Abstract
Among approaches aimed at reducing Lyme disease risk in the environment, those targeting reservoirs of Borrelia burgdorferi Johnson are promising because they have the potential to reduce both the density of questing Ixodes scapularis Say (Acari: Ixodidea) ticks and the prevalence of B. burgdorferi in the tick population. In this 4-yr field study, we treated a population of wild small mammals with 2 densities of fluralaner baits and investigated the effect of the treatment on 3 parameters of the endemic cycle of B. burgdorferi: (i) the prevalence of infected Peromyscus mice (PIM), (ii) the density of questing nymphs (DON), and (iii) the prevalence of infected questing nymphs (NIP). We demonstrated that fluralaner baiting is effective at reducing tick infestation of Peromyscus mice, the main reservoir of B. burgdorferi in central and northeastern North America, in the laboratory and the field. Results from this study showed a significant decrease in B. burgdorferi infection in mice (odds ratio: 0.37 [CI95: 0.17 to 0.83]). A reduction in the DON between 45.4% [CI95: 22.4 to 61.6] and 62.7% [CI95: 45.9 to 74.2] occurred in treated area when compared with control areas. No significant effect was reported on the NIP. These results confirm the hypothesis that fluralaner baits have an effect on B. burgdorferi endemic cycle, with the potential to reduce the density of B. burgdorferi-infected ticks in the environment. Further studies performed in various habitats and public health intervention contexts are needed to refine and operationalize this approach for reducing Lyme disease risk in the environment.
Keywords: Borrelia burgdorferi, Lyme disease, Ixodes scapularis, Peromyscus mouse, fluralaner
Introduction
Lyme disease is a tick-borne disease of major concern for public health in temperate area of the northern hemisphere (Piesman and Gern 2004). In addition to being the most frequent tick-borne disease in Europe and North America, Lyme disease is currently expanding northward in Canada and northern European countries bringing risk to new animal and human populations (Leighton et al. 2012, Ogden et al. 2013, Bouchard et al. 2015, 2019, Vandekerckhove et al. 2019). In Canada, the emergence of the causal agent of Lyme disease, Borrelia burgdorferi sensu stricto Johnson (hereafter B. burgdorferi), is linked with the northward geographic expansion of its vector, the tick Ixodes scapularis Say, endemic area due to climate and ecological changes (Leighton et al. 2012, Ogden et al. 2013). A key driver of this progression is climate warming, resulting in a more suitable environment for I. scapularis ticks for a longer period of the year (Ogden et al. 2005, Leighton et al. 2012). Warmer temperature and more days per year with temperature >0 °C favor activity and reproduction of I. scapularis ticks, allowing them to complete their life cycle faster and establish endemic populations (Ogden et al. 2004, Ogden and Lindsay 2016). The growing threat and increasing public health burden of Lyme disease and other emerging tick-borne diseases create a strong need for innovative approaches to reduce tick-borne disease risk in the environment.
An important driver of B. burgdorferi endemic cycle in the environment is the frequency with which immature stages of I. scapularis feed on infected small mammals (Bouchard et al. 2019). Because ticks do not transmit B. burgdorferi transovarially from the female adults to its offspring, larvae must feed upon an infected host to become infected themselves (Patrican 1997, Piesman and Gern 2004, Tsao 2009). In central and eastern North America, Peromyscus mice, namely the white-footed mouse, P. leucopus Rafinesque, and the deer mouse, P. maniculatus Wagner, are major reservoirs for B. burgdorferi (Donahue et al. 1987, Mather et al. 1989, Rand et al. 1993, Dumas et al. 2022). For instance, they can infect between 80% and 90% of immature I. scapularis feeding on them, they usually feed an important proportion of immature I. scapularis stages questing in the environment and they can become permanent carrier at least for some B. burgdorferi strains (Mather et al. 1989, Rand et al. 1993, Lindsay et al. 1997, Hanincová et al. 2008, Dumas et al. 2022). Other vertebrates, such as shrews, chipmunks and some species of birds, act as reservoir for B. burgdorferi but they usually infect a smaller proportion of ticks feeding on them, are less abundant in the environment or host a smaller fraction of immature I. scapularis ticks (Mather et al. 1989, LoGiudice et al. 2003, Brunner et al. 2008, Dumas et al. 2022). This makes Peromyscus mice strategic species for approaches targeting reservoirs of B. burgdorferi in order to disrupt its endemic cycle and reduce the density of infected ticks (DIT).
Two categories of approaches have been used to reduce the DIT by targeting small mammal reservoirs of B. burgdorferi (Eisen and Dolan 2016). First, several authors have targeted small mammal populations with vaccines against outer surface protein A (OspA), reducing the prevalence of B. burgdorferi-infected ticks in the environment by up to 25% (Tsao et al. 2001, 2004, Gomes-Solecki 2014, Richer et al. 2014). Other approaches have targeted small mammals with acaricides or acaropathogen fungi such as permethrin, fipronil, or Met52 (Hornbostel et al. 2005, Eisen and Dolan 2016). In contrast to methods using vaccines, killing ticks infesting reservoir hosts has the potential to affect other tick-borne pathogens transmitted by small mammals such as Anaplasma phagocytophilum or Babesia microtii (Eisen and Dolan 2016, Eisen 2023).
Killing ticks infesting small mammals has the potential to affect the endemic cycle of B. burgdorferi through 3 mechanisms. First, killing nymphs infesting small mammals may reduce the transmission of B. burgdorferi between vectors and reservoirs, and result in a lower B. burgdorferi prevalence among small mammals. Second, killing larvae and nymphs may reduce the prevalence of B. burgdorferi-infected ticks, as an important fraction of nymphs and adults are infected after biting a small mammal, especially a Peromyscus mouse (Mather et al. 1989, Dumas et al. 2022). Conversely, untreated nymphs and adults would likely have bitten non-reservoir or less competent reservoir species at their previous stage resulting in a lower B. burgdorferi prevalence. Third, as small mammals usually feed an important fraction of I. scapularis larvae and nymphs, killing immature ticks that feed on them may subsequently result in a reduction in the density of nymphs and adults in the environment.
Isoxazolines kill ticks infesting animals mainly by blocking the opening of γ-aminobutyric acid-gated chloride channels (Gassel et al. 2014). Fluralaner is an acaricide of the isoxazolines family commercialized in 2014 on the veterinary drugs market for tick bite prevention in dogs and cats (Zhou et al. 2022). A dose of 25 mg/kg of fluralaner can kill >90% of adult Ixodes spp. ticks infesting domestic animals with a duration of effect of more than 2 months (Wengenmayer et al. 2014). As with permethrin and fipronil, fluralaner baits were effective at killing I. scapularis ticks infesting small mammals in laboratory (Pelletier et al. 2020) and/or field trials (Pelletier et al. 2022). In the laboratory, fluralaner killed more than 90% of infesting I. scapularis larvae on P. maniculatus mice in 48 h with doses of 12.5 and 50 mg/kg, even though this effect was limited to the first 4 days following administration of the treatment (Pelletier et al. 2020). These results contrast with a duration of effect of fluralaner reported in domestic animal such as dogs (Wengenmayer et al. 2014). In a previous field trial, fluralaner successfully reduced counts of larval and nymphal I. scapularis infesting Peromyscus mice by up to 86% depending on treatment intensity and tick stage (Pelletier et al. 2022).
This non-randomized controlled study took place in southern Québec, Canada, where Lyme disease has been emerging since 2008 when the first locally acquired cases were diagnosed (Bourré-Tessier et al. 2011). It aimed to determine if the reduction in the number of I. scapularis larvae and nymphs infesting Peromycus spp. mice previously observed by Pelletier et al. (2022) had an effect on the endemic cycle of B. burgdorferi. Consequently, based on the field work described in Pelletier et al. (2022), the effect of fluralaner baiting of small mammals was assessed through 3 critical parameters of the B. burgdorferi endemic cycle after a 4-yr deployment: (i) the prevalence of B. burgdorferi-infected Peromycus mice (PIM), (ii) the density of questing nymphs (DON), and (iii) the prevalence of B. burgdorferi-infected nymphs (NIP).
Material and Methods
Field Activities
Experimental Area
The study took place between 2016 and 2019 on the Farnham National Defense Facility located in southern Québec, Canada, close to the border with the USA. This study was performed in the same experimental area and plots described by Pelletier et al (2022). The environment of the experimental area was homogeneous and composed of maple and oak forests with a thick dead leaf litter. Briefly, the experimental area was divided into 3 zones (C, T1, and T2) designed to limit mixing between the small mammal populations. Four plots were located within zones C and T1, 3 within zone T2 (Pelletier et al. 2022). Experimental plots were 75 × 75 m in area and separated by at least 100 m with respect to Peromyscus mice average home range to limit the movement of small mammals between sites (Wolff 1985).
Treatment
Fluralaner baits were distributed in the field through mouse-size Protecta RTU stations (Bell Laboratories Inc., Murray Hill, NJ, USA), with one bait per station. Stations were placed at designated locations for the whole treatment period. Fluralaner baits were a mixture of peanut butter and the commercial formulation Bravecto (Merck Animal Health, Madison, NJ) with a final concentration of 4.8 mg of fluralaner per gram of bait (Pelletier et al. 2022). In treatment zone T1, bait stations were deployed within experimental plots at a density of 2.1 baits/1,000 m2 (n = 12 stations per 5,625 m2 plot) in 2016 but density was increased to 4.4 (n = 25) between 2017 and 2019. New experimental plots located in treatment zone T2 were added to the study in 2017 at a density of 2.1 baits/1,000 m2 and this bait density was maintained until 2019 (Pelletier et al. 2022). Stations were filled with a 250- to 500-mg of bait containing 1.2 to 2.4 mg of fluralaner, enough to provide a dose of 50 to 100 mg/kg to a 25-g Peromyscus mice were it to eat the entire bait (Pelletier et al. 2022). The treatment was deployed over a 6-wk period each year between July 10 to 15 and August 23 to 26, spanning the peak in larval I. scapularis questing activity, with bait stations refilled each week (Pelletier et al. 2022). From 2017 to 2019, 2,176 baits each made up of 250 to 500 mg of bait mixture containing 1.2 to 2.4 mg of fluralaner were administered in zones T1 and T2. No bait or stations were deployed in zone C.
Small Mammal Sampling
Small mammals sampling was performed as described by Pelletier et al. (2022). Mammals were captured with Sherman live-traps (H.B. Sherman Traps, Tallahassee, FL) in 2016, 2017, and 2018 both before (Pre-Tx) and during (Tx) treatment application. Mice were weighted to extrapolate age, sexed, microchipped, and an ear biopsy was taken on each first capture mouse for B. burgdorferi testing (Pelletier at al. 2022). Mouse handling was done under general anesthesia as described by Pelletier et al. (2022) and was performed in accordance with the Canadian Council for Animal Care and the Ministère des forêts, de la faune et des parcs (SEG: 2016SF2052R16, 2017-05-11-2232-16-SF, and 2018-3-22-2369-16-S-F) guidelines and regulation. The handling protocol was approved by the institutional animal ethics committee of Université de Montréal (16-Rech-1845, 17-Rech-1836, and 18-Rech-1836). After handling, mice were released at their capture location.
Tick Sampling
Host-seeking ticks in experimental plots were sampled between 2016 and 2019 by dragging a 1-m2 piece of white flannel cloth across the forest floor (Rulison et al. 2013). In 2016, each zone was sampled twice (once in June and once in July). Starting in 2017, 2 additional sampling periods were added (May and August) for a total of 4 sampling periods at monthly intervals throughout the summer. Between 2016 and 2017, two 75 m2 transects were sampled per plot in each period for a surface of 150 m2 per plot-period. In 2018 and 2019, 3 transects of 75 m2 were added to each plot to bring the sampled surface to 375 m2 per plot-period. After each 25 m2 of dragging, host-seeking ticks were collected for identification and pathogen testing. During a typical sampling day, the effort was divided equally among the plots to control for the effect of environmental factors such as temperature and relative humidity on the DON.
Sample Testing
Ixodes scapularis nymphs and Peromyscus mouse ear biopsies were stored in 70% ethanol at room temperature until DNA extraction. All tested nymphs were first identified with a taxonomic key (Lindquist et al. 2016). If the number of nymphs collected was ≤30, all ticks were tested, but when the number of nymphs sampled was >30 on a specific plot during a sampling period, a subsample of nymphs was randomly selected for testing. DNA was extracted with DNeasy 96 tissue kits (Qiagen, Hilden, Germany) or DNeasy Blood & Tissue kit (Qiagen) according to the manufacturer’s protocol. Samples were screened for B. burgdorferi using a multiplex real-time PCR following 2 different protocols. The first protocol was performed at the National Microbiology Laboratory (NML, Winnipeg, MB, Canada). First, samples were screened for Borrelia spp. with mRNA-23S gene in a duplex real-time quantitative polymerase chain reaction (qPCR) as Tokarz et al. (2017) and then, B. burgdorferi were discriminated from B. miyamotoi with a duplex assay with OspA gene as described by Courtney et al. (2004). Our research team developed a second protocol allowing us to perform tick testing locally due to limited laboratory capacity for tick testing at the NML during the COVID-19 pandemic. The complete description of this protocol and the PCR conditions are reported in the Supplementary materials. Briefly, samples were screened for Borrelia spp. with primers amplifying the 23s-rRNA gene as Courtney et al. (2004), and, if positives, subsequently tested for B. burgdorferi with primers amplifying the OspA gene as Tokarz et al. (2017). Results of the first PCR reaction were confirmed by the presence of a 75-bp band by agarose gel electrophoresis. The presence of B. burgdorferi was confirmed with a qPCR reaction using Universal SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) and the CFX Opus 96 real-time PCR system (Bio-Rad Laboratories). A sample was confirmed positive when the quantification threshold was reached within 30 amplification cycles and a melting peak was detected at 76 °C.
Statistical Analyses
Outcomes
The 3 parameters (PIM, DON, and NIP) measured to assess the effect of the treatment on B. burgdorferi endemic cycle were used as outcome variables for statistical modeling. To test the effects of the treatment on the PIM and the NIP, generalized linear models (GLM) with a binomial error distribution and with infection status as the outcome were built (respectively, models 1 and 3). For the DON, the number of nymphs collected per 100 m2 was analyzed with a negative binomial GLM to account for overdispersion (model 2).
Predictor of Treatment Effect
To evaluate the association between the 3 outcome variables and the effect of the treatment, we used the cumulative treatment variable. Cumulative Bait Density (CBD) represents the sum of annual bait densities administrated at a given location across all previous years. For example, plots from every zone during their first year in the study cumulated no treatment (CBD = 0.0 baits per 1,000 m2). However, plots of treated zones T1 and T2 cumulated 2.1 baits/1,000 m2 during their second year in the study. By the third year of the study, zone T1 plots cumulated 6.5 baits/1,000 m2 (2.1 plus 4.4) and plots of zone T2 plots cumulated 4.2 (2.1 plus 2.1). Being untreated controls, plots of zone C cumulated no treatment. In model 1 (exploring PIM), CBD was included in the analyses as a 3-level factor (0.0, 2.1, and 6.5 baits/1,000 m2) because mice were not captured in the fourth year of the study, whereas in models 2 (exploring NIP) and 3 (exploring DON) it was included as a 5-level factor (0.0, 2.1, 4.2, 6.5, and 10.9 baits/1,000 m2).
Covariates
To adjust the effect of the treatment for potential confounding factors, covariates were included in each model. In model 1, the calendar year (2016, 2017, or 2018), period (Pre-Tx or Tx), the zone of capture (C, T1, or T2), mouse age (subadult or adult), and sex (male or female) were tested as covariates. Mice were attributed to an age category based on weight: <17 g = subadult (aggregated category of juvenile and subadult) and ≥17 g = adult (Martell 1983, Linzey 1989). In model 2, the sampling zone (C, T1, or T2), the calendar year (2016, 2017, 2018, or 2019), the calendar month (May, June, July, or August), tick cohort (years 2016 and 2018, and years 2017 and 2019) and the sampling plot were tested as potential covariates. In model 3, covariates zone (C, T1, or T2), year (2016, 2017, 2018, or 2019), month (May, June, July, or August), and plot were tested. Spatial (zone and plot) and temporal (cohort, year, and period) covariates were included to control for unmeasured and related ecological factors that may confound treatment estimates. If included, the covariates plot was included as a random factor. The month variable was included to consider the sampling period difference between years, e.g., in the first year of the study, no sampling period for ticks was performed in May and August.
Model Selection and Fit
The 3 models followed the same selection methodology. First, univariate association between covariates and the outcomes were tested using χ² tests. Covariate with a significant univariate association (P < 0.2) with the outcome were included in multivariate analyses. If significantly associated with the outcome during univariate analyses, the covariate plot was included in the multivariate analyses as a random intercept in mixed GLM (GLMM). At the beginning of the multivariate modeling process, the full set of variables was tested for multicollinearity with variance inflation factor. Covariates presenting a variance inflation factor 95% confidence interval (CI95) including values > 10 were excluded. The final model was then selected using manual backward stepwise selection and log-likelihood ratio χ² test (LRT; P < 0.05). Model fit was evaluated with response and Pearson residual plots. Response residual uniformity and homoscedasticity were tested with Kolmogorov–Smirnov and quantile regression tests, respectively (P > 0.05). Goodness of fit of each model was evaluated with Pearson residual deviance χ² tests (P > 0.05). Residual spatial dependency was tested on the response residuals using Moran’s I test (P > 0.05).
Software
Statistical analyses were performed with R version 4.1.1 using packages DHARMa, emmeans, ggplot2, glmmTMB, lme4, and performance (Wickham 2009, Bates et al. 2015, Brooks et al. 2017, Hartig 2017, Lüdecke et al. 2021, R Core Team 2021).
Results
Prevalence of Infected Mice (Model 1)
A total of 282 Peromyscus spp. mice were captured and ear biopsies collected and tested for the presence of B. burgdorferi. The PIM over all the study was 51.8%. During the study period, the prevalence increased by 35.5% in zone C while it decreased by 17.0% in zone T1 (Table 1). Between 2017 and 2018, prevalence increased in zone T2 by 14.1% while it increased by 18.9% in zone C (Table 1). Concurrently, in zone T1, treated at the highest bait density, the PIM decreased by 1.4% (Table 1). The prevalence of B. burgdorferi in mice was higher during the capture period before application of the treatment in all zones (Table 1). Conversely, the observed prevalence decreased from 61.8% when mice were not exposed to treatment to 47.2% when they were exposed to a CBD of 2.1 baits/1,000 m2 and 38.6% for a CBD of 6.5 baits/1,000 m2.
Table 1.
Prevalence of B. burgdorferi-infected Peromyscus spp. mice per zone and period of capture
| Year | Zone | Deployed bait density | Cumulative bait density | Pre-Tx | Tx | Total | |||
|---|---|---|---|---|---|---|---|---|---|
| Prev. (%) [Bb+/n]a | Nb. of adults | Prev. (%) [Bb+/n]a | Nb. of adults | Prev. (%) [Bb+/n]a | Nb. of adults | ||||
| 2016 | C | 0.0 | 0.0 | 41.2 [7/17] | 5 | 41.2 [7/17] | 5 | ||
| T1 | 2.1 | 0.0 | 55.6 [10/18] | 14 | 55.6 [10/18] | 14 | |||
| 2017 | C | 0.0 | 0.0 | 65.4 [17/26] | 18 | 47.4 [9/19] | 9 | 57.8 [26/45] | 27 |
| T1 | 4.4 | 2.1 | 53.6 [15/28] | 18 | 22.7 [5/22] | 8 | 40.0 [20/50] | 26 | |
| T2 | 2.1 | 0.0 | 53.8 [7/13] | 8 | 30.8 [4/13] | 5 | 42.3 [11/26] | 13 | |
| 2018 | C | 0.0 | 0.0 | 83.3 [20/24] | 22 | 68.4 [13/19] | 16 | 76.7 [33/43] | 38 |
| T1 | 4.4 | 6.5 | 41.7 [10/24] | 14 | 35.0 [7/20] | 13 | 38.6 [17/44] | 27 | |
| T2 | 2.1 | 2.1 | 60.0 [12/20] | 17 | 52.6 [10/19] | 15 | 56.4 [22/39] | 32 | |
| Total | 60.0 [81/135] | 97 | 44.2 [65/147] | 85 | 51.8 [146/282] | 182 |
Nb. = number, Prev. = prevalence, Pre-Tx = mice captured before application of the treatment, Tx = mice captured during application of the treatment.
In 2016, there was not enough animal captured during the Pre-Tx period to allow data interpretation.
aThe number of B. burgdorferi-infected mice (Bb+) divided by the number of mice captured (n).
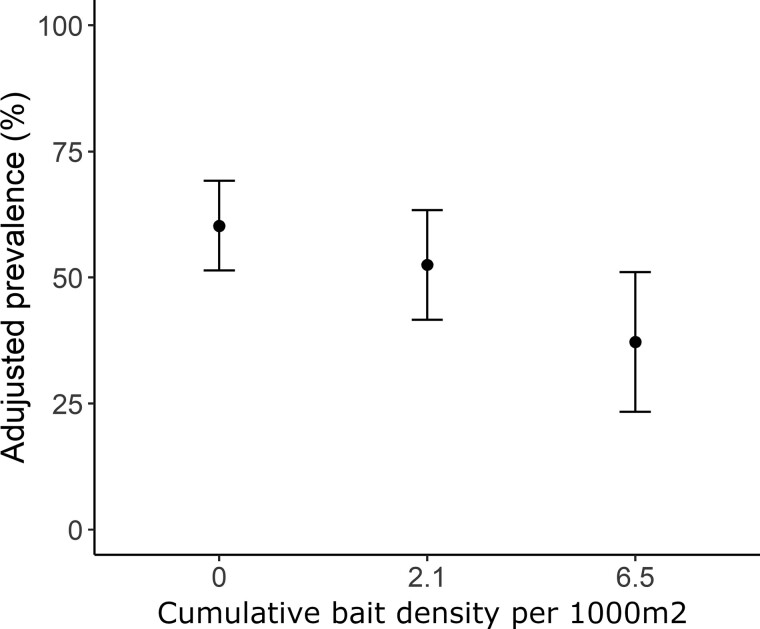
Among the potential covariates, the variables period, zone and age had significant univariate associations with the odds of a mouse being infected with B. burgdorferi (Supplementary Table S1). No covariate showed high multicollinearity (Supplementary Table S1). During multivariate analyses, the variable zone (LRT, 2.17, P = 0.338) and period (LRT, 3.13, P = 0.077) were sequentially excluded from the final model. Mice exposed to a CBD of 6.5 baits/1,000 m2 had significantly lower odds (OR: 0.37 [0.17 to 0.83]) of being infected compared with untreated mice (Table 2). In contrast, mice exposed to a CBD of 2.1 baits/1,000 m2 showed no significant effect of treatment (Table 2). The adjusted mouse infection prevalence predicted by the final model decreased from 60.3% [CI95: 51.4% to 69.2%] with no treatment to 52.5% [CI95: 41.6% to 63.4%] at a CBD value of 2.1 baits/1,000 m2 and to 37.2% [CI95: 23.4% to 51.0%] at the value of 6.5 baits/1,000 m2 (Figure 1).
Table 2.
Estimates (β) from the binomial GLMM built to investigate the relation between the treatment and Peromycus mouse infection by B. burgdorferi
| Variables | Categories | β (SE) | OR (CI 95%) | P value |
|---|---|---|---|---|
| Cumulative treatment (ref. 0) | 2.1 | −0.44 (0.32) | 0.64 (0.74 to 8.96) | 0.173 |
| 6.5 | −0.98 (0.41) | 0.37 (0.17 to 0.83) | 0.015 | |
| Age (ref. subadults) | Adults | 2.33 (0.34) | 10.36 (5.30 to 20.25) | <0.001 |
CI95 = 95% confidence interval, OR = odds ratio, SE = standard error.
Figure 1.
The effect of the cumulative bait density per 1,000 m2 between 2016 and 2018 on Peromyscus mouse infection by B. burgdorferi. Black points are the values predicted by the final binomial GLMM and error bars represent ± CI95.
Density of Questing Nymphs (Model 2)
A mean density of 4.79 nymphs per 100 m2 was collected over all site visits during the study (Table 3). The zone C, T1, and T2 had mean densities of 3.44, 5.60, and 5.64 nymphs/100 m2 over the study period, respectively. The cohorts of ticks that were questing as nymphs were more abundant during years 2017 and 2019 (4.73 nymphs/100 m2) than during years 2016 and 2018 (3.66 nymphs/100 m2). Observed DON decreased from 11.8 and 11.7 nymphs/100 m2 to 7.5 and 3.2 in zones T1 and T2 after being exposed to a bait density of 2.1 during their first year in the study, respectively (Table 3). The density remained low in both zones after this first reduction. In contrast, the DON was reduced by only 0.67 nymphs/100 m2 in zone C after its first year in the study (Table 3).
Table 3.
The density of I. scapularis nymphs and the prevalence of infected I. scapularis nymphs per year
| Year | Zone | Deployed bait density | Cumulative bait density | Nymphs | Effortsa | DON (nymphs/efforts) | NIP (%) [Bb+/tested]b |
|---|---|---|---|---|---|---|---|
| 2016 | C | 0.0 | 0.0 | 71 | 11.25 | 6.31 | 22.5 [16/71] |
| T1 | 2.1 | 0.0 | 142 | 12.00 | 11.83 | 12.7 [18/142] | |
| 2017 | C | 0.0 | 0.0 | 237 | 42.00 | 5.64 | 29.6 [34/115] |
| T1 | 4.4 | 2.1 | 317 | 42.00 | 7.54 | 30.0 [39/130] | |
| T2 | 2.1 | 0.0 | 251 | 21.50 | 11.67 | 32.9 [50/152] | |
| 2018 | C | 0.0 | 0.0 | 173 | 67.50 | 2.56 | 40.3 [48/119] |
| T1 | 4.4 | 6.5 | 215 | 67.50 | 3.19 | 33.5 [60/179] | |
| T2 | 2.1 | 2.1 | 155 | 48.25 | 3.21 | 26.2 [34/130] | |
| 2019 | C | 0.0 | 0 | 173 | 69.00 | 2.51 | 37.7 [57/151] |
| T1 | 4.4 | 10.9 | 391 | 68.75 | 5.69 | 35.1 [101/288] | |
| T2 | 2.1 | 4.2 | 266 | 49.50 | 5.37 | 36.0 [59/164] | |
| Total | 2391 | 499.25 | 4.79 | 31.4 [516/1641] |
DON = density of questing nymphs, NIP = prevalence of infected nymphs.
aThe total surface sampled in 100 m2.
bThe number of B. burgdorferi-infected nymphs (Bb+) divided by the number nymphs tested.
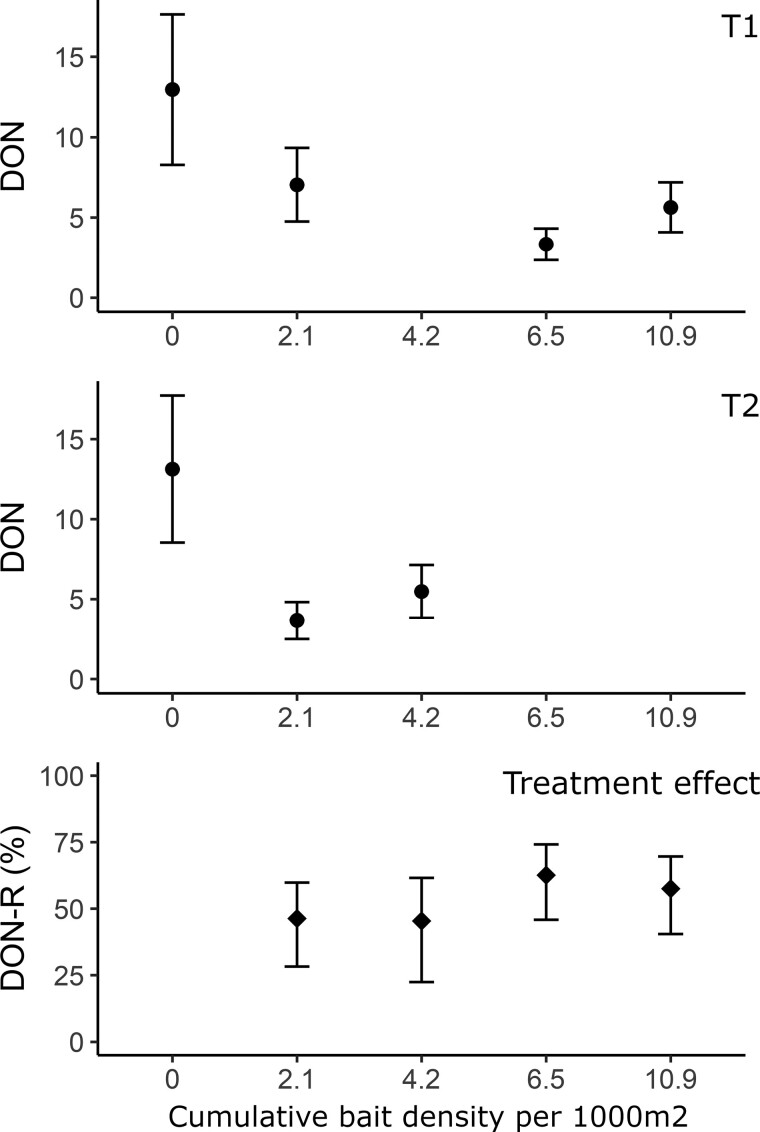
No potential covariates were excluded during univariate analysis, but the variable year was excluded at the beginning of the multivariate analysis due to high risk of multicollinearity (Supplementary Table S2). No variables were excluded during the backward selection. CBD was significantly associated with a reduction in DON (Table 4). After the first year of treatment and the deployment of a bait density of 2.1 baits/1,000 m2, the adjusted DON (nymphs/100 m2) decreased from 13.0 [CI95: 8.3 to 17.6] to 7.0 [CI95: 4.8 to 19.3] in plots of zone T1 and from 13.1 [CI95: 8.5 to 17.7] to 3.7 [CI95: 2.5 to 4.8] in zone T2 (Figure 2a and b). After this reduction, the DON in treated zones remains low compared to the first year, notwithstanding the increase in CBD (Figure 2a and b). The treatment, when compared to areas that received no treatment, was associated with a reduction of the DON (henceforth termed DON-R) between 45.4% [CI95: 22.4% to 61.6%] with a CBD of 4.2/1,000 m2 and 62.7% [45.9% to 74.2%] with a CBD of 6.5/1,000 m2 (Figure 2c). To explore whether the effect of the treatment increases as a function of the CBD, each treatment category was compared with its previous category using a Tukey adjusted post-hoc test. Only the CBD of 2.1 bait/1,000 m2 showed a significant DON-R (46.3% [CI95: 28.2% to 59.8%]) with the previous category (P < 0.001). Cumulative Bait Densities of 4.2 bait/1,000 m2 (P = 1.000), 6.5 bait/1,000 m2 (P = 0.515), and 10.9 bait/1,000 m2 (DON-R: 57.5% [CI95: 40.5% to 69.7%]; P = 0.944) were not significantly different.
Table 4.
Estimates of the negative binomial GLMM built to investigation the association between the density of I. scapularis nymphs and the treatment
| Variables | Categories | β (SE) | RD (CI 95%) | P value |
|---|---|---|---|---|
| Cumulative bait density (ref. 0) | 2.1 | −0.62 (0.14) | 0.54 (0.40 to 0.72) | <0.001 |
| 4.2 | −0.61 (0.18) | 0.54 (0.38 to 0.78) | <0.001 | |
| 6.5 | −0.98 (0.19) | 0.38 (0.26 to 0.54) | <0.001 | |
| 10.9 | −0.86 (0.17) | 0.42 (0.30 to 0.59) | <0.001 | |
| Zone (ref. C) | T1 | 0.84 (0.22) | 2.32 (1.49 to 3.56) | <0.001 |
| T2 | 1.13 (0.22) | 3.10 (2.01 to 4.76) | <0.001 | |
| Cohort (2016 and 2018) | 2017 and 2019 | 0.48 (0.10) | 1.61 (1.33 to 1.95) | <0.001 |
| Month (ref. May) | June | 3.15 (0.22) | 23.33 (15.33 to 35.52) | <0.001 |
| July | 2.98 (0.22) | 19.69 (12.49 to 29.96) | <0.001 | |
| August | 2.27 (0.22) | 9.68 (6.30 to 14.88) | <0.001 |
CI95 = 95% confidence interval, RD = relative density, SE = standard error.
Figure 2.
The effect of the cumulative bait density per 1000 m2 between 2016 and 2019 on the density of questing nymphs (DON). Upper and lower panels show the adjusted DON per 100 m2 ± CI95 predicted by the final GLMM in zone T1 and T2, respectively. Lower panel shows the DON reduction (DON-R) ± CI95 of cumulative treatment values. DON-R ± CI95 were computed by subtracting the null hypothesis (relative density (RD) between no treatment and cumulative treatment = 1) from each CBD values RD ± CI95 with no treatment (Table 4). Computed that way, it represents the proportion of DON-R for each cumulative treatment values in comparison with no treatment deployment.
Prevalence of Infected Nymphs (Model 3)
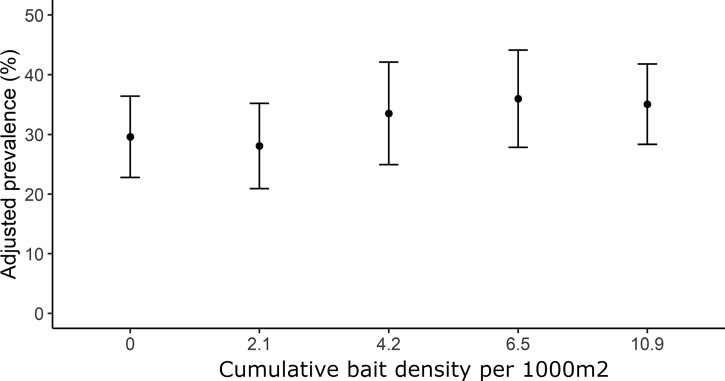
During the study, the NIP was at 31.4% with the lowest prevalence (16.0%) in 2016, and the highest (36.0%) in 2019 (Table 3). The prevalence increased in zone C and T1 between 2016 and 2017 but remained stable in all zones between 2017 and 2019 (Table 3). Overall, during the study period, prevalence in zones C, T1, and T2, was 29.4%, 29.5%, and 32.1%, respectively (Table 3). The variable zone was excluded during univariate analyses (Supplementary Table S3). No covariates showed high multicollinearity or were excluded during backward selection (Supplementary Table S3). The treatment showed no effect on NIP (Figure 3; Table 5).
Figure 3.
The effect of the cumulative bait density per 1,000 m2 between 2016 and 2019 on prevalence infection by B. burgdorferi in I. scapularis nymphs. Black dots and error bars are respectively the predicted values ± CI95 computed from the final GLMM.
Table 5.
Estimates of the binomial GLMM built to investigate the association between the prevalence of B. burgdorferi-infected I. scapularis nymphs and the treatment
| Variables | Categories | β (SE) | OR (CI 95%) | P value |
|---|---|---|---|---|
| Cumulative bait density (ref. 0) | 2.1 | −0.29 (0.43) | 0.75 (0.53 to 1.07) | 0.108 |
| 4.2 | −0.25 (0.18) | 0.78 (0.49 to 1.23) | 0.284 | |
| 6.5 | −0.03 (0.19) | 0.97 (0.62 to 1.52) | 0.883 | |
| 10.9 | −0.08 (0.17) | 0.92 (0.61 to 1.39) | 0.705 | |
| Year (ref. 2016) | 2017 | 1.02 (0.23) | 2.77 (1.78 to 4.32) | <0.001 |
| 2018 | 1.22 (0.25) | 3.38 (2.06 to 5.55) | <0.001 | |
| 2019 | 1.20 (0.26) | 3.32 (2.01 to 5.48) | <0.001 | |
| Month (ref. May) | June | −1.07 (0.39) | 0.34 (0.16 to 0.73) | 0.006 |
| July | −0.84 (0.39) | 0.43 (0.20 to 0.92) | 0.029 | |
| August | −1.14 (0.40) | 0.32 (0.15 to 0.71) | 0.005 |
CI95 = 95% confidence interval, OR = odds ratio, SE = standard error.
Discussion
In this study, we aimed to evaluate the effects of fluralaner treatment of small mammals on 3 parameters of the B. burgdorferi endemic cycle: (i) PIM, (ii) DON, and (iii) NIP. The treatment resulted in a significant reduction in mouse infection by B. burgdorferi after the application of 2.1 baits/1,000 m2 during the first year followed by the deployment of 4.4 baits/1,000 m2 the second year. The single application of the treatment at a bait density of 2.1 baits/1,000 m2 resulted in a reduction of 46% in the DON the following year. In the following years, the treatment reduced the DON by 45% to 68% but there was no significant effect due to cumulating more treatment.
The results also showed that the treatment was not associated with a reduction in the NIP. In the current literature, Mather et al. (1988) and Dolan et al. (2004) showed a reduction of the pathogen presence in ticks using an acaricide alone on small mammal populations. Dolan et al. (2004) administrated topical fipronil to small mammals during 3 yr in a residential community in southeastern Connecticut. The treatment was associated with a 45% to 96% reduction in I. scapularis larvae and nymphs feeding on P. leucopus, and a significant reduction of 16% in the NIP (Dolan et al. 2004). Mather et al. (1988) treated small mammals with permethrin, reducing infestation of P. leucopus mice by 97% in treated area and it resulted in a NIP reduction of 22%. In our experimental area, we previously showed that fluralaner baiting reduces infestation of Peromyscus spp. mice by 68% and 86% for larvae at 2.1 and 4.4 baits per 1,000 m2, respectively; and by 72% for nymphs at the highest bait density (Pelletier et al. 2022). Those results are similar to those of Dolan et al. (2004) yet did not result in a reduction in the NIP.
Infection of ticks by B. burgdorferi is mainly driven by the host community composition. It is plausible that transmission of B. burgdorferi to I. scapularis ticks varies between habitats or even between specific sites. While Dumas et al. (2022) and Mather et al. (1989) showed that most nymphs at their study sites get infected after feeding on Peromyscus mice, other studies highlighted the potential role of eastern chipmunks or shrews in the endemic cycle of B. burgdorferi (Telford et al. 1990, Slajchert et al. 1997, Brisson et al. 2008). Dumas et al. (2022) and Elias et al. (2011) also illustrated the contribution of birds to the endemic cycle of B. burgdorferi even if this contribution is smaller than that of Peromyscus mice. In Dumas et al. (2022), up to 20% of infected nymphs acquired their infection by feeding on birds. An absence of reduction in the NIP may be explained by a significant contribution of other competent reservoirs to the transmission of B. burgdorferi. As we do not know how the treatment is distributed among small mammal populations, a failure to effectively treat a significant proportion of the Peromyscus mouse reservoir host population is another hypothesis that could explain a lack of effect on NIP.
The distribution of I. scapularis larvae within the host population when the present study took place may explain the significant effect of the treatment on the DON. On this experimental site, the killing of 68% to 86% of larvae infesting Peromyscus mice (Pelletier et al. 2022) resulted in a significant reduction of questing nymphs in the following years. In this regard, it is plausible that Peromyscus mice feed an important fraction of I. scapularis larvae in the experimental plots during this study as shown by studies of Dumas et al (2022) and Mather et al (1989). The significant reduction in the DON is similar to the observations made by Deblinger and Rimmer (1991), Schulze et al. (2017), Jordan and Schulze (2019), Mandli et al. (2021), and Keesing et al. (2022). Keesing et al. (2022) deployed a topical fipronil treatment targeting small mammals and showed that the occurrence of questing nymphs was 50% lower for treated households. Nevertheless, the reduction in DON observed in the present study is lower than the one observed in previous studies (Deblinger and Rimmer 1991, Schulze et al. 2017, Jordan and Schulze 2019). For example, Jordan and Schulze (2019) deployed a topical permethrin treatment that resulted in a reduction of 79% to 84% in questing nymphs. This difference may be caused by different distributions of I. scapularis larvae on the host community between experimental sites. For example, in this study, the distribution of the treatment within the mouse population is unknown. Differences in the proportion of mice that consumed the treatment and received sufficiently high doses of acaricide could explain differences between studies. However, results from studies in this field remain difficult to compare directly due to differences in data processing, in experimental design, treatment application approach, and drugs’ mechanisms of action.
An effect of an acaricidal treatment alone on Peromyscus mouse infection by B. burgdorferi was also observed by Dolan et al. (2004). Their study showed that a lower number of young and untreated mice became infected when the treatment was deployed in the field (Dolan et al. 2004). The reduction of the PIM can occur through 2 mechanisms: (i) the treatment killed nymphs infesting Peromyscus mice quickly enough to impair B. burgdorferi transmission and (ii) it reduced the DON in the environment in such a way that rates of encounter of mice with infected nymphs were reduced. Results presented by Dolan et al. (2004) illustrate the first mechanism, i.e., the deployment of the treatment reduced the likelihood of B. burgdorferi transmission between I. scapularis nymphs and mice. The current study’s findings likely reflect the second mechanism. Unlike Dolan et al. (2004), our results suggest no immediate impact of the treatment on the PIM during treatment administration. However, a significant reduction in PIM was observed after 2 yr in zone T1, coinciding with a CBD of 6.5 baits/1,000 m2. Our observations also suggest that a significant reduction may have occurred in zone T1 after cumulating 2.1 baits/1,000 m2, but the effect observed is negated by an absence of effect in zone T2. It suggests an interaction between the environment and the effect of the treatment.
Differences in the treatment effects between studies may also be caused by factors related to study design and treatment administration protocols. In this study, we deliberately chose, as a first experimental step, to deploy the treatment at a small scale. It is plausible that mice or nymphs unexposed to the treatment were sampled in the area classified as treated, which contributed to mitigate the observed effect. In their study, Hinckley et al. (2021) showed no effect of a topical fipronil treatment on the DIT nor on pathogen infection rate or human-tick encounter. It was hypothesized that approaches targeting small mammals with acaricide may be sensitive to the spatial distribution and extent of the treatment, and that the deployment over broad geographic areas rather than small isolated patches may be more effective (Dolan et al. 2004, Hinckley et al. 2021). Another element of design that likely influenced the treatment effect is the intensity of treatment administration over time. In this study, the treatment was administrated between mid-July and the end of August. It is plausible that this timing was sufficient to kill an important fraction of feeding larvae, the future questing nymphs, but insufficient to prevent infection of mice by nymphs as shown by Dolan et al. (2004).
The fact that mouse biopsies and nymphs were not tested by the same analysis protocol is a limitation of this study as it may have mitigated the observed effect of the treatment. Overall, using 2 protocols may have caused a differential classification bias. About 78 out of 123 mouse biopsies with a CBD of 0.0 baits/1,000 m2 were tested using the NML protocol, whereas no biopsies with a CBD of 2.1 baits/1,000 m2 and all biopsies with a CBD of 6.5 baits/1,000 m2 were tested using this protocol. It is therefore plausible that use of 2 protocols may have reduced the difference between the CBD of 0.0 baits/1,000 m2 and the CBD of 2.1 baits/1,000 m2 while increasing the difference with the CBD of 6.5 baits/1,000 m2, but likely had no significant impact on the difference between CBD of 0.0 baits/1,000 m2 and 6.5. baits/1,000 m2. The fact that all nymphs tested in 2016 were tested by a different protocol may partly explain the difference in NIP between 2016 and 2017 to 2019. Of the 750 nymphs with a CBD of 0.0 baits/1,000 m2, 213 were tested with the NML protocol and only this CBD had nymphs tested with this protocol. There is little chance of any impact on the NIP results, however, as the covariate year, nesting the effect of having a different protocol, was included to adjust treatment estimates.
Several other factors may also affect effectiveness measurement and limit repeatability of experimental testing of the approach tested in this study. For example, populations of questing nymphs are highly susceptible to weather and the density of Peromycus spp. mouse populations are highly variable in nature (Ogden and Lindsay 2016, Krebs et al. 2018, Sullivan and Sullivan 2023). Measurement of treatment effect is also susceptible to the stochasticity inherent to tick collections. Accordingly, we preferred to include fewer sites but sampled them more intensively. However, this choice places limits on the generalizability of the study results to other ecological contexts.
Conclusion
This study tested the administration of an isoxazoline acaricide to rodent reservoirs of B. burgdorferi in the field and demonstrated a significant effect on the local endemic cycle of the bacterium. This effect was observed thought a reduction in the PIM and in the DON. A 45% to 63% reduction in the DON without a significant change in the NIP would likely translate to a 45% to 63% decrease in the Density of Infected Nymphs (DIN). While these results suggest that this approach has the potential to reduce the DIN, the primary environmental indicator of Lyme disease risk, the current study was performed at a small scale and in a homogeneous environment. To refine, operationalize, and comprehensively evaluate this approach, further studies in different habitats and public health intervention contexts are necessary. This will allow the assessment not only the effectiveness of isoxazoline administration for reducing DIN but also help in identifying any potential unintended ecological consequences of this approach (Wells and Collins 2022). Interventions aiming at reducing the DIT in the environment are intrinsically limited since they target only one component of Lyme disease risk (Eisen et al. 2012, Eisen 2021). Accordingly, evaluation of the efficacy of integrated interventions combining application of isoxazoline treatment of small mammals along with other preventive approaches targeting human behavior and other environmental drivers of the DIT is a promising avenue for future research.
Supplementary data
Supplementary data are available at Journal of Medical Entomology online.
Acknowledgments
We thank the Université de Montréal, the Institut national de santé publique du Québec (INSPQ) and the Natural Sciences and Engineering Research Council of Canada (Discovery Grant 03793-2014 to Patrick Leighton) for funding. We also thank the Natural Sciences and Engineering Research Council of Canada for a Doctoral Canadian Graduate Scholarship (CGS-D) supporting Jérôme Pelletier. We are very grateful to Robert Werbiski and Franck Siriex, from the Canadian Armed Forces, for their collaboration in making this study possible. We also thank all field assistants who participated in this study; this work would not have been possible without their dedicated efforts.
Contributor Information
Jérôme Pelletier, Département de pathologie et microbiologie, Faculté de médecine vétérinaire, Université de Montréal, Saint-Hyacinthe, Québec, Canada; Groupe de recherche en épidémiologie des zoonoses et santé publique, Faculté de médecine vétérinaire, Université de Montréal, Saint-Hyacinthe, Québec, Canada; Centre de recherche en santé publique de l’Université de Montréal et du CIUSSS du Centre-Sud-de-l’Île-de-Montréal, Université de Montréal, Montréal, Québec, Canada.
Catherine Bouchard, Groupe de recherche en épidémiologie des zoonoses et santé publique, Faculté de médecine vétérinaire, Université de Montréal, Saint-Hyacinthe, Québec, Canada; Public Health Risk Science Division, National Microbiology Laboratory, Public Health Agency of Canada, Saint-Hyacinthe, Québec, Canada.
Cecile Aenishaenslin, Département de pathologie et microbiologie, Faculté de médecine vétérinaire, Université de Montréal, Saint-Hyacinthe, Québec, Canada; Groupe de recherche en épidémiologie des zoonoses et santé publique, Faculté de médecine vétérinaire, Université de Montréal, Saint-Hyacinthe, Québec, Canada; Centre de recherche en santé publique de l’Université de Montréal et du CIUSSS du Centre-Sud-de-l’Île-de-Montréal, Université de Montréal, Montréal, Québec, Canada.
Antonia Dibernardo, One Health division, National Microbiology Laboratory, Public Health Agency of Canada, Winnipeg, Manitoba, Canada.
Gabrielle Dimitri Masson, Département de pathologie et microbiologie, Faculté de médecine vétérinaire, Université de Montréal, Saint-Hyacinthe, Québec, Canada; Groupe de recherche en épidémiologie des zoonoses et santé publique, Faculté de médecine vétérinaire, Université de Montréal, Saint-Hyacinthe, Québec, Canada.
Christopher Fernandez-Prada, Département de pathologie et microbiologie, Faculté de médecine vétérinaire, Université de Montréal, Saint-Hyacinthe, Québec, Canada; Groupe de recherché sur les maladies infectieuses en production animale, Faculté de Médecine Vétérinaire, Université de Montréal, Saint-Hyacinthe, Québec, Canada.
Simon Gagnon, Département de pathologie et microbiologie, Faculté de médecine vétérinaire, Université de Montréal, Saint-Hyacinthe, Québec, Canada; Groupe de recherché sur les maladies infectieuses en production animale, Faculté de Médecine Vétérinaire, Université de Montréal, Saint-Hyacinthe, Québec, Canada.
Ana Victoria Ibarra Meneses, Département de pathologie et microbiologie, Faculté de médecine vétérinaire, Université de Montréal, Saint-Hyacinthe, Québec, Canada; Groupe de recherché sur les maladies infectieuses en production animale, Faculté de Médecine Vétérinaire, Université de Montréal, Saint-Hyacinthe, Québec, Canada.
Robbin Lindsay, One Health division, National Microbiology Laboratory, Public Health Agency of Canada, Winnipeg, Manitoba, Canada.
Nicholas Ogden, Public Health Risk Science Division, National Microbiology Laboratory, Public Health Agency of Canada, Saint-Hyacinthe, Québec, Canada.
Jean-Philippe Rocheleau, Groupe de recherche en épidémiologie des zoonoses et santé publique, Faculté de médecine vétérinaire, Université de Montréal, Saint-Hyacinthe, Québec, Canada; Département de santé animale, Cégep de Saint-Hyacinthe, Saint-Hyacinthe, Québec, Canada.
Patrick Leighton, Département de pathologie et microbiologie, Faculté de médecine vétérinaire, Université de Montréal, Saint-Hyacinthe, Québec, Canada; Groupe de recherche en épidémiologie des zoonoses et santé publique, Faculté de médecine vétérinaire, Université de Montréal, Saint-Hyacinthe, Québec, Canada; Centre de recherche en santé publique de l’Université de Montréal et du CIUSSS du Centre-Sud-de-l’Île-de-Montréal, Université de Montréal, Montréal, Québec, Canada.
Author contributions
Jerome Pelletier (Conceptualization [lead], Data curation [lead], Formal analysis [lead], Investigation [lead], Methodology [lead], Project administration [lead], Software [lead], Visualization [lead], Writing—original draft [lead], Writing—review & editing [lead]), Catherine Bouchard (Conceptualization [lead], Data curation [supporting], Formal analysis [supporting], Funding acquisition [lead], Investigation [equal], Methodology [lead], Project administration [lead], Resources [equal], Software [equal], Supervision [lead], Validation [lead], Visualization [equal], Writing—original draft [equal], Writing—review & editing [equal]), Cecile Aenishaenslin (Conceptualization [equal], Investigation [equal], Methodology [equal], Writing—review & editing [equal]), Antonia Dibernardo (Methodology [supporting], Resources [supporting]), Gabriel Dimitri Masson (Investigation [equal], Methodology [equal], Project administration [supporting]), Christopher Fernandez-Prada (Formal analysis [supporting], Methodology [supporting], Resources [supporting]), Simon Gagnon (Formal analysis [supporting]), Ana Victoria Ibarra Meneses (Formal analysis [supporting], Methodology [supporting]), Robbin Lindsay (Conceptualization [lead], Methodology [lead]), Nicholas Ogden (Funding acquisition [lead], Investigation [lead], Methodology [lead]), Jean-Philippe Rocheleau (Conceptualization [lead], Formal analysis [lead], Funding acquisition [lead], Investigation [equal], Methodology [lead], Project administration [supporting], Supervision [lead], Validation [lead], Writing—original draft [lead], Writing—review & editing [lead]), and Patrick Leighton (Funding acquisition [lead], Supervision [lead], Writing—original draft [lead], Writing—review & editing [lead])
Data availability
Data are available on reasonable request.
References Cited
- Bates D, Maechler M, Bolker B, et al. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67(1):1–48. [Google Scholar]
- Bouchard C, Leonard E, Koffi JK, et al. 2015. The increasing risk of Lyme disease in Canada. Can. Vet. J. 56(7):693–699. 10.1051/vetres/2009019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C, Dibernardo A, Koffi JK, et al. 2019. Increased risk of tick-borne diseases with climate and environmental changes. Can. Commun. Dis. Rep. 45(4):81–89. 10.14745/ccdr.v45i04a02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourré-Tessier J, Milord F, Pineau C, et al. 2011. Indigenous Lyme disease in Quebec. J. Rheumatol. 38(1):183. [DOI] [PubMed] [Google Scholar]
- Brisson D, Dykhuizen DE, Ostfeld RS.. 2008. Conspicuous impacts of inconspicuous hosts on the Lyme disease epidemic. Proc. Biol. Sci. 275(1631):227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks ME, Kristensen K, van Benthem KJ, et al. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9(2):378–400. [Google Scholar]
- Brunner JL, LoGiudice K, Ostfeld RS.. 2008. Estimating reservoir competence of Borrelia burgdorferi hosts: prevalence and infectivity, sensitivity, and specificity. J. Med. Entomol. 45(1):139–147. 10.1603/0022-2585(2008)45[139:ercobb]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- Courtney JW, Kostelnik LM, Zeidner NS, et al. 2004. Multiplex real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J. Clin. Microbiol. 42(7):3164–3168. 10.1128/JCM.42.7.3164-3168.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblinger RD, Rimmer DW.. 1991. Efficacy of a permethrin-based acaricide to reduce the abundance of Ixodes dammini (Acari: Ixodidae). J. Med. Entomol. 28(5):708–711. 10.1093/jmedent/28.5.708 [DOI] [PubMed] [Google Scholar]
- Hartig F. DHARMa: residual diagnostics for hierarchical (multi-level / mixed) regression models. R packages version 0.1.5; 2017. http://florianhartig.github.io/DHARMa/
- Dolan MC, Maupin GO, Schneider BS, et al. 2004. Control of immature Ixodes scapularis (Acari: Ixodidae) on rodent reservoirs of Borrelia burgdorferi in a residential community of southeastern Connecticut. J. Med. Entomol. 41(6):1043–1054. [DOI] [PubMed] [Google Scholar]
- Donahue JG, Piesman J, Spielman A.. 1987. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am. J. Trop. Med. Hyg. 36(1):92–96. 10.4269/ajtmh.1987.36.92 [DOI] [PubMed] [Google Scholar]
- Dumas A, Bouchard C, Dibernardo A, et al. 2022. Transmission patterns of tick-borne pathogens among birds and rodents in a forested park in southeastern Canada. PLoS One 17(4):e0266527. 10.1371/journal.pone.0266527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L. 2021. Control of ixodid ticks and prevention of tick-borne diseases in the United States: the prospect of a new Lyme disease vaccine and the continuing problem with tick exposure on residential properties. Ticks Tick Borne Dis. 12(3):101649. 10.1016/j.ttbdis.2021.101649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L. 2023. Rodent-targeted approaches to reduce acarological risk of human exposure to pathogen-infected Ixodes ticks. Ticks Tick Borne Dis. 14(2):102119. 10.1016/j.ttbdis.2023.102119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, Dolan MC.. 2016. Evidence for personal protective measures to reduce human contact with blacklegged ticks and for environmentally-based control methods to suppress host-seeking blacklegged ticks and reduce infection with Lyme disease spirochetes in tick vectors and rodent reservoirs. J. Med. Entomol. 53(5):1063–1092. 10.1093/jme/tjw103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Piesman J, Zielinski-Gutierrez E, et al. 2012. What do we need to know about disease ecology to prevent Lyme disease in the northeastern United States? J. Med. Entomol. 49(1):11–22. 10.1603/me11138 [DOI] [PubMed] [Google Scholar]
- Elias SP, Smith RP Jr, Morris SR, et al. 2011. Density of Ixodes scapularis ticks on Monhegan Island after complete deer removal: a question of avian importation? J. Vector Ecol. 36(1):11–23. 10.1111/j.1948-7134.2011.00136.x [DOI] [PubMed] [Google Scholar]
- Gassel M, Wolf C, Noack S, et al. 2014. The novel isoxazoline ectoparasiticide fluralaner: selective inhibition of arthropod γ-aminobutyric acid- and L-glutamate-gated chloride channels and insecticidal/acaricidal activity. Insect Biochem. Mol. Biol. 45(1):111–124. 10.1016/j.ibmb.2013.11.009 [DOI] [PubMed] [Google Scholar]
- Gomes-Solecki M. 2014. Blocking pathogen transmission at the source: reservoir targeted OspA-based vaccines against Borrelia burgdorferi. Front. Cell. Infect. Microbiol. 4:136. 10.3389/fcimb.2014.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanincová K, Ogden NH, Diuk-Wasser M, et al. 2008. Fitness variation of Borrelia burgdorferi sensu stricto strains in mice. Appl. Environ. Microbiol. 74(1):153–157. 10.1128/AEM.01567-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley AF, Niesobecki SA, Connally NP, et al. 2021. Prevention of Lyme and other tickborne diseases using a rodent-targeted approach: a randomized controlled trial in Connecticut. Zoonoses Public Health. 68(6):578–587. 10.1111/zph.12844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbostel V, Ostfeld RS, Benjamin MA.. 2005. Effectiveness of Metarhizium anisopliae (Deuteromycetes) against Ixodes scapularis (Acari: Ixodidae) engorging on Peromyscus leucopus. J. Vector Ecol. 30(1):91–101. [PubMed] [Google Scholar]
- Jordan RA, Schulze TL.. 2019. Ability of two commercially available host-targeted technologies to reduce abundance of Ixodes scapularis (Acari: Ixodidae) in a residential landscape. J. Med. Entomol. 56(4):1095–1101. 10.1093/jme/tjz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing F, Mowry S, Bremer W, et al. 2022. Effects of tick-control interventions on tick abundance, human encounters with ticks, and incidence of tick-borne diseases in residential neighborhoods, New York, USA. . Emerg. Infect. Dis. 28(5):957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs CJ, Boonstra R, Kenney AJ, et al. 2018. Hares and small rodent cycles: a 45-year perspective on predator-prey dynamics in the Yukon boreal forest. Aust. Zool. 39(4):724–732. 10.7882/az.2018.012 [DOI] [Google Scholar]
- Leighton PA, Koffi JK, Pelcat Y, et al. 2012. Predicting the speed of tick invasion: An empirical model of range expansion for the Lyme disease vector Ixodes scapularis in Canada. J. Appl. Ecol. 49(2):457–464. 10.1111/j.1365-2664.2012.02112.x [DOI] [Google Scholar]
- Lindquist EE, Galloway TD, Artsob H, Lindsay LR, Dredot M, Wood H, Robbins RG.. A handbook to the ticks of Canada (Ixodida: Ixodidae, Argasidae). Canada: Biological Survey of Canada; 2016 [Google Scholar]
- Lindsay LR, Barker IK, Surgeoner GA, et al. 1997. Duration of Borrelia burgdorferi infectivity in white-footed mice for the tick vector Ixodes scapularis under laboratory and field conditions in Ontario. J. Wildl. Dis. 33(4):766–775. 10.7589/0090-3558-33.4.766 [DOI] [PubMed] [Google Scholar]
- Linzey AV. 1989. Response of the white-footed mouse (Peromyscus leucopus) to the transition between disturbed and undisturbed habitats. Can. J. Zool. 67(2):505–512. 10.1139/z89-073 [DOI] [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt KA, et al. 2003. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl. Acad. Sci. U.S.A. 100(2):567–571. 10.1073/pnas.0233733100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdecke D, Ben-Shachar MS, Patil I, et al. 2021. An R package for assessment, comparison and testing of statistical models. J. Open Sour. Softw. 6(60):3139. [Google Scholar]
- Mandli JT, Lee X, Bron GM, et al. 2021. Integrated tick management in south central Wisconsin: impact of invasive vegetation removal and host-targeted acaricides on the density of questing Ixodes scapularis (Acari: Ixodidae) nymphs. J. Med. Entomol. 58(6):2358–2367. 10.1093/jme/tjab131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell AM. 1983. 1983. Demography of southern red-backed voles (Clethrionomys gapperi) and deer mice (Peromyscus maniculatus) after logging in north-central Ontario. Can. J. Zool. 61(5):958–969. 10.1139/z83-129 [DOI] [Google Scholar]
- Mather TN, Ribeiro JMC, Moore SI, et al. 1988. Reducing transmission of Lyme disease spirochetes in a suburban setting. Ann. N. Y. Acad. Sci. 539(1):402–403. 10.1111/j.1749-6632.1988.tb31885.x [DOI] [Google Scholar]
- Mather TN, Wilson ML, Moore SI, et al. 1989. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi). Am. J. Epidemiol. 130(1):143–150. 10.1093/oxfordjournals.aje.a115306 [DOI] [PubMed] [Google Scholar]
- Ogden NH, Lindsay LR.. 2016. 2016. Effects of climate and climate change on vectors and vector-borne diseases: ticks are different. Trends Parasitol. 32(8):646–656. 10.1016/j.pt.2016.04.015 [DOI] [PubMed] [Google Scholar]
- Ogden NH, Lindsay LR, Beauchamp G, et al. 2004. Investigation of relationships between temperature and developmental rates of tick Ixodes scapularis (Acari: Ixodidae) in the laboratory and field. J. Med. Entomol. 41(4):622–633. 10.1603/0022-2585-41.4.622 [DOI] [PubMed] [Google Scholar]
- Ogden NH, Bigras-Poulin M, O’Callaghan, BIK, et al. 2005. A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. Int. J. Parasitol. 35(4):375–389. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Lindsay LR, Leighton PA.. 2013. Predicting the rate of invasion of the agent of Lyme disease Borrelia burgdorferi. J. Appl. Ecol. 50(2):510–518. 10.1111/1365-2664.12050 [DOI] [Google Scholar]
- Patrican LA. 1997. Absence of Lyme disease spirochetes in larval progeny of naturally infected Ixodes scapularis (Acari:Ixodidae) fed on dogs. J. Med. Entomol. 34(1):52–55. 10.1093/jmedent/34.1.52 [DOI] [PubMed] [Google Scholar]
- Pelletier J, Rocheleau J-P, Aenishaenslin C, et al. 2020. Evaluation of fluralaner as an oral acaricide to reduce tick infestation in a wild rodent reservoir of Lyme disease. Parasit. Vectors. 13(1):73. 10.1186/s13071-020-3932-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J, Rocheleau J-P, Aenishaenslin C, et al. 2022. Fluralaner baits reduce the infestation of Peromyscus spp. mice (Rodentia: Cricetidae) by Ixodes scapularis (Acari: Ixodidae) larvae and nymphs in a natural environment. J. Med. Entomol. 59(6):2080–2089. 10.1093/jme/tjac106 [DOI] [PubMed] [Google Scholar]
- Piesman J, Gern L.. 2004. Lyme borreliosis in Europe and North America. Parasitology. 129(Suppl):S191–S220. 10.1017/s0031182003004694 [DOI] [PubMed] [Google Scholar]
- R Core Team. 2021. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical. [Google Scholar]
- Rand PW, Lacombe EH, Smith RP Jr, et al. 1993. Competence of Peromyscus maniculatus (Rodentia: Cricetidae) as a reservoir host for Borrelia burgdorferi (Spirochaetares: Spirochaetaceae) in the wild. J. Med. Entomol. 30(3):614–618. [DOI] [PubMed] [Google Scholar]
- Richer LM, Brisson D, Melo R, et al. 2014. Reservoir targeted vaccine against Borrelia burgdorferi: a new strategy to prevent Lyme disease transmission. J. Infect. Dis. 209(12):1972–1980. 10.1093/infdis/jiu005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulison EL, Kuczaj I, Pang G, et al. 2013. 2013. Flagging versus dragging as sampling methods for nymphal Ixodes scapularis (Acari: Ixodidae). J. Vector Ecol. 38(1):163–167. 10.1111/j.1948-7134.2013.12022.x [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, Williams M, et al. 2017. Evaluation of the SELECT Tick Control System (TCS), a host-targeted bait box to reduce exposure to Ixodes scapularis (Acari: Ixodidae) in a Lyme disease endemic area of New Jersey. J. Med. Entomol. 54(4):1019–1024. 10.1093/jme/tjx044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slajchert T, Kitron UD, Jones CJ, et al. 1997. Role of the eastern chipmunk (Tamias striatus) in the epizootiology of Lyme borreliosis in northwestern Illinois, USA. J. Wildl. Dis. 33(1):40–46. 10.7589/0090-3558-33.1.40 [DOI] [PubMed] [Google Scholar]
- Sullivan TP, Sullivan DS.. 2023. Population fluctuations of the deer mouse (Peromyscus maniculatus) in old-field and bunchgrass-sagebrush habitats: the role of agricultural setting and optimum habitat. Ecologies. 4(2):406–425. 10.3390/ecologies4020026 [DOI] [Google Scholar]
- Telford SR 3rd, Mather TN, Adler GH, et al. 1990. Short-tailed shrews as reservoirs of the agents of Lyme disease and human babesiosis. J. Parasitol. 76(5):681–683. [PubMed] [Google Scholar]
- Tokarz R, Tagliafierro T, Cucura DM, et al. 2017. Detection of Anaplasma phagocytophilum, Babesia microti, Borrelia burgdorferi, Borrelia miyamotoi, and Powassan virus in ticks by a multiplex real-time reverse transcription-PCR assay. mSphere. 2(2):e00151–e00117. 10.1128/mSphere.00151-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao JI. 2009. Reviewing molecular adaptations of Lyme borreliosis spirochetes in the context of reproductive fitness in natural transmission cycles. Vet. Res. 40(2):36. 10.1051/vetres/2009019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao JI, Barbour AG, Luke CJ, et al. 2001. OspA immunization decreases transmission of Borrelia burgdorferi spirochetes from infected Peromyscus leucopus mice to larval Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 1(1):65–74. [DOI] [PubMed] [Google Scholar]
- Tsao JI, Wootton JT, Bunikis J, et al. 2004. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc. Natl. Acad. Sci. U.S.A. 101(52):18159–18164. 10.1073/pnas.0405763102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove O, De Buck E, Van Wijngaerden E.. 2019. Lyme disease in western Europe: an emerging problem? A systematic review. Acta Clin. Belg. 76(3):244–252. 10.1080/17843286.2019.1694293 [DOI] [PubMed] [Google Scholar]
- Wells C, Collins CMT.. 2022. A rapid evidence assessment of the potential risk to the environment presented by active ingredients in the UK’s most commonly sold companion animal parasiticides. Environ. Sci. Pollut Res. Int. 29(30):45070–45088. 10.1007/s11356-022-20204-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengenmayer C, Williams H, Zschiesche E, et al. 2014. The speed of kill of fluralaner (Bravecto™) against Ixodes ricinus ticks on dogs. Parasit Vectors. 7:525. 10.1186/s13071-014-0525-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham, H. ggplot2: elegant Graphics for Data Analysis. New York (USA): Springer-Verlag; 2009. [Google Scholar]
- Wolff JO. 1985. The effects of density, food, and interspecific interference on home range size in Peromyscus leucopus and Peromyscus maniculatus. Can. J. Zool. 63(11):2657–2662. 10.1139/z85-397 [DOI] [Google Scholar]
- Zhou X, Hohman AE, Hsu WH.. 2022. Current review of isoxazoline ectoparasiticides used in veterinary medicine. J. Vet. Pharmacol. Ther. 45(1):1–15. 10.1111/jvp.12959 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.