Abstract
Gene expression is an intricate biological process that bridges gap between the genotype and the phenotype. Canonical and hereditable epigenetic mechanisms, such as histone and DNA modifications, regulate the release of genetic information encoded in DNA without altering the underlying sequence. Many other non-canonical players, such as chromatin regulators and noncoding RNAs, are also involved in regulating gene expression. Recently, RNA modifications (epitranscriptomics) have been shown to hold enormous potential in shaping cellular transcriptomes. However, their co-transcriptional nature and uncertain heritability mean that they fall outside the current definition of epigenetics, sparking an ongoing debate in the field. Here we will discuss the relationship between canonical and non-canonical epigenetic mechanisms that govern gene expression and offer our perspective on whether (or not) epitranscriptomic modifications can be classified as epigenetic mechanisms.
Keywords: Epigenetics, Epitranscriptomics, Methylation, DNA, RNA, Epigenetic memory
Main text
Unraveling the mechanisms by which genetic variation translates into phenotypic diversity at the cell, tissue, or organism level has long remained a major challenge in biology. Initially, genetic mutations were considered the exclusive source of trait diversity. However, examples of phenotypic variability in cells and humans bearing the same genetic code (e.g., cell differentiation and homozygous twins) called this idea into question. In 1942, the developmental biologist C. H. Waddington inferred the existence of mechanisms that act “on top of” (hence the prefix “epi” from Greek) genetics, defining epigenetics as “the branch of biology which studies the causal interactions between genes and their products, which bring the phenotype into being” (Waddington, 1942). Over time, the concept of epigenetics has broadened, moving beyond developmental and evolutionary biology to encompass all chromatin-associated (hereditable) mechanisms that regulate gene expression without altering the DNA sequence. According to this definition, deposition of 5-methylcytosine (5mC) at gene promoters and histone post-translational modifications (hPTMs) were recognized among the first examples of epigenetic traits (reviewed in [1]). Subsequently, the discovery of other mechanisms influencing the cellular transcriptome beyond chromatin regulation has increased the complexity of the epigenetic landscape. For instance, the existence of topology-associated domains that drive gene expression through inter- and intra-chromatinic interactions and the presence of noncoding RNAs, such as microRNA (miRNA), long noncoding RNA (lncRNA), and circular RNA(circRNA) that participate in the transduction of genetic information by either sponging, scaffolding, or localizing transcripts, are all examples of newly epimechanisms influencing gene expression beyond genomic changes (reviewed in [1]). More recently, RNA modifications have added a further layer of complexity to link the “epigenotype” with the specific phenotype.
Epitranscriptomic modifications, such as 5mC, 7-methyl-guanine (m7G), and pseudouridine, control the fate of thousands of RNAs, and their deposition is dynamically regulated by various effectors, including writers, erasers, and readers (reviewed in [2]).
Among the several modifications N6-methyladenosine (m6A) is the most abundant and widespread epimark on RNA molecules, and it governs multiple steps of RNA metabolism by regulating the processing, stability, and translation of RNAs in nearly every biological process, ultimately affecting gene expression post-transcriptionally (reviewed in [3]). Since the current definition of epigenetics primarily focuses on the various mechanisms that regulate gene expression without altering the DNA sequence, the question arises as to whether epitranscriptomics can be considered a part of epigenetics. We believe that the answer to this controversy hinges on the definition of epigenetics, with a key point being the heritability of epigenetic memory. Typically, transgenerational epigenetic inheritance (TEI) involves the propagation of epigenetic traits across generations in the absence of continuous environmental cues. Although TEI is widely recognized in plants, fungi, and worms (reviewed in [4]), its existence in animals remains uncertain. In mammals, the transmission of DNA methylation at CpG islands (e.g., 5mC) and hPTMs through TEI has been proposed. Recently, de novo methylation of CpG-free DNA introduced by the transposase technology at the Ankrd26 promoter was found to generate an obese phenotype that was maintained over multiple mice generations, showing that metabolic phenotypes associated with a specific DNA methylation signature are inherited in vivo [5]. Propagation of hPTM information was also confirmed in vitro. The deregulation of mini-chromosome maintenance complex component 2, a DNA helicase responsible for correct histone segregation and hPTM transmission, was shown to induce aberrant inheritance of histone methylation marks that impaired embryonic cell differentiation [6]. Therefore, evidence of the potential transmissibility of 5mC and hPTMs, together with their influence on cell transcription, supports their inclusion in the definition of epigenetics. Conversely, the transmissibility of RNA-based epitraits is more controversial. Intriguingly, the injection of RNAs (e.g., miRNAs, transfer RNA (tRNA)-derived small RNAs) into mice sperm or transfer RNA fragments (tfRNA) into fertilized eggs was sufficient to reproduce and propagate parental phenotypes, such as white-tail color, metabolic defects due to high-fat diet, mice gigantism, and stress behavior (reviewed in [7]). RNA marks have also been proposed to participate in TEI. Cytosine methylation of tRNAs in mice sperm by DNA methyltransferase 2 (DNMT2) was found essential for the induction and propagation of the white-tail and hypertrophic phenotypes in mice [8]. Similarly, TEI of susceptibility to seminomas was increased in mice with reduced cytosine deamination due to the loss of the RNA modifier apolipoprotein B catalytic polypeptide 1 (APOBEC1) [9]. Together, these findings highlight the fact that sperm RNAs, and their related modifications, may act as potential carriers of epimemory. Concerning m6A, although numerous studies have highlighted its crucial role in meiosis and embryonic development, a precise mechanism has not yet been proposed. Interestingly, maternal inheritance of m6A marks was recently reported in mice embryos, where the presence of m6A on a subset of maternally transmitted transcripts was correlated to the enhancement of their translation [10]. However, the high dynamism and likely stability of RNA modifications and DNA marks (such as hPTMs and CpG methylation) still leaves many questions unanswered as to how the epitranscriptomic signature may be transmitted to offspring.
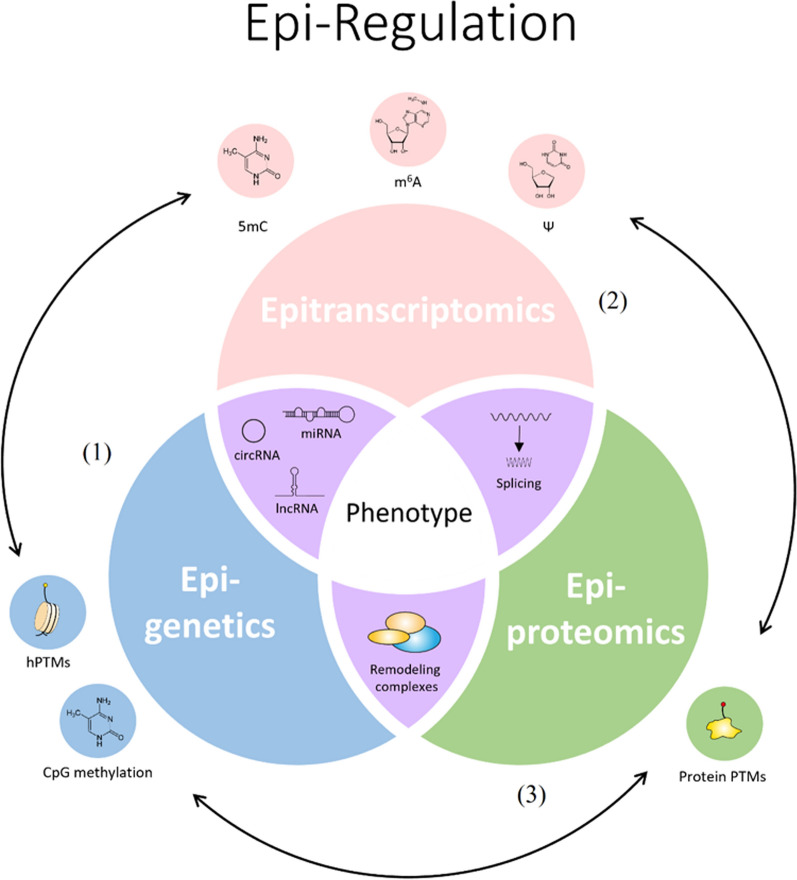
Signatures of the RNA modifications 5mC and 2-methyl-guanine (m2G) were found altered in high-fat diet mice progeny, and their aberrant deposition, in combination with tfRNAs, DNA methylation, and hPTMs, was proposed to mediate the acquired metabolic phenotype in mice [11]. These findings suggest that the transmission of epitraits is a multifactorial event, likely driven by multiple epilayers rather than a single epigenetic cue. Thus, limiting the concept of epigenetics to inheritance and gene expression ignores the dynamic interplay of pathways acting on chromatin (epigenetics), RNA (epitranscriptomics), and proteins (epiproteomics). These pathways work together “on top of” the resulting phenotype. Expanding on the original definition of epigenetics by Waddington, we are convinced that epigenetics, epitranscriptomics, and epiproteomics are strongly interconnected in a “cell epiregulation” network that defines the cellular gene function output and fosters phenotypic variability (Fig. 1). For example, the fact that cellular methyltransferases, despite targeting different substrates (DNA, RNA, and histones), all use S-adenosyl methionine (SAM) as the methyl donor, hints at the existence of a common regulatory network. According to this model, multiple layers of regulation such as (i) mechanisms acting at DNA level (e.g., 5mC and hPTMs) that influence transcription initiation, (ii) noncoding RNA species and RNA marks governing stability, abundance, and functionality of transcripts, (iii) chromatin tridimensional organization in TADs, and (vi) post-translational modifications that regulate protein activity, all cooperate to define cellular gene activity (Fig. 1). Epitranscriptomics can therefore (and for the time being) be considered part of the “cell epiregulation” system, which also includes epigenetics and epiproteomics. In line with this view, epiregulation is the blueprint of gene activity, and its interconnected branches (i.e., epigenetics, epitranscriptomics, and epiproteomics) only represent the layers and substrates on which epiregulation acts. Examples of transcriptional interplay such as the functional interaction of epitranscriptomic factors with histone marks (e.g., METTL14 binding H3K36me3) [12], the spread of Polycomb repressive complexes (PRC) on chromatin by lncRNAs [13], the influence of m6A on miRNA biogenesis, and crosstalk between different hPTMs, different RNA marks, and each other (reviewed in [14]) indicate that we cannot consider these mechanisms distinct. Instead, they are tightly interconnected as part of one unique process (epiregulation) in which they intersect to define gene activity and ultimately shape the cell phenotype.
Fig. 1.
Epiregulation: Unified view of interconnected epigenetic, epitranscriptomic, and epiproteomic mechanisms that shape gene activity and cell phenotype. (1) Mechanisms acting at DNA level (e.g., 5mC and hPTMs) that influence transcription initiation. (2) RNA marks governing the stability, abundance, and functionality of transcripts. (3) PTM versus hPTM that regulate protein activity
At this point, the question again arises: is epitranscriptomics epigenetics?
Considering the substrate “on top of” which they act, we cannot recognize epitranscriptomics as epigenetics. However, considering the effects “on top of” the phenotype that are determined by gene activity, we can assert that epitranscriptomics, epigenetics and epiproteomics are two sides of the same coin, both participating in a complex network of interconnections that we define “cell epiregulation.”
This concept is supported and strengthened by the immense clinical implications both as epibiomarkers of human diseases (but not restricted to) such as cancer and as therapeutic opportunities. Undoubtedly the next future also with the availability of always more defined technologies will clarify further the deep epi-interplay that shapes the normal and the pathological phenotype and its hereditability.
Acknowledgements
We thank C. Fisher for linguistic editing.
Abbreviations
- m2G
2-Methyl-guanine
- 5mC
5-Methylcytosine
- APOBEC1
Apolipoprotein B catalytic polypeptide 1
- circRNA
Circular RNA
- DNMT2
DNA methyltransferase 2
- hPTMs
Histone post-translational modifications
- lncRNA
Long non-coding RNA
- miRNA
MicroRNA
- m6A
N6-methyladenosine
- ncRNAs
Non-coding RNAs
- PRC
Polycomb repressive complexes
- SAM
S-adenosyl methionine
- tsRNA
TRNA-derived small RNAs
- TADs
Topology-associated domains
- tfRNA
Transfer RNA fragments
- TEI
Transgenerational epigenetic inheritance
Author contributions
Project administration and supervision: N.D.G., G.B., and L.A.; conceptualization and writing: N.D.G. and G.B; review and editing: L.A. All the authors read and approved the final manuscript.
Funding
This work was supported by PNRR-MAD-2022-12376723; PNRR-CN3, National Centre for Gene Therapy and Drugs Based on RNA Technology, cod: CN000000041. NextGenerationEU—CUP: B83C22004960002. EPI-MET Fondo Crescita Sostenibile—Accordi per l’Innovazione D.M. 31.12.2021, D.D. 18.03.2022 no. 34; project no. F/310034/03/X56 (VANVITELLI). PRIN P2022F3YRF; Bando di Ateneo per il finanziamento di progetti di ricerca fondamentale ed applicata dedicato ai giovani ricercatori D.R. no. 834 of 30/09/2022: IDEA (CUP: B63C22001470005). VALERE: Vanvitelli per la Ricerca Program: EPInhibitDRUGre (CUP: B66J20000680005). N.D.G. was supported by PON Ricerca e Innovazione 2014–2020.
Availability of data and materials
No datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nunzio Del Gaudio, Email: nunzio.delgaudio@unicampania.it.
Lucia Altucci, Email: lucia.altucci@unicampania.it.
References
- 1.Allis CD, Jenuwein T. The molecular hallmarks of epigenetic control. Nat Rev Genet. 2016;17(8):487–500. 10.1038/nrg.2016.59 [DOI] [PubMed] [Google Scholar]
- 2.Delaunay S, Helm M, Frye M. RNA modifications in physiology and disease: towards clinical applications. Nat Rev Genet. 2024;25(2):104–22. 10.1038/s41576-023-00645-2 [DOI] [PubMed] [Google Scholar]
- 3.Sendinc E, Shi Y. RNA m6A methylation across the transcriptome. Mol Cell. 2023;83(3):428–41. 10.1016/j.molcel.2023.01.006 [DOI] [PubMed] [Google Scholar]
- 4.Bošković A, Rando OJ. Transgenerational epigenetic Inheritance. Annu Rev Genet. 2018;52:21–41. 10.1146/annurev-genet-120417-031404 [DOI] [PubMed] [Google Scholar]
- 5.Takahashi Y, Morales Valencia M, Yu Y, Ouchi Y, Takahashi K, Shokhirev MN, et al. Transgenerational inheritance of acquired epigenetic signatures at CpG islands in mice. Cell. 2023;186(4):715-31.e19. 10.1016/j.cell.2022.12.047 [DOI] [PubMed] [Google Scholar]
- 6.Wenger A, Biran A, Alcaraz N, Redó-Riveiro A, Sell AC, Krautz R, et al. Symmetric inheritance of parental histones governs epigenome maintenance and embryonic stem cell identity. Nat Genet. 2023;55(9):1567–78. 10.1038/s41588-023-01476-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boscardin C, Manuella F, Mansuy IM. Paternal transmission of behavioural and metabolic traits induced by postnatal stress to the 5th generation in mice. Environ Epigenet. 2022;8(1):dvac024. 10.1093/eep/dvac024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiani J, Grandjean V, Liebers R, Tuorto F, Ghanbarian H, Lyko F, et al. RNA-mediated epigenetic heredity requires the cytosine methyltransferase Dnmt2. PLoS Genet. 2013;9(5):e1003498. 10.1371/journal.pgen.1003498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson VR, Heaney JD, Tesar PJ, Davidson NO, Nadeau JH. Transgenerational epigenetic effects of the Apobec1 cytidine deaminase deficiency on testicular germ cell tumor susceptibility and embryonic viability. Proc Natl Acad Sci USA. 2012;109(41):E2766–73. 10.1073/pnas.1207169109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu W, Ding Y, Meng J, Gu L, Liu W, Li L, et al. Reading and writing of mRNA m(6)A modification orchestrate maternal-to-zygotic transition in mice. Genome Biol. 2023;24(1):67. 10.1186/s13059-023-02918-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science (New York, NY). 2016;351(6271):397–400. 10.1126/science.aad7977 [DOI] [PubMed] [Google Scholar]
- 12.Huang H, Weng H, Zhou K, Wu T, Zhao BS, Sun M, et al. Histone H3 trimethylation at lysine 36 guides m(6)A RNA modification co-transcriptionally. Nature. 2019;567(7748):414–9. 10.1038/s41586-019-1016-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schertzer MD, Braceros KCA, Starmer J, Cherney RE, Lee DM, Salazar G, et al. lncRNA-Induced spread of polycomb controlled by genome architecture, RNA abundance, and CpG Island DNA. Mol Cell. 2019;75(3):523-37.e10. 10.1016/j.molcel.2019.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fico A, Di Croce L, Matarazzo MR. Interplay between DNA and RNA modifications: a constantly evolving process. Epigenomes. 2020;4(4):26. 10.3390/epigenomes4040026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.