Abstract
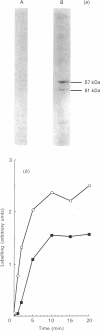
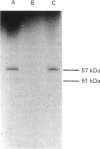
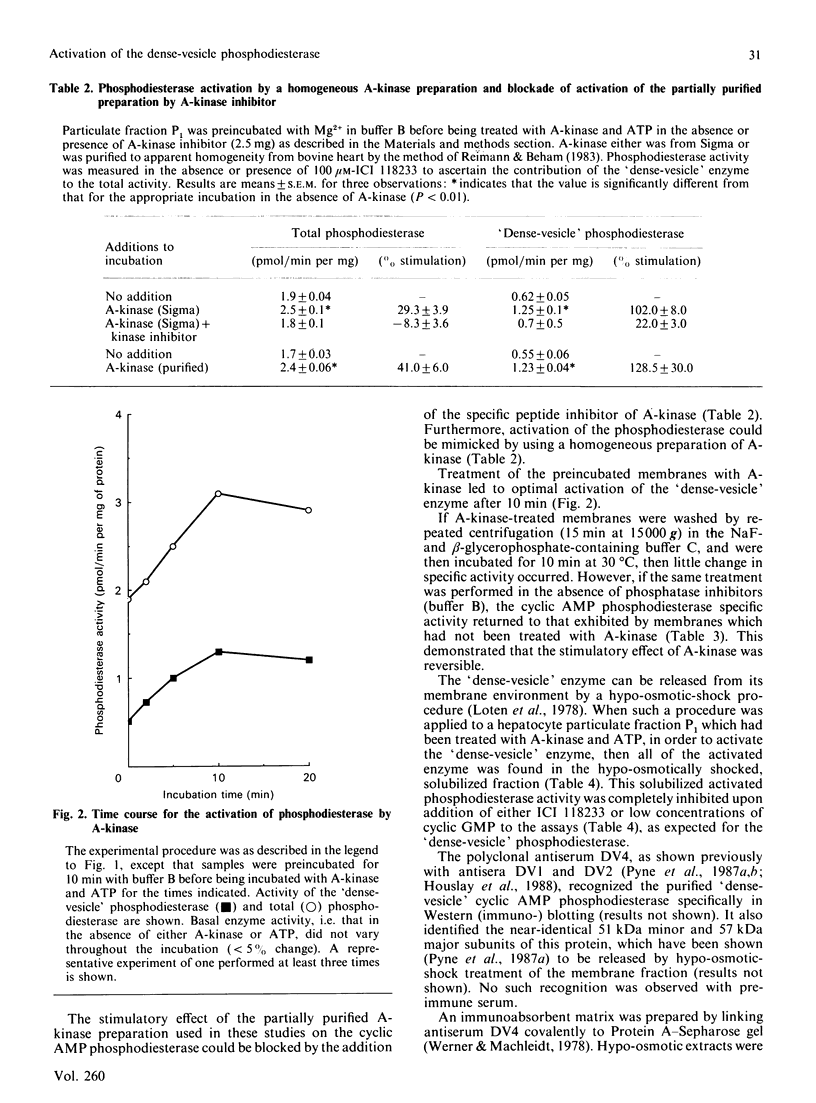
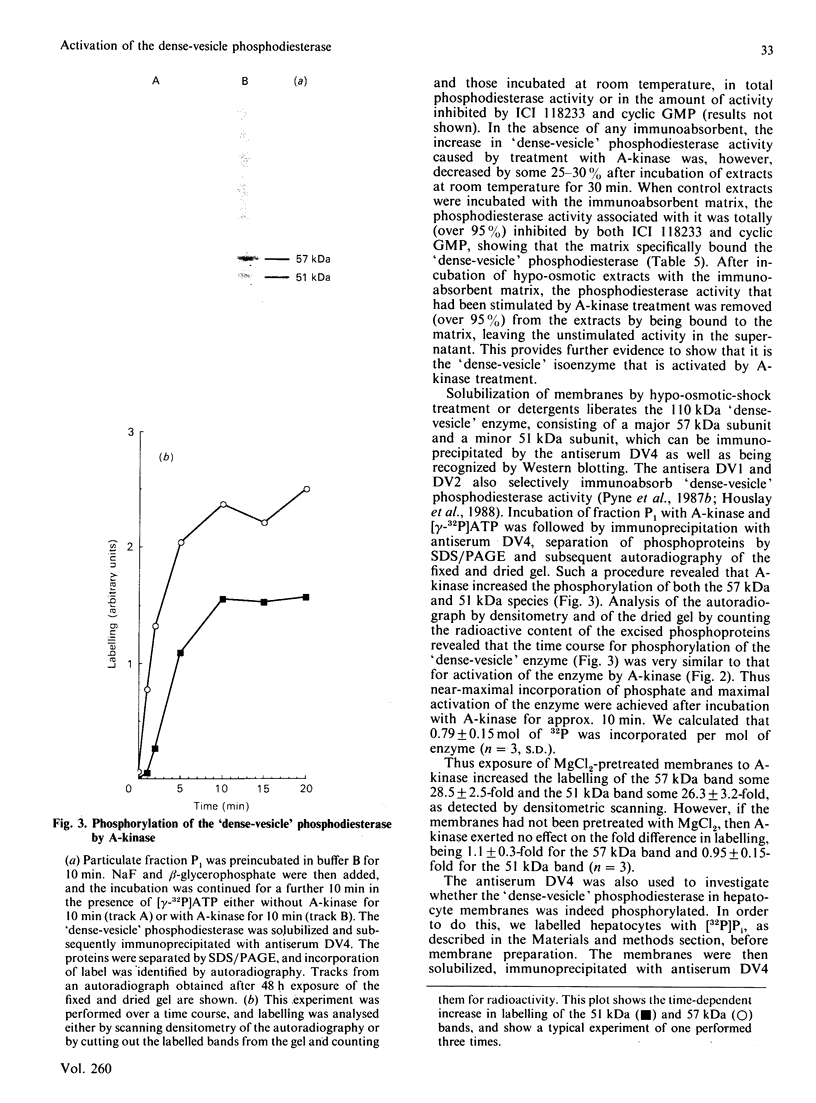
Incubation of a hepatocyte particulate fraction with ATP and the isolated catalytic unit of cyclic AMP-dependent protein kinase (A-kinase) selectively activated the high-affinity 'dense-vesicle' cycle AMP phosphodiesterase. Such activation only occurred if the membranes had been pre-treated with Mg2+. Mg2+ pre-treatment appeared to function by stimulating endogenous phosphatases and did not affect phosphodiesterase activity. Using the antiserum DV4, which specifically immunoprecipitated the 51 and 57 kDa components of the 'dense-vesicle' phosphodiesterase from a detergent-solubilized membrane extract, we isolated a 32P-labelled phosphoprotein from 32P-labelled hepatocytes. MgCl2 treatment of such labelled membranes removed 32P from the immunoprecipitated protein. Incubation of the Mg2+-pre-treated membranes with [32P]ATP and A-kinase led to the time-dependent incorporation of label into the 'dense-vesicle' phosphodiesterase, as detected by specific immunoprecipitation with the antiserum DV4. The time-dependences of phosphodiesterase activation and incorporation of label were similar. It is suggested (i) that phosphorylation of the 'dense-vesicle' phosphodiesterase by A-kinase leads to its activation, and that such a process accounts for the ability of glucagon and other hormones, which increase intracellular cyclic AMP concentrations, to activate this enzyme, and (ii) that an as yet unidentified kinase can phosphorylate this enzyme without causing any significant change in enzyme activity but which prevents activation and phosphorylation of the phosphodiesterase by A-kinase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyes S., Allan E. H., Loten E. G. Energy-dependent activation and magnesium--dependent inactivation of hepatocyte hormone-sensitive phosphodiesterase. Biochim Biophys Acta. 1981 Jan 7;672(1):21–28. doi: 10.1016/0304-4165(81)90275-0. [DOI] [PubMed] [Google Scholar]
- Carling D., Zammit V. A., Hardie D. G. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 1987 Nov 2;223(2):217–222. doi: 10.1016/0014-5793(87)80292-2. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in the hormonal control of enzyme activity. Eur J Biochem. 1985 Sep 16;151(3):439–448. doi: 10.1111/j.1432-1033.1985.tb09121.x. [DOI] [PubMed] [Google Scholar]
- Degerman E., Belfrage P., Newman A. H., Rice K. C., Manganiello V. C. Purification of the putative hormone-sensitive cyclic AMP phosphodiesterase from rat adipose tissue using a derivative of cilostamide as a novel affinity ligand. J Biol Chem. 1987 Apr 25;262(12):5797–5807. [PubMed] [Google Scholar]
- Francis S. H., Kono T. Hormone-sensitive cAMP phosphodiesterase in liver and fat cells. Mol Cell Biochem. 1982 Feb 5;42(2):109–116. doi: 10.1007/BF00222697. [DOI] [PubMed] [Google Scholar]
- Harrison S. A., Reifsnyder D. H., Gallis B., Cadd G. G., Beavo J. A. Isolation and characterization of bovine cardiac muscle cGMP-inhibited phosphodiesterase: a receptor for new cardiotonic drugs. Mol Pharmacol. 1986 May;29(5):506–514. [PubMed] [Google Scholar]
- Heyworth C. M., Wallace A. V., Houslay M. D. Insulin and glucagon regulate the activation of two distinct membrane-bound cyclic AMP phosphodiesterases in hepatocytes. Biochem J. 1983 Jul 15;214(1):99–110. doi: 10.1042/bj2140099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth C. M., Wallace A. V., Wilson S. R., Houslay M. D. An assessment of the ability of insulin-stimulated cyclic AMP phosphodiesterase to decrease hepatocyte intracellular cyclic AMP concentrations. Biochem J. 1984 Aug 15;222(1):183–187. doi: 10.1042/bj2220183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkirk T. J., Denton R. M. Studies on the specific activity of [gamma-32P]ATP in adipose and other tissue preparations incubated with medium containing [32P]phosphate. Biochim Biophys Acta. 1986 Feb 21;885(2):195–205. doi: 10.1016/0167-4889(86)90089-3. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Pyne N. J., Cooper M. E. Isolation and characterization of insulin-stimulated, high-affinity cAMP phosphodiesterases from rat liver. Methods Enzymol. 1988;159:751–760. doi: 10.1016/0076-6879(88)59071-7. [DOI] [PubMed] [Google Scholar]
- Krebs E. G. The phosphorylation of proteins: a major mechanism for biological regulation. Fourteenth Sir Frederick Gowland Hopkins memorial lecture. Biochem Soc Trans. 1985 Oct;13(5):813–820. doi: 10.1042/bst0130813. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loten E. G., Assimacopoulos-Jeannet F. D., Exton J. H., Park C. R. Stimulation of a low Km phosphodiesterase from liver by insulin and glucagon. J Biol Chem. 1978 Feb 10;253(3):746–757. [PubMed] [Google Scholar]
- Manganiello V. C. Subcellular localization and biological function of specific cyclic nucleotide phosphodiesterases. J Mol Cell Cardiol. 1987 Oct;19(10):1037–1040. doi: 10.1016/s0022-2828(87)80575-8. [DOI] [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. A peripheral and an intrinsic enzyme constitute the cyclic AMP phosphodiesterase activity of rat liver plasma membranes. Biochem J. 1980 May 1;187(2):381–392. doi: 10.1042/bj1870381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G., Gierschik P., Spiegel A. M., Klee W. A. The GTP-binding regulatory proteins of neuroblastoma x glioma, NG108-15, and glioma, C6, cells. Immunochemical evidence of a pertussis toxin substrate that is neither Ni nor No. FEBS Lett. 1986 Jan 20;195(1-2):225–230. doi: 10.1016/0014-5793(86)80165-x. [DOI] [PubMed] [Google Scholar]
- Pyne N. J., Anderson N., Lavan B. E., Milligan G., Nimmo H. G., Houslay M. D. Specific antibodies and the selective inhibitor ICI 118233 demonstrate that the hormonally stimulated 'dense-vesicle' and peripheral-plasma-membrane cyclic AMP phosphodiesterases display distinct tissue distributions in the rat. Biochem J. 1987 Dec 15;248(3):897–901. doi: 10.1042/bj2480897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne N. J., Cooper M. E., Houslay M. D. The insulin- and glucagon-stimulated 'dense-vesicle' high-affinity cyclic AMP phosphodiesterase from rat liver. Purification, characterization and inhibitor sensitivity. Biochem J. 1987 Feb 15;242(1):33–42. doi: 10.1042/bj2420033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann E. M., Beham R. A. Catalytic subunit of cAMP-dependent protein kinase. Methods Enzymol. 1983;99:51–55. doi: 10.1016/0076-6879(83)99039-0. [DOI] [PubMed] [Google Scholar]
- Smith S. A., Elliott K. R., Pogson C. I. Differential effects of tryptophan on glucose synthesis in rats and guinea pigs. Biochem J. 1978 Dec 15;176(3):817–825. doi: 10.1042/bj1760817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Werner S., Machleidt W. Isolation of precursors of cytochrome oxidase from Neurospora crassa: application of subunit-specific antibodies and protein A from Staphylococcus aureus. Eur J Biochem. 1978 Sep 15;90(1):99–105. doi: 10.1111/j.1432-1033.1978.tb12579.x. [DOI] [PubMed] [Google Scholar]
- Wilson S. R., Wallace A. V., Houslay M. D. Insulin activates the plasma-membrane and dense-vesicle cyclic AMP phosphodiesterase in hepatocytes by distinct routes. Biochem J. 1983 Oct 15;216(1):245–248. doi: 10.1042/bj2160245. [DOI] [PMC free article] [PubMed] [Google Scholar]