Abstract
Background
Asthma is a chronic inflammatory condition, and choline may alleviate airway inflammation and oxidative stress but studies on the association between dietary choline and asthma remain limited. The purpose of this study is to investigate the associations between dietary choline intake and asthma, as well as pulmonary inflammation and lung function in children and adults.
Methods
In our research, we employed the data of the National Health and Nutrition Examination Survey (NHANES) from 2009 to 2018, including 7,104 children and 16,580 adults. We used fractional exhaled nitric oxide (FENO) to assess pulmonary inflammation and forced expiratory volume in one second (FEV1), forced vital capacity (FVC), the FEV1/FVC ratio, peak expiratory flow rate (PEF), predicted FEV1% and predicted FVC% to assess lung function. Binary logistic regression, linear regression, and the restricted cubic splines were used to analyze the associations between dietary choline intake and asthma and pulmonary inflammation and lung function.
Results
In children, we observed the positive associations between the natural logarithmic transformation of choline (ln-choline) and ln-FEV1 [ β:0.011; 95%CI: (0.004,0.018)] and ln-FVC [ β:0.009; 95%CI: (0.002,0.016)]. In adult males, the ln-choline was positively associated with ln-FEV1[ β:0.018; 95%CI: (0.011,0.024)], ln-FVC [ β:0.020; 95%CI: (0.014,0.026)], ln-PEF [ β:0.014; 95%CI: (0.007,0.022)], ln-predicted FEV1% [ β: 0.007; 95%CI: (0.001, 0.013)] and ln-predicted FVC%[ β: 0.010; 95%CI: (0.005, 0.015)] and negatively associated with FENO [ β: -0.029; 95%CI: (-0.049, -0.009)]. In unadjusted and partially adjusted models, adult females with ln-choline in the highest quartile had 25.2% (95%CI:9.4-38.3%) and 23.8% (95%CI:7.6-37.1%) decreased odds of asthma compared to those with the lowest quartile group. In the dose-response relationships of dietary choline and pulmonary inflammation and lung function indicators in adults, there existed threshold and saturation effects.
Conclusion
The associations between dietary choline and lung function indicators such as FEV1 and FVC are positive in children and adults. The association between dietary choline and pulmonary inflammation is negative only in adults.
Keywords: Diet, Choline, Asthma, Lung, NHANES
Introduction
Asthma is a chronic condition characterized primarily by episodic wheezing, coughing and breathlessness resulting from airway hyperresponsiveness and inflammation [1, 2]. Asthma ranks among the mainly prevalent chronic lung diseases in the United States and about 7.9% of adults and 8.1% of children in the country endure the burdens [3]. Additionally, disparities in asthma prevalence are observed based on age and gender. In the United States, a higher incidence of asthma is observed among boys (9.0%) than among girls (7.1%) within individuals under the age of 18. However, after the age of 18, the incidence is higher in women (10.0%) compared to men (5.7%) [4, 5].
A substantial amount of natural choline is found in foods such as beef, chicken, fish, milk, eggs, soybeans, peanuts, and so on [6, 7]. DNA methylation is a mechanism involved in the epigenetics of asthma, recognized as the interaction between genes and the environment [8–10]. Essential to the one-carbon metabolic pathway, choline is crucial for the synthesis of S-adenosylmethionine (SAM), a versatile methyl donor for DNA methylation processes [11–13]. Choline may influence the expression of susceptibility genes, thereby affecting the pathogenesis of asthma. Additionally, choline may reduce airway inflammation and oxidative stress. A recent animal study reported that choline chloride may attenuate allergic airway disease by inhibiting airway hyperresponsiveness, inflammation, and oxidative stress [14]. In addition, some clinical studies have documented that choline supplementation significantly reduces markers of inflammation, suggesting its potential utility as an adjunctive therapeutic approach for asthma [15, 16].
However, research on the correlation between dietary choline and asthma remains limited. To better learn about the relationship, a large-scale, population-based study was conducted. This study focused on participants, including both children (6–19 years) and adults (20–79 years), who were involved in the National Health and Nutrition Examination Survey (NHANES) from 2009 to 2018. The primary aim was to explore the relationship between dietary choline intake and asthma, as well as its impact on pulmonary inflammation and lung function.
Methods
Study population
The data for this research were obtained from NHANES. All NHANES procedures were approved by ethical scrutiny and all participants provided informed consents. Additional information is available at https://www.cdc.gov/nchs/nhanes/.
Our research utilized data from NHANES spanning the years 2009–2010,2011–2012, 2013–2014, 2015–2016, 2017–2018. From 2009 to 2018, a total of 49,693 samples were involved in the NHANES. Individuals were excluded based on the following criteria: (1) aged < 6 years old and aged > 79 years old (n = 9,844) (2) missing two 24-hour dietary recall data (n = 9,532) (3) lacking self-reported asthma status and two reproducible fractional exhaled nitric oxide (FENO) and complete spirometry data (n = 96) (4) missing covariates data (n = 5,964) (5) individuals with extreme total energy consumption (less than 600 or more than 6000 kcal/d for women and less than 800 or more than 8000 kcal/d for men) and women who were pregnant or lactating were excluded from the study (n = 573). Finally, this research included 23,684 participants, comprising 7,104 children and 16,580 adults. Among them, participants from 2009 to 2012 had FENO and spirometry data and were included in the pulmonary inflammation and lung function analysis. The flow chart of the screening process is shown in Fig. 1.
Fig. 1.
Flowchart of participants selection from NHANES 2009–2018
Dietary choline intake
To assess dietary choline intake, a rigorous methodology was employed involving two 24-hour dietary recall interviews, in accordance with the Food and Nutrient Database for Dietary Studies provided by the United States Department of Agriculture [17, 18]. Following the 24-hour dietary recall, 24-hour supplement usage was recorded. The actual dietary choline intake in this study was determined by taking the average of the two days’ total choline intake, which includes both dietary and supplementary intake.
Outcomes
The outcomes of this research included asthma, pulmonary inflammation and lung function. Asthma was diagnosed using a validated medical condition questionnaire during the personal interview. Participants who still had asthma during the interview were considered as asthma patients and those who did not have current asthma served as control subjects. For the assessment of pulmonary inflammation, the average of two reproducible fractional exhaled nitric oxide (FENO) measurements was computed. For the evaluation of lung function, pre-bronchodilator spirometry data were employed, encompassing forced expiratory volume in one second (FEV1), forced vital capacity (FVC), the FEV1/FVC ratio, peak expiratory flow rate (PEF), predicted FEV1%, and predicted FVC%. To ensure data accuracy, only spirometry data with quality grades A and B were considered during the testing process. Raw FEV1 and FVC values were transformed into predicted percentages using the Hankinson equation, based on age, gender, height, and race (White, Black or Mexican, Hispanic, or other).
Covariates
Covariates in this study included age, gender, race, marital status, education level, poverty/income ratio (PIR), obesity status, physical activity, blood cotinine(ng/mL), family history of asthma, female menopausal status, average intake of dietary folate equivalents [(DFEs), µg/d], vitamin B6 (mg/d) and vitamin B12 (µg/d), history of diabetes and hypertension.
Marital status was classified into three categories: married/living with a partner, widowed/divorced/separated, and never married. The educational level was categorized as below high school, high school, and above high school. For children aged 6–19 years, the educational level was defined as that of the family member who owns or rents the domicile. The PIR was divided into two groups of PIR < 1.3 and PIR ≥ 1.3, based on eligibility for benefits through the Supplemental Nutrition Assistance Program (SNAP) [19]. Participants were categorized as non-obese or obese according to their BMI. ln children, BMI < 85th percentile was non-obese and BMI ≥ 85th percentile was obese; ln adults, BMI < 25 kg/m2 was considered non-obese and ≥ 25 kg/m2 was considered obese. In the physical activity category, children and adults who followed the recommendations of the physical activity guidelines were considered active, while those failed to do so were considered inactive [20]. Menopause status was determined using a self-reported reproductive health questionnaire, adult females who had not menstruated in the last 12 months due to hysterectomy or menopause/life changes were classified as postmenopausal. The intake of DFE, vitamin B6, and vitamin B12 involved the mean from two 24-hour periods, encompassing both dietary and supplementary usages. The standards for defining diabetes and hypertension followed the guidelines of the American Diabetes Association Professional Practice Committee and 2018 ESC/ESH Guidelines [21, 22].
Statistical analysis
The continuous variables were presented as medians (interquartile ranges) and compared using the Mann-Whitney U test. The categorical variables were expressed as numbers (percentages), compared with the chi-square test. The data of dietary choline and pulmonary inflammation, and lung function were transformed into the natural logarithm to improve the skewed distribution. The natural logarithmic conversion of choline (named ln-choline) was divided into quartiles and we analyzed their associations with asthma through binary logistic regression. Additionally, for the linear regression analysis of the relationships between dietary choline and pulmonary inflammation and lung function, the quartiles of ln-choline were treated as continuous variables. Three types of models were constructed. In Model 1, no adjustment for any covariates. Model 2 adjusted for age, gender, and race. Model 3 adjusted for all covariates. To investigate the dose-response relationships between dietary choline intake, asthma, pulmonary inflammation, and lung function, restricted cubic spline models were employed. When non-linearity was detected, we employed piecewise regression models to ascertain both the threshold and saturation effects.
All of these analyses were performed by the statistical software SPSS (Version 24.0 for Windows; SPSS Inc.) and R program (Version 3.4.2), the p-value < 0.05 was considered of statistical significance.
Results
Baseline characteristics of the participants
The characteristics of the study population are depicted in Tables 1 and 2. Among the 7,104 children and 16,158 adults, 843 children and 1,455 adults had asthma. Compared to children without asthma, those with asthma were more likely to be non-Hispanic black, have a PIR < 1.3, obese, have higher blood cotinine and a family history of asthma, higher FENO, lower FEV1, FEV1/FVC, predicted FEV1%, and predicted FVC%. In adults, those with asthma were more likely to be female and non-Hispanic white or black, with a PIR < 1.3, obese and inactive in physical activity, have higher blood cotinine and a family history of asthma, higher FENO and lower lung function indicators.
Table 1.
Characteristics of NHANES participants by asthma status in children
| Characteristics | Controls | Asthma | P-value | |
|---|---|---|---|---|
| n = 6261 | n = 843 | |||
| Age (years) | 12.00 (9.00,16.00) | 12.00 (9.00,16.00) | 0.726 | |
| Gender: | 0.108 | |||
| Male | 3146 (50.25) | 449 (53.26) | ||
| Female | 3115 (49.75) | 394 (46.74) | ||
| Race: | < 0.001 | |||
| Mexican American | 1514 (24.18) | 127 (15.07) | ||
| Other Hispanic | 630 (10.06) | 86 (10.20) | ||
| Non-Hispanic white | 1849 (29.53) | 220 (26.10) | ||
| Non-Hispanic black | 1397 (22.31) | 308 (36.54) | ||
| Others | 871 (13.91) | 102 (12.10) | ||
| Education level: | 0.009 | |||
| Less than high school | 1632 (26.07) | 181 (21.47) | ||
| High school | 1746 (27.89) | 264 (31.32) | ||
| More than high school | 2883 (46.05) | 398 (47.21) | ||
| PIR: | < 0.001 | |||
| < 1.3 | 2715 (43.36) | 421 (49.94) | ||
| ≥ 1.3 | 3546 (56.64) | 422 (50.06) | ||
| Obesity status: | < 0.001 | |||
| Non-obese | 5366 (85.71) | 672 (79.72) | ||
| Obese | 895 (14.29) | 171 (20.28) | ||
| Physical activity category: | 0.599 | |||
| inactive | 3077 (49.15) | 423 (50.18) | ||
| active | 3184 (50.85) | 420 (49.82) | ||
| Blood cotinine (ng/mL) | 0.03 (0.01,0.17) | 0.06 (0.01,0.43) | < 0.001 | |
| Family history of asthma: | < 0.001 | |||
| No | 4515 (72.11) | 274 (32.50) | ||
| Yes | 1746 (27.89) | 569 (67.50) | ||
| DFEs (µg/d) |
513.50 (357.50,748.00) |
522.50 (364.75,803.00) |
0.150 | |
| Vitamin B6 (mg/d) | 1.77 (1.26,2.50) | 1.82 (1.29,2.59) | 0.115 | |
| Vitamin B12 (µg/d) | 5.54 (3.38,8.87) | 5.78 (3.20,9.51) | 0.439 | |
| History of diabetes: | 0.985 | |||
| No | 6221 (99.36) | 837 (99.29) | ||
| Yes | 40 (0.64) | 6 (0.71) | ||
| History of hypertension: | 0.195 | |||
| No | 6193 (98.91) | 829 (98.34) | ||
| Yes | 68 (1.09) | 14 (1.66) | ||
| Choline (mg/d) |
240.10 (178.40,324.60) |
242.35 (175.80,323.21) |
0.694 | |
| FENO (ppb) | 11.00 (7.00,17.50) | 15.00 (8.50,33.00) | < 0.001 | |
| FEV1 (mL) | 2582.00 (1785.00,3393.50) | 2494.00 (1734.00,3240.00) | 0.028 | |
| FVC (mL) | 2969.00 (2090.75,3908.25) | 2983.00 (2087.00,3831.00) | 0.598 | |
| FEV1/FVC | 0.87 (0.83,0.91) | 0.84 (0.79,0.88) | < 0.001 | |
| PEF (mL/s) |
5943.50 (4223.00,7719.50) |
5945.00 (4075.00,7628.00) |
0.299 | |
| Predicted FEV1% |
101.03 (91.92,109.86) |
95.58 (86.61,106.26) | < 0.001 | |
| Predicted FVC% | 98.81 (89.48,107.75) | 94.59 (84.96,104.96) | < 0.001 | |
Notes PIR: poverty/income ratio; DFEs: dietary folate equivalents; FENO: fractional exhaled nitric oxide; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; PEF: peak expiratory flow rate
Table 2.
Characteristics of NHANES participants by asthma status in adults
| Characteristics | Controls n = 15,125 |
Asthma n = 1455 |
P-value |
|---|---|---|---|
| Age (years) | 48.00 (34.00,62.00) | 50.00 (35.00,62.00) | 0.259 |
| Gender: | < 0.001 | ||
| Male | 7803 (51.59) | 492 (33.81) | |
| Female | 7322 (48.41) | 963 (66.19) | |
| Race: | < 0.001 | ||
| Mexican American | 2232 (14.76) | 141 (9.69) | |
| Other Hispanic | 1540 (10.18) | 127 (8.73) | |
| Non-Hispanic white | 6241(41.26) | 672 (46.19) | |
| Non-Hispanic black | 3150 (20.83) | 372 (25.57) | |
| Others | 1962 (12.97) | 143 (9.83) | |
| Marital status: | < 0.001 | ||
| Married/Living with partner | 9326 (61.66) | 751 (51.62) | |
| Widowed/Divorced/Separated | 2954 (19.53) | 390 (26.80) | |
| Never married | 2845 (18.81) | 314 (21.58) | |
| Education level: | 0.440 | ||
| Less than high school | 2973 (19.66) | 293 (20.14) | |
| High school | 3385 (22.38) | 343 (23.57) | |
| More than high school | 8767 (57.96) | 819 (56.29) | |
| PIR: | < 0.001 | ||
| < 1.3 | 4534 (29.98) | 607 (41.72) | |
| ≥ 1.3 | 10,591 (70.02) | 848 (58.28) | |
| Obesity status: | < 0.001 | ||
| Non-obese | 4280 (28.30) | 296 (20.34) | |
| Obese | 10,845 (71.70) | 1159 (79.66) | |
| Physical activity category: | 0.001 | ||
| inactive | 5720 (37.82) | 613 (42.13) | |
| active | 9405 (62.18) | 842 (57.87) | |
| Blood cotinine(ng/mL) | 0.04 (0.01,5.78) | 0.07 (0.01,98.70) | < 0.001 |
| Family history of asthma: | < 0.001 | ||
| No | 12,243 (80.95) | 764 (52.51) | |
| Yes | 2882 (19.05) | 691 (47.49) | |
| Menopausal status* | 0.004 | ||
| Premenopausal | 3602 (49.19) | 426 (44.24) | |
| Postmenopausal | 3720 (50.81) | 537 (55.76) | |
| DFE (µg/d) |
569.50 (369.50,951.50) |
538.50 (329.00,918.75) |
0.003 |
| Vitamin B6 (mg/d) | 2.26 (1.50,3.77) | 2.13 (1.33,3.64) | 0.001 |
| Vitamin B12 (µg/d) | 6.14 (3.18,13.59) | 6.11 (3.07,13.62) | 0.604 |
| History of diabetes: | < 0.001 | ||
| No | 12,677 (83.81) | 1111 (76.36) | |
| Yes | 2448 (16.19) | 344 (23.64) | |
| History of hypertension: | < 0.001 | ||
| No | 9046 (59.81) | 710 (48.80) | |
| Yes | 6079 (40.19) | 745 (51.20) | |
| Choline(mg/d) |
307.95 (223.15,418.25) |
277.15 (201.88,388.67) |
< 0.001 |
| FENO (ppb) | 13.00 (8.50,20.00) | 15.00 (8.50,24.50) | 0.002 |
| FEV1 (mL) | 3047.00 (2461.25,3716.00) | 2611.00 (2048.00,3240.50) | < 0.001 |
| FVC (mL) | 3854.00 (3165.25,4707.50) | 3473.00 (2819.50,4238.50) | < 0.001 |
| FEV1/FVC | 0.80 (0.74,0.84) | 0.76 (0.69,0.82) | < 0.001 |
| PEF (mL/s) |
8064.50 (6641.50,9805.00) |
7025.00 (5583.50,8620.00) |
< 0.001 |
| Predicted FEV1% | 97.64 (88.15,106.97) | 89.01 (75.90,99.68) | < 0.001 |
| Predicted FVC% | 98.83 (89.54,107.76) | 94.25 (84.41,104.83) | < 0.001 |
Notes “*” means the descriptions of the menopausal status included only adult females (N = 8,285)
PIR: poverty/income ratio; DFEs: dietary folate equivalents; FENO: fractional exhaled nitric oxide; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; PEF: peak expiratory flow rate
The associations between dietary choline and asthma in children and adults
The results of logistic regression were shown in Tables 3, 4, 5 for children, adult males, and adult females respectively. In children and adult males, we did not observe the association between dietary choline and asthma. In adult females, the negative relationship between dietary choline and asthma was observed in Model 1 and Model 2 but eliminated in Model 3. Adult females with ln-choline levels in the highest quartile had 25.2% (95%CI:9.4-38.3%) and 23.8% (95%CI:7.6-37.1%) decreased odds of asthma compared to those with the lowest quartile group in Model 1 and Model 2 respectively.
Table 3.
Logistic regression analysis of the association between dietary choline and asthma in children
| Ln-Choline (mg/d) | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95%CI) | P-value | OR (95%CI) | P-value | |
|
Quartile1 (3.41–5.18) |
1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
|
Quartile2 (5.19–5.49) |
0.888 (0.723,1.090) |
0.255 |
0.917 (0.745,1.128) |
0.413 |
0.922 (0.742,1.144) |
0.460 |
|
Quartile3 (5.50–5.79) |
1.047 (0.859,1.277) |
0.649 |
1.078 (0.881,1.319) |
0.468 |
1.109 (0.895,1.375) |
0.343 |
|
Quartile4 (5.80–7.65) |
0.928 (0.756,1.138) |
0.471 |
0.965 (0.781,1.192) |
0.739 |
0.979 (0.778,1.230) |
0.853 |
Notes OR: odds ratio. Model 1: unadjusted; Model 2: adjusted for age, gender and race. Model 3: adjusted for age, gender, race, education level, PIR, obesity status, physical activity, blood cotinine, family history of asthma, folate DFE, vitamin B6, vitamin B12, history of diabetes and hypertension
Table 4.
Logistic regression analysis of the association between dietary choline and asthma in adult males
| Ln-Choline (mg/d) | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | P-value | OR (95%CI) | P-value | OR (95%CI) | P-value | |
|
Quartile1 (3.88–5.58) |
1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
|
Quartile2 (5.59–5.89) |
0.793 (0.612,1.026) |
0.078 |
0.798 (0.616,1.034) |
0.088 |
0.862 (0.660,1.124) |
0.273 |
|
Quartile3 (5.90–6.19) |
0.785 (0.606,1.016) |
0.066 |
0.806 (0.622,1.045) |
0.104 |
0.868 (0.665,1.133) |
0.297 |
|
Quartile4 (6.19–7.70) |
0.999 (0.781,1.279) |
0.996 |
1.042 (0.813,1.335) |
0.748 |
1.114 (0.860,1.441) |
0.413 |
Notes OR: odds ratio. Model 1: unadjusted; Model 2: adjusted for age and race. Model 3: adjusted for age, race, marital status, education level, PIR, obesity status, physical activity, blood cotinine, family history of asthma, folate DFE, vitamin B6, vitamin B12, history of diabetes and hypertension
Table 5.
Logistic regression analysis of the association between dietary choline and asthma in adult females
| Ln-Choline (mg/d) | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | P-value | OR (95%CI) | P-value | OR (95%CI) | P-value | |
|
Quartile1 (3.12–5.26) |
1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |||
|
Quartile2 (5.27–5.57) |
0.841 (0.699,1.012) |
0.067 |
0.845 (0.702,1.018) |
0.076 |
0.901 (0.743,1.094) |
0.292 |
|
Quartile3 (5.58–5.85) |
0.851 (0.708,1.024) |
0.088 |
0.857 (0.712,1.032) |
0.103 |
0.942 (0.775,1.145) |
0.549 |
|
Quartile4 (5.86–7.38) |
0.748 (0.617,0.906) |
0.003 |
0.762 (0.629,0.924) |
0.006 |
0.827 (0.676,1.013) |
0.067 |
Notes OR: odds ratio. Model 1: unadjusted; Model 2: adjusted for age and race. Model 3: adjusted for age, race, marital status, education level, PIR, obesity status, physical activity, blood cotinine, family history of asthma, menopausal status, folate DFE, vitamin B6, vitamin B12, history of diabetes and hypertension
The associations between dietary choline and pulmonary inflammation and lung function in children and adults
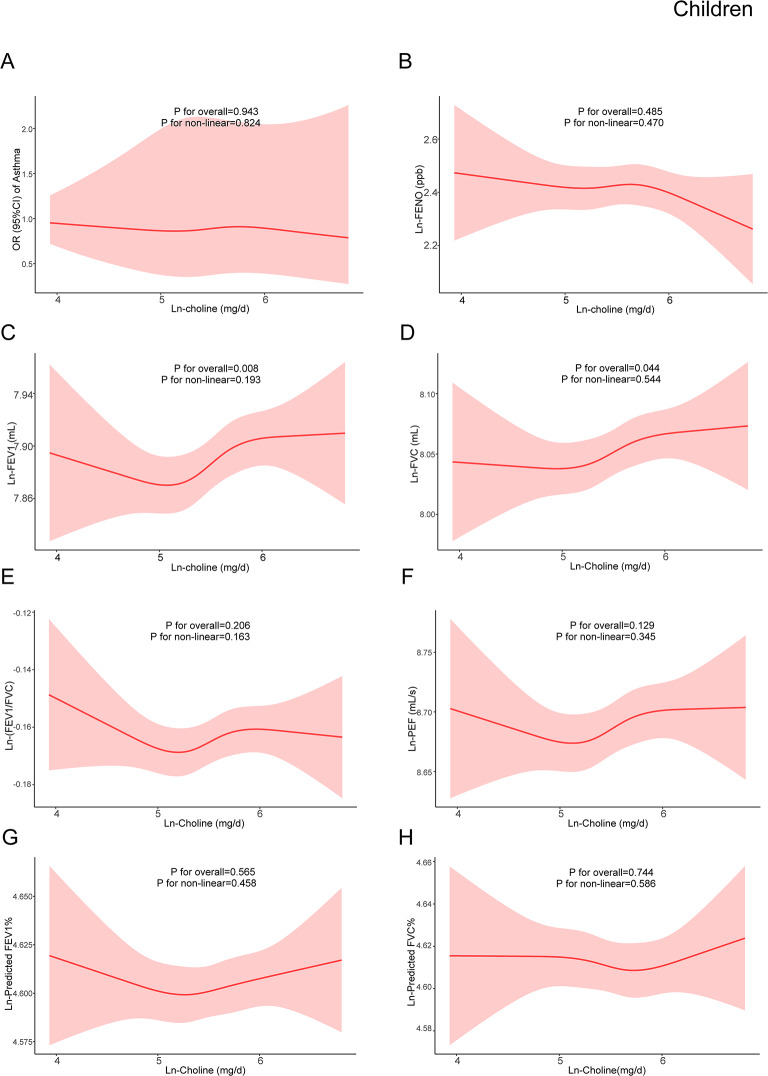
The results of linear regression were shown in Tables 6, 7 and 8 for children, adult males and adult females respectively. In all models of children, ln-choline was found to be significantly positively correlated with ln-FEV1 and ln-FVC. In Model 3, each quartile increment of ln-choline was associated with increased ln-FEV1 [β:0.011; 95%CI: (0.004,0.018)] and ln-FVC [β:0.009; 95%CI: (0.002,0.016)]. Similarly, the positive associations between dietary choline and FEV1 and FVC were presented in adult males. In Model 3, each quartile increment of ln-choline was positively associated with ln-FEV1[β:0.018; 95%CI: (0.011,0.024)], ln-FVC [β:0.020; 95%CI: (0.014,0.026)]. In addition, the associations between ln-choline and ln-PEF [β:0.014; 95%CI: (0.007,0.022)], ln-predicted FEV1% [β: 0.007; 95%CI: (0.001, 0.013)] and ln-predicted FVC% [β: 0.010; 95%CI: (0.005, 0.015)] were positive. We also observed the negative association between ln-choline and ln-FENO [β: -0.029; 95%CI: (-0.049, -0.009)]. In all models of adult females, the association between ln-choline and ln-FVC was positive while the relationship between ln-choline and ln-(FEV1/FVC) was negative.
Table 6.
Linear regression analysis of the association between dietary choline and pulmonary inflammation and lung function indicators in children
| Ln-Choline (mg/d) |
Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β (95%CI) | P-value | β (95%CI) | P-value | β (95%CI) | P-value | |
| Ln-FENO |
0.023 (-0.003,0.049) |
0.085 |
0.003 (-0.023,0.029) |
0.835 |
-0.010 (-0.037,0.016) |
0.446 |
| Ln-FEV1 |
0.043 (0.029,0.056) |
< 0.001 |
0.013 (0.006,0.020) |
< 0.001 |
0.011 (0.004,0.018) |
0.002 |
| Ln-FVC |
0.043 (0.029,0.056) |
< 0.001 |
0.010 (0.004,0.017) |
0.003 |
0.009 (0.002,0.016) |
0.009 |
|
Ln-(FEV1/ FVC) |
-0.0002 (-0.0028,0.0025) |
0.910 |
0.0025 (-0.0002,0.0051) |
0.069 |
0.002 (-0.001,0.005) |
0.125 |
| Ln-PEF |
0.037 (0.023,0.050) |
< 0.001 |
0.011 (0.003,0.018) |
0.007 |
0.00776 (-0.00007,0.01559) |
0.052 |
|
Ln-Predicted FEV1% |
0.004 (-0.001,0.008) |
0.102 |
0.002 (-0.002,0.007) |
0.340 |
0.002 (-0.003,0.007) |
0.423 |
|
Ln-Predicted FVC% |
-0.0003 (-0.0045,0.0040) |
0.897 |
-0.001 (-0.006,0.003) |
0.515 |
-0.001 (-0.006,0.003) |
0.542 |
Notes FENO: fractional exhaled nitric oxide; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; PEF: peak expiratory flow rate. Model 1: unadjusted; Model 2: adjusted for age, gender and race; Model 3: adjusted for age, gender, race, education level, PIR, obesity status, physical activity, blood cotinine, family history of asthma, folate DFE, vitamin B6, vitamin B12, history of diabetes and hypertension
Table 7.
Linear regression analysis of the association between dietary choline and pulmonary inflammation and lung function indicators in adult males
| Ln-Choline (mg/d) |
Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β (95%CI) | P-value | β (95%CI) | P-value | β (95%CI) | P-value | |
| Ln-FENO |
-0.027 (-0.048, -0.006) |
0.012 |
-0.023 (-0.044, -0.002) |
0.036 |
-0.029 (-0.049, -0.009) |
0.005 |
| Ln-FEV1 |
0.035 (0.027,0.044) |
< 0.001 |
0.023 (0.017,0.029) |
< 0.001 |
0.018 (0.011,0.024) |
< 0.001 |
| Ln-FVC |
0.035 (0.028,0.042) |
< 0.001 |
0.025 (0.019,0.031) |
< 0.001 |
0.020 (0.014,0.026) |
< 0.001 |
|
Ln-(FEV1/ FVC) |
0.001 (-0.003,0.004) |
0.794 |
-0.002 (-0.005,0.001) |
0.219 |
-0.002 (-0.006,0.001) |
0.185 |
| Ln-PEF |
0.028 (0.020,0.036) |
< 0.001 |
0.021 (0.014,0.029) |
< 0.001 |
0.014 (0.007,0.022) |
< 0.001 |
|
Ln-Predicted FEV1% |
0.012 (0.006,0.018) |
< 0.001 |
0.011 (0.005,0.016) |
< 0.001 |
0.007 (0.001,0.013) |
0.014 |
|
Ln-Predicted FVC% |
0.014 (0.009,0.018) |
< 0.001 |
0.013 (0.008,0.018) |
< 0.001 |
0.010 (0.005,0.015) |
< 0.001 |
Notes FENO: fractional exhaled nitric oxide; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; PEF: peak expiratory flow rate. Model 1: unadjusted; Model 2: adjusted for age and race. Model 3: adjusted for age, race, marital status, education level, PIR, obesity status, physical activity, blood cotinine, family history of asthma, folate DFE, vitamin B6, vitamin B12, history of diabetes and hypertension
Table 8.
Linear regression analysis of the association between dietary choline and pulmonary inflammation and lung function indicators in adult females
| Ln-Choline (mg/d) |
Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β (95%CI) | P-value | β (95%CI) | P-value | β (95%CI) | P-value | |
| Ln-FENO |
0.004 (-0.017,0.025) |
0.708 |
0.0004 (-0.0199,0.0207) |
0.927 |
-0.0191 (-0.0388,0.0005) |
0.056 |
| Ln-FEV1 |
0.007 (-0.002,0.016) |
0.143 |
0.011 (0.004,0.017) |
0.001 |
0.005 (-0.002,0.011) |
0.156 |
| Ln-FVC |
0.013 (0.005,0.020) |
0.002 |
0.015 (0.009,0.021) |
< 0.001 |
0.010 (0.004,0.016) |
0.001 |
|
Ln-(FEV1/ FVC) |
-0.006 (-0.009, -0.002) |
0.001 |
-0.004 (-0.007, -0.001) |
0.009 |
-0.005 (-0.008, -0.002) |
0.001 |
| Ln-PEF |
0.012 (0.004,0.021) |
0.005 |
0.015 (0.007,0.023) |
< 0.001 |
0.006 (-0.002,0.014) |
0.116 |
|
Ln-Predicted FEV1% |
-0.002 (-0.008,0.004) |
0.537 |
-0.001 (-0.007,0.005) |
0.711 |
-0.005 (-0.011,0.001) |
0.077 |
|
Ln-Predicted FVC% |
0.002 (-0.003,0.007) |
0.426 |
0.003 (-0.002,0.008) |
0.294 |
-0.0004 (-0.0053,0.0046) |
0.885 |
Notes FENO: fractional exhaled nitric oxide; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; PEF: peak expiratory flow rate. Model 1: unadjusted; Model 2: adjusted for age and race. Model 3: adjusted for age, race, marital status, education level, PIR, obesity status, physical activity, blood cotinine, family history of asthma, menopausal status, folate DFE, vitamin B6, vitamin B12, history of diabetes and hypertension
Dose-response relationship
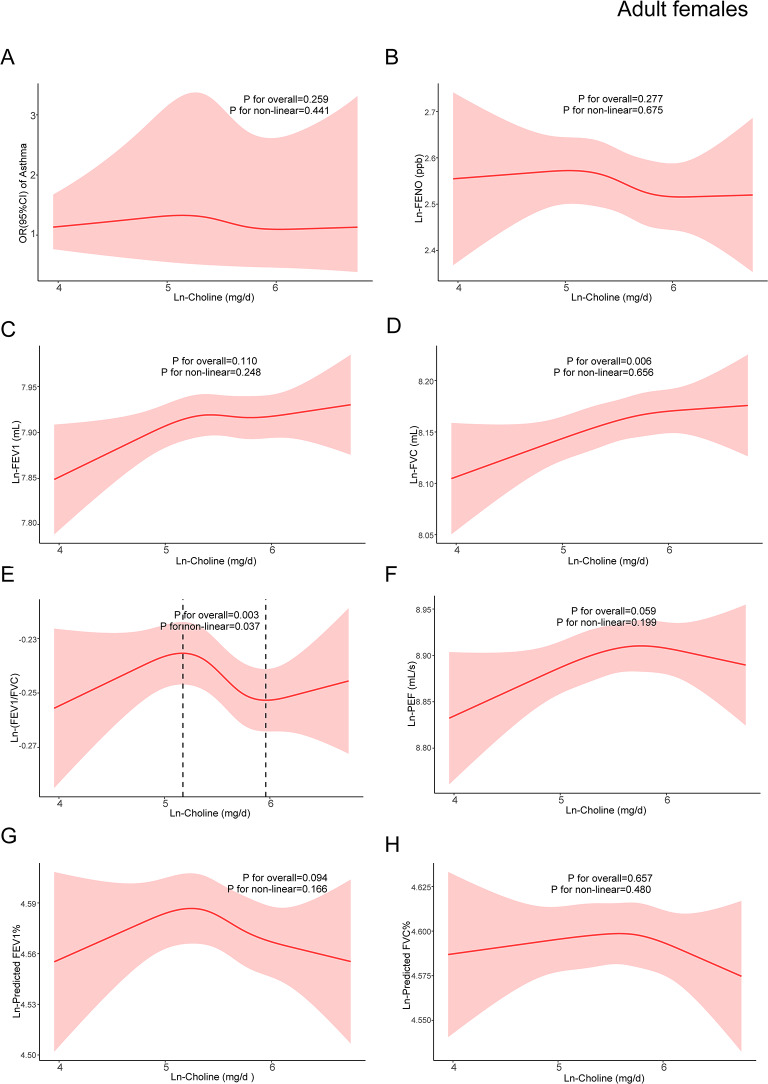
Based on model 3, restricted cubic spline models were constructed to explore the dose-response relationships between dietary choline and asthma, pulmonary inflammation, and lung function. RCSs demonstrated non-linear relationships between ln-choline and ln-FENO, ln-FEV1, ln-FVC, ln-PEF, ln-predicted FEV1%, and ln-predicted FVC% in adult males (Fig. 2). A non-linear association between ln-choline and ln-(FEV1/FVC) in adult females was also observed (Fig. 3). Among children, no non-linear association was found (Fig. 4).
Fig. 2.
The dose-response relationship between dietary choline intake and asthma, pulmonary inflammation and lung function indicators in adult males. The red line represented the estimated β or OR and the red shading around it represented its 95% confidence intervals. OR: odds ratio; FENO: fractional exhaled nitric oxide; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; PEF: peak expiratory flow rate. (A)The relationship between ln-choline and asthma in adult males. (B) The relationship between ln-choline and ln-FENO in adult males. (C) The relationship between ln-choline and ln-FEV1 in adult males. (D) The relationship between ln-choline and ln-FVC in adult males. (E) The relationship between ln-choline and ln-(FEV1/FVC) in adult males. (F) The relationship between ln-choline and ln-PEF in adult males. (G) The relationship between ln-choline and ln-predicted FEV1% in adult males. (H) The relationship between ln-choline and ln-predicted FVC% in adult males. Adjusted for age, race, marital status, education level, PIR, obesity status, physical activity, blood cotinine, family history of asthma, folate DFE, vitamin B6, vitamin B12, history of diabetes and hypertension
Fig. 3.
The dose-response relationship between dietary choline intake and asthma, pulmonary inflammation and lung function indicators in adult females. The red line represented the estimated β or OR and the red shading around it represented its 95% confidence intervals. OR: odds ratio; FENO: fractional exhaled nitric oxide; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; PEF: peak expiratory flow rate. (A)The relationship between ln-choline and asthma in adult females. (B) The relationship between ln-choline and ln-FENO in adult females. (C) The relationship between ln-choline and ln-FEV1 in adult females. (D) The relationship between ln-choline and ln-FVC in adult females. (E) The relationship between ln-choline and ln-(FEV1/FVC) in adult females. (F) The relationship between ln-choline and ln-PEF in adult females. (G) The relationship between ln-choline and ln-predicted FEV1% in adult females. (H) The relationship between ln-choline and ln-predicted FVC% in adult females. Adjusted for age, race, marital status, education level, PIR, obesity status, physical activity, blood cotinine, family history of asthma, menopausal status, folate DFE, vitamin B6, vitamin B12, history of diabetes and hypertension
Fig. 4.
The dose-response relationship between dietary choline intake and asthma, pulmonary inflammation and lung function indicators in children. The red line represented the estimated β or OR and the red shading around it represented its 95% confidence intervals. OR: odds ratio; FENO: fractional exhaled nitric oxide; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; PEF: peak expiratory flow rate. (A)The relationship between ln-choline and asthma in children. (B) The relationship between ln-choline and ln-FENO in children. (C) The relationship between ln-choline and ln-FEV1 in children. (D) The relationship between ln-choline and ln-FVC in children. (E) The relationship between ln-choline and ln-(FEV1/FVC) in children. (F) The relationship between ln-choline and ln-PEF in children. (G) The relationship between ln-choline and ln-predicted FEV1% in children. (H) The relationship between ln-choline and ln-predicted FVC% in children. Adjusted for age, gender, race, education level, PIR, obesity status, physical activity, blood cotinine, family history of asthma, folate DFE, vitamin B6, vitamin B12, history of diabetes and hypertension
In adult males, the relationship between ln-choline and ln-FENO showed an inverted U-shaped curve, with 5.63 mg/d as the turning point (Table 9). When ln-choline was < 5.63 mg/d, the association was not significant, and a negative association was observed after ln-choline reached 5.63 mg/d [β: -0.161; 95%CI: (-0.247, -0.075)]. The association between ln-choline and ln-FVC showed a S-shaped curve with two turning points at 5.38 mg/d and 6.32 mg/d. When the ln-choline was < 5.38 mg/d, the association was insignificant. A threshold effect was observed beyond 5.38 mg/d, which was positively associated with ln-FVC [β:0.079; 95%CI: (0.051,0.106)]. After reaching 6.32 mg/d, the β value had a significant decline and became insignificant [β:0.024; 95%CI: (-0.060,0.107)]. The associations between ln-choline and ln-FEV1, ln- PEF, ln-predicted FEV1%, and ln-predicted FVC% in adult males showed similar S-shaped curve (Table 9). The turning points of the association between ln-choline and ln-(FEV1/FVC) in adult females were 5.18 mg/d and 5.96 mg/d. When ln-choline was between 5.18 mg/d and 5.96 mg/d (Table 10), ln-PEF decreased with the increment in ln-choline [ β: -0.011; 95%CI: (-0.019, -0.003)].
Table 9.
The threshold and saturation effects of ln-choline on pulmonary inflammation and lung function indicators in adult males
| Ln-choline | β (95%CI) | P-value |
|---|---|---|
| Ln-FENO | ||
| < 5.63 | 0.050(-0.119,0.220) | 0.559 |
| > 5.63 | -0.161(-0.247, -0.075) | < 0.001 |
| Ln-FEV1 | ||
| < 5.48 | -0.118(-0.195, -0.042) | 0.003 |
| 5.48–6.27 | 0.067(0.029,0.105) | 0.001 |
| > 6.27 | 0.007(-0.074,0.088) | 0.868 |
| Ln-FVC | ||
| < 5.38 | -0.075(-0.162,0.012) | 0.092 |
| 5.38–6.32 | 0.079(0.051,0.106) | < 0.001 |
| > 6.32 | 0.024(-0.060,0.107) | 0.578 |
| Ln-PEF | ||
| < 5.40 | -0.066(-0.170,0.038) | 0.211 |
| 5.40–6.17 | 0.069(0.023,0.115) | 0.003 |
| > 6.17 | -0.065(-0.139,0.010) | 0.088 |
| Ln-Predicted FEV1% | ||
| < 5.55 | -0.085(-0.146, -0.025) | 0.006 |
| 5.55–6.23 | 0.025(-0.017,0.066) | 0.244 |
| > 6.23 | 0.015(-0.052,0.082) | 0.656 |
| Ln-Predicted FVC% | ||
| < 5.41 | -0.033(-0.099,0.034) | 0.336 |
| 5.41–6.27 | 0.046(0.020,0.072) | 0.001 |
| > 6.27 | 0.011(-0.050,0.073) | 0.716 |
Notes FENO: fractional exhaled nitric oxide; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; PEF: peak expiratory flow rate
Table 10.
The threshold and saturation effect of ln-choline on ln-(FEV1/FVC) in adult females
| Ln-choline | β (95%CI) | P-value |
|---|---|---|
| Ln-(FEV1/FVC) | ||
| < 5.18 | 0.018(-0.013,0.049) | 0.264 |
| 5.18–5.96 | -0.011(-0.019, -0.003) | 0.008 |
| > 5.96 | -0.004(-0.062,0.054) | 0.888 |
Notes FEV1: forced expiratory volume in one second; FVC: forced vital capacity
Discussion
As far as we know, there are few epidemiological studies examining the associations between dietary choline and asthma and pulmonary inflammation and lung function. Our results indicated positive associations between dietary choline intake and lung function indicators, such as FEV1 and FVC in children and adults. Additionally, a negative relationship was observed between dietary choline and pulmonary inflammation in adult males.
The restricted cubic spline analysis revealed a S-shaped curve of the association between ln-choline and ln-FVC in adult males, ln-FVC increased with the increment in ln-choline when ln-choline between 5.38 mg/d and 6.32 mg/d (equivalent to 217.02-555.57 mg/d of choline). Similarly, threshold and saturation effects were observed in the associations between ln-choline and ln-FENO, ln-FEV1, ln-(FEV1/FVC), ln-PEF, ln-predicted FEV1% and ln-predicted FVC% in adults. Moreover, according to the National Academies of Medicine, the recommended adequate intake (AI) of choline varies across different age groups [23]. It is advised to aim for 250 mg/d for children aged 4–8 years, 375 mg/d for those aged 9–13 years, 550 mg/d for boys aged 14–18 years and 400 mg/d for girls. In adults, the recommended AI stands at 550 mg/d for men and 425 mg/d for women. In our study, the actual dietary choline intake was below these established recommendations. Consequently, it is critical for children and adults to increase dietary choline intake, which may be associated with a reduced risk of asthma, lower levels of pulmonary inflammation, and improved lung function.
The results of several studies are similar to our study. A study involving 76 adults demonstrated that choline therapy could alleviate airway inflammation and improve lung function [15]. Another case-control study with 23 asthma adults found that the participants receiving choline treatment experienced improvements in lung function and reductions in airway responsiveness [24]. Additionally, a case-control study in the United States involving 1,345 preschoolers aged 2–5 years showed no significant correlation between dietary choline and childhood asthma [25].
Asthma is mediated by chronic airway inflammation and oxidative stress. Reactive oxygen species (ROS) released from inflammatory cells are a central factor in oxidative stress [26, 27]. Dietary choline supplementation may reduce ROS production and decrease the eosinophilic infiltration and reactive oxidant species [28]. Regrettably, the protective effect of dietary choline against asthma was observed only in unadjusted and partially adjusted models. However, we observed that dietary choline could relieve pulmonary inflammation and improve lung function. Choline is involved in the synthesis of the acetylcholine and the phosphatidylcholine. Phosphatidylcholine is crucial for maintaining homeostasis of active substance in the lungs and improving lung function [6, 29, 30]. A choline-deficiency diet can limit the mRNA expression of key genes encoding extracellular matrix, such as col1a1, col3a1, and elastin, which in turn affects extracellular matrix homeostasis and the regulation of lung function [31].
Our study has several strengths compared to previous studies. Firstly, a large and nationally representative sample was used, including individuals aged 6–79 years. Secondly, we employed the restricted cubic spline models to further explore the dose-response relationship. Finally, we observed the threshold and saturation effects in the models, providing the effective range of dietary choline. Nevertheless, there are still some limitations in this research. Due to the nature of the cross-sectional study, we can’t conclude the causal relationships between dietary choline and asthma and pulmonary inflammation and lung function. Additionally, we used data from interviews such as self-reported dietary intake, which may be biased by recalling and reporting. Finally, although numerous confounding variables had been adjusted in our research, there are still potential confounding variables that we hadn’t taken into account.
Conclusion
In conclusion, higher intake of dietary choline was associated with better lung function indicators such as FEV1 and FVC in children and adults. Only in adults, choline demonstrated protective effects on pulmonary inflammation. Choline can be a potential dietary strategy for preventing asthma, alleviating pulmonary inflammation and improving lung function, further mechanism and prospective studies are needed to confirm our results.
Acknowledgements
Not applicable.
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- FENO
Fractional exhaled nitric oxide
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- PEF
Peak expiratory flow rate
- PIR
Poverty/income ratio
- BMI
Body mass index
- DFEs
Dietary folate equivalents
Author contributions
Q.D and Y.L designed the study. Q.D wrote the manuscript. Q.D , T.H, Y.G and S.J collected, analyzed, and interpreted the data. Y.L and Y.H critically reviewed, edited, and approved the manuscript. All authors read and approved the final manuscript.
Funding
This manuscript was supported by the Key Projects of the Anhui Provincial Department of Education (grant #KJ2021A0836 and GXXT-2021-087) and the Key scientific research project of Wannan Medical College (grant #WK2020Z07).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
All protocols were approved by the ethics review board of the National Center for Health Statistics, and written informed consents were obtained from the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yue’e Huang, Email: 19990008@wnmc.edu.cn.
Yali Liang, Email: 20100030@wnmc.edu.cn.
References
- 1.Sockrider M, Fussner L. What is asthma? Am J Respir Crit Care Med. 2020;202(9):P25–6. 10.1164/rccm.2029P25. 10.1164/rccm.2029P25 [DOI] [PubMed] [Google Scholar]
- 2.Agache I, Akdis CA. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J Clin Invest. 2019;129(4):1493–503. 10.1172/JCI124611. 10.1172/JCI124611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pate CA, Zahran HS, Qin X, Johnson C, Hummelman E, Malilay J. Asthma surveillance — United States, 2006–2018. MMWR Surveill Summ. 2021;70(5):1–32. 10.15585/mmwr.ss7005a1. 10.15585/mmwr.ss7005a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu L, Freishtat RJ, Gordish-Dressman H, et al. Natural progression of childhood asthma symptoms and strong influence of sex and puberty. Ann Am Thorac Soc. 2014;11(6):939–44. 10.1513/AnnalsATS.201402-084OC. 10.1513/AnnalsATS.201402-084OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowdhury NU, Guntur VP, Newcomb DC, Wechsler ME. Sex and gender in asthma. Eur Respir Rev. 2021;30(162):210067. 10.1183/16000617.0067-2021. 10.1183/16000617.0067-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiedeman A, Barr S, Green T, Xu Z, Innis S, Kitts D. Dietary choline intake: current state of knowledge across the life cycle. Nutrients. 2018;10(10):1513. 10.3390/nu10101513. 10.3390/nu10101513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis ED, Zhao YY, Richard C, et al. Measurement of the abundance of choline and the distribution of choline-containing moieties in meat. Int J Food Sci Nutr. 2015;66(7):743–8. 10.3109/09637486.2015.1088942. 10.3109/09637486.2015.1088942 [DOI] [PubMed] [Google Scholar]
- 8.Ntontsi P, Photiades A, Zervas E, Xanthou G, Samitas K. Genetics and epigenetics in Asthma. Int J Mol Sci. 2021;22(5):2412. 10.3390/ijms22052412. 10.3390/ijms22052412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lovinsky-Desir S, Miller RL. Epigenetics, asthma, and allergic diseases: a review of the latest advancements. Curr Allergy Asthma Rep. 2012;12(3):211–20. 10.1007/s11882-012-0257-4. 10.1007/s11882-012-0257-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu CJ, Söderhäll C, Bustamante M, et al. DNA methylation in childhood asthma: an epigenome-wide meta-analysis. Lancet Respir Med. 2018;6(5):379–88. 10.1016/S2213-2600(18)30052-3. 10.1016/S2213-2600(18)30052-3 [DOI] [PubMed] [Google Scholar]
- 11.Zeisel S, Choline. Other Methyl-Donors and Epigenetics. Nutrients. 2017;9(5):445. 10.3390/nu9050445. 10.3390/nu9050445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mentch SJ, Locasale JW. One-carbon metabolism and epigenetics: understanding the specificity. Ann N Y Acad Sci. 2016;1363(1):91–8. 10.1111/nyas.12956. 10.1111/nyas.12956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clare CE, Brassington AH, Kwong WY, Sinclair KD. One-Carbon Metabolism: linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu Rev Anim Biosci. 2019;7(1):263–87. 10.1146/annurev-animal-020518-115206. 10.1146/annurev-animal-020518-115206 [DOI] [PubMed] [Google Scholar]
- 14.Bansal P, Singh N, Joshi J, Arora N, Gaur SN. Choline chloride attenuates the allergic airway disease by inhibiting the lysophosphatidylcholine induced response in mouse model. Curr Res Pharmacol Drug Discov. 2022;3:100109. 10.1016/j.crphar.2022.100109. 10.1016/j.crphar.2022.100109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta AK, Singh BP, Arora N, Gaur SN. Choline attenuates immune inflammation and suppresses oxidative stress in patients with asthma. Immunobiology. 2010;215(7):527–34. 10.1016/j.imbio.2009.09.004. 10.1016/j.imbio.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 16.Detopoulou P, Panagiotakos DB, Antonopoulou S, Pitsavos C, Stefanadis C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study. Am J Clin Nutr. 2008;87(2):424–30. 10.1093/ajcn/87.2.424. 10.1093/ajcn/87.2.424 [DOI] [PubMed] [Google Scholar]
- 17.Jieru P, Zhang S, Cai L, et al. Dietary choline intake and health outcomes in U.S. adults: exploring the impact on cardiovascular disease, cancer prevalence, and all-cause mortality. J Health Popul Nutr. 2024;43(1):59. 10.1186/s41043-024-00528-0. 10.1186/s41043-024-00528-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jun S, Cowan AE, Dodd KW, et al. Association of food insecurity with dietary intakes and nutritional biomarkers among US children, National Health and Nutrition Examination Survey (NHANES) 2011–2016. Am J Clin Nutr. 2021;114(3):1059–69. 10.1093/ajcn/nqab113. 10.1093/ajcn/nqab113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fadeyev K, Nagao-Sato S, Reicks M. Nutrient and Food Group Intakes among U.S. children (2–5 years) Differ by Family income to poverty ratio, NHANES 2011–2018. Int J Environ Res Public Health. 2021;18(22):11938. 10.3390/ijerph182211938. 10.3390/ijerph182211938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62. 10.1136/bjsports-2020-102955. 10.1136/bjsports-2020-102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Supplement1):S17–38. 10.2337/dc22-S002. 10.2337/dc22-S002 [DOI] [PubMed] [Google Scholar]
- 22.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104. 10.1093/eurheartj/ehy339. 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 23.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Vitamins OB. and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academies Press (US); 1998. Accessed January 10, 2024. http://www.ncbi.nlm.nih.gov/books/NBK114310/ [PubMed]
- 24.Gupta SK, Gaur SN. A placebo controlled trial of two dosages of LPC antagonist–choline in the management of bronchial asthma. Indian J Chest Dis Allied Sci. 1997;39(3):149–56. [PubMed] [Google Scholar]
- 25.Qu Y, Pan C, Guo S, Wu H. Dietary intake and asthma in preschoolers: a logistic Lasso Regression Analysis. Front Pediatr. 2022;10:870529. 10.3389/fped.2022.870529. 10.3389/fped.2022.870529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mims JW. Asthma: definitions and pathophysiology. Int Forum Allergy Rhinol. 2015;5(S1):S2–6. 10.1002/alr.21609. 10.1002/alr.21609 [DOI] [PubMed] [Google Scholar]
- 27.Al-Harbi NO, Nadeem A, Al-Harbi MM, et al. Oxidative airway inflammation leads to systemic and vascular oxidative stress in a murine model of allergic asthma. Int Immunopharmacol. 2015;26(1):237–45. 10.1016/j.intimp.2015.03.032. 10.1016/j.intimp.2015.03.032 [DOI] [PubMed] [Google Scholar]
- 28.Mehta AK, Arora N, Gaur SN, Singh BP. Choline supplementation reduces oxidative stress in mouse model of allergic airway disease. Eur J Clin Invest. 2009;39(10):934–41. 10.1111/j.1365-2362.2009.02190.x. 10.1111/j.1365-2362.2009.02190.x [DOI] [PubMed] [Google Scholar]
- 29.Agassandian M, Mallampalli RK. Surfactant phospholipid metabolism. Biochim Biophys Acta BBA - Mol Cell Biol Lipids. 2013;1831(3):612–25. 10.1016/j.bbalip.2012.09.010. 10.1016/j.bbalip.2012.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chroneos Z, Sever-Chroneos Z, Shepherd V. Pulmonary surfactant: an immunological perspective. Cell Physiol Biochem. 2010;25(001):013–26. 10.1159/000272047. 10.1159/000272047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jubinville É, Milad N, Maranda-Robitaille M, et al. Critical importance of dietary methionine and choline in the maintenance of lung homeostasis during normal and cigarette smoke exposure conditions. Am J Physiol-Lung Cell Mol Physiol. 2020;319(2):L391–402. 10.1152/ajplung.00353.2019. 10.1152/ajplung.00353.2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.