranes. II. Identification of membrane-associated cytoskeletal proteins. J Cell Biol. 1983 Jan;96(1):230–239. doi: 10.1083/jcb.96.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Wall D. A., Ma A. Isolation of rat hepatocyte plasma membranes. I. Presence of the three major domains. J Cell Biol. 1983 Jan;96(1):217–229. doi: 10.1083/jcb.96.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay D. G., Khan M. N., Posner B. I., Bergeron J. J. In vivo uptake of insulin into hepatic Golgi fractions: application of the diaminobenzidine-shift protocol. Biochem Biophys Res Commun. 1984 Sep 28;123(3):1144–1148. doi: 10.1016/s0006-291x(84)80252-1. [DOI] [PubMed] [Google Scholar]
- Low M. G., Finean J. B. Modification of erythrocyte membranes by a purified phosphatidylinositol-specific phospholipase C (Staphylococcus aureus). Biochem J. 1977 Feb 15;162(2):235–240. doi: 10.1042/bj1620235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Paiement J., Beaufay H., Godelaine D. Coalescence of microsomal vesicles from rat liver: a phenomenon occurring in parallel with enhancement of the glycosylation activity during incubation of stripped rough microsomes with GTP. J Cell Biol. 1980 Jul;86(1):29–37. doi: 10.1083/jcb.86.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundler R., Sarcione S. L., Alberts A. W., Vagelos P. R. Evidence against phospholipid asymmetry in intracellular membranes from liver. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3350–3354. doi: 10.1073/pnas.74.8.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trams E. G., Lauter C. J. On the sidedness of plasma membrane enzymes. Biochim Biophys Acta. 1974 Apr 29;345(2):180–197. doi: 10.1016/0005-2736(74)90257-0. [DOI] [PubMed] [Google Scholar]


