Abstract
Purpose:
Postoperative radiation therapy (RT) is an underused standard-of-care intervention for patients with prostate cancer and recurrence/adverse pathologic features after radical prostatectomy. Although stereotactic body RT (SBRT) is a well-studied and convenient option for definitive treatment, data on the postprostatectomy setting are extremely limited. The purpose of this study was to evaluate short-term physician-scored genitourinary (GU) and gastrointestinal (GI) toxicities and patient-reported outcomes after postprostatectomy SBRT.
Methods and Materials:
The SCIMITAR trial was a phase 2, dual-center, open-label, single-arm trial that enrolled patients with postoperative prostate-specific antigen >0.03 ng/mL or adverse pathologic features. Coprimary endpoints were 4-year biochemical recurrence–free survival, physician-scored acute and late GU and GI toxicities by the Common Terminology Criteria for Adverse Events (version 4.03) scale, and patient-reported quality-of-life (QOL) outcomes, as represented by the Expanded Prostate Cancer Index-26 and the International Prostate Symptom Score. Patients received SBRT 30 to 34 Gy/5 fractions to the prostate bed ± bed boost ± pelvic nodes with computed tomography (CTgRT) or magnetic resonance imaging guidance (MRgRT) in a nonrandomized fashion. Physician-scored toxicities and patient-reported QOL outcomes were collected at baseline and at 1, 3, and 6 months of follow-up. Univariable and multivariable analyses were performed to evaluate predictors of toxicities and QOL outcomes.
Results:
One hundred participants were enrolled (CTgRT, n = 69; MRgRT, n = 31). The median follow-up was 29.5 months (CTgRT: 33.3 months, MRgRT: 22.6 months). The median (range) prostate bed dose was 32 (30-34) Gy. Acute and late grade 2 GU toxicities were both 9% while acute and late grade 2 GI toxicities were 5% and 0%, respectively. Three patients had grade 3 toxicity (n = 1 GU, n = 2 GI). No patient receiving MRgRT had grade 3 GU or grade ≥2 GI toxicity. Compared with CTgRT, MRgRT was associated with a 30.5% (95% confidence interval, 11.6%-49.5%) reduction in any-grade acute GI toxicity (P = .006). MRgRT was independently associated with improved any-grade GI toxicity and improved bowel QOL.
Conclusions:
Postprostatectomy SBRT was well tolerated at short-term follow-up. MRgRT may decrease GI toxicity. Longer toxicity and/or efficacy follow-up and randomized studies are needed.
Introduction
For patients with biochemical recurrence after radical prostatectomy and those at high risk of biochemical recurrence, postoperative radiation therapy (RT) directed to the prostate bed and/or pelvic lymph nodes is recommended, with early salvage RT generally preferred.1–9 However, all forms of postoperative RT remain underused.10,11 This may in part be due to the protracted course of therapy, as conventional fractionation (1.8 Gy/fraction over 30-33 daily fractions) remains standard. This places both logistical and financial burdens on patients.12
For definitive RT, moderately hypofractionated RT (>2.4 Gy/fraction) and ultrahypofractionated RT (>5 Gy/fraction) are considered standard of care.5,13–17 Stereotactic body RT (SBRT) is a form of ultrahypofractionated RT that uses sophisticated radiation planning and delivery technologies to deliver ≤5 treatments. Potential SBRT benefits include leveraging radiobiology (with prostate cancer cells thought to experience greater death with high doses per fraction18,19), increased patient convenience, greater access to care, and lower health care costs.20–23 Whereas moderately hypofractionated postprostatectomy RT has shown favorable results in several phase 2 studies and 1 phase 3 study,24–35 postprostatectomy SBRT has only been evaluated in 2 small single-institution phase 1 studies.36,37
Despite an acceptable toxicity profile, concerns regarding the highly deformable and mobile prostate bed clinical target volume (which is adjacent to the bladder, rectum, and vesicourethral anastomosis), have precluded further study in the phase 2 setting. However, now a standard option in definitive treatment of intact prostate cancer, further exploration of postprostatectomy SBRT is warranted, particularly given the aforementioned advantages and the context of widespread underutilization. Patient-reported quality of life (QOL) may be affected by SBRT and needs to be preserved before SBRT can be adopted in the postprostatectomy setting. Moreover, the emerging technology of magnetic resonance imaging (MRI)–guided RT (MRgRT) offers several theoretical advantages over standard computed tomography (CT)–guided RT (CTgRT), including enhanced prostate bed visualization, precise visualization of the boundary between the prostate bed and surrounding organs at risk (OARs), the ability to track organ motion in real time, and the capacity to perform online adaptive planning.38
This multicenter phase 2 study was designed to evaluate postprostatectomy SBRT with prostate bed doses of 30 to 34 Gy in 5 fractions in terms of oncologic efficacy, physician-scored toxicity, and patient-reported outcomes. We also prespecified an exploratory comparison of toxicity profiles for patients treated with CTgRT versus MRgRT.
Methods and Materials
Study design
The Stereotactic Intensity Modulated Radiotherapy After Radical Prostatectomy (SCIMITAR) trial was a dual-center (University of California, Los Angeles [UCLA] and University of Southern California [USC], Los Angeles, California, USA) phase 2 single-arm trial activated in February 2018 (NCT03541850) that evaluated the toxicity, QOL, and treatment efficacy of postoperative SBRT for prostate cancer. The study was approved by the institutional research boards of the participating centers. All participants were provided written informed consent forms before trial enrollment in a manner that was consistent with the Declaration of Helsinki.39 Participation was entirely voluntary with no compensation or incentive offered.
Study endpoints
The primary endpoint of the study was the efficacy of SBRT in the postoperative setting, defined as 4-year biochemical recurrence–free survival (BCRFS). A coprimary endpoint was physician-scored toxicity, represented by the rates of acute (<90 days post-SBRT) and late (≥90 days after SBRT) genitourinary (GU) and gastrointestinal (GI) toxicity per the Common Terminology Criteria for Adverse Events (CTCAE), version 4.03. Physicians were not blinded to the radiation platform assignment (ie, CTgRT vs MRgRT). A second coprimary endpoint was patient-reported outcome profiles, as represented by changes in the urinary incontinence, urinary irritative/obstruction, bowel, and sexual function domains of the Expanded Prostate Cancer Index-26 (EPIC-26)40 QOL instrument and longitudinal changes in the International Prostate Symptom Score (IPSS).41 A comparison of the toxicity and QOL profiles between patients treated with CTgRT versus MRgRT was a prespecified exploratory analysis. This publication focuses on the toxicity and patient-reported QOL-related primary endpoints and exploratory endpoint.
Eligibility
Eligible patients must have had a history of clinical localized adenocarcinoma of the prostate treated with radical prostatectomy. Additionally, patients must have had at least one of the following: (1) adverse pathologic features at the time of prostatectomy (positive surgical margin, pathologic T3/T4 disease, pathologic Gleason score 8-10 disease or presence of tertiary Gleason grade 5 disease), (2) rising prostate-specific antigen (PSA) on at least 2 consecutive measurements, with both >0.03 ng/mL, or (3) a Decipher genomic classifier score42 >0.45. A CT scan, MRI of the pelvis and a bone scan within 120 days before enrollment were required. Positron emission tomography was strongly encouraged.
Postoperative SBRT
Before the commissioning of an MRI linear accelerator at UCLA in March 2020, all patients were treated with CTgRT using a NovalisTx or TrueBeam (Varian, Inc, Palo Alto, CA). Subsequent to that, all patients treated at UCLA were offered treatment on the 0.35T MRI-guided MRIdian (ViewRay, Inc, Mountain View, CA); of these, 4 of 37 (11%) declined due to claustrophobia (n = 2) or pacemaker (n = 2). All USC patients were treated with CTgRT. Pelvic nodal RT and 6 months of androgen-deprivation therapy (ADT) were used at the discretion of the treating physician. Specific bladder and rectum preparation protocols, described previously, were in place for simulation and for each fraction.43,44 A planning CT was performed with patients immobilized with a custom vacuum lock bag. Patients treated with MRgRT underwent an additional 0.35T MRI simulation as previously described.45 Clinical target volumes (CTV) of the prostate bed (CTVPB) and pelvic lymph node volume (CTVN, when treated) were delineated in accordance with the Radiation Therapy Oncology Group (RTOG) consensus guideline.46 If gross tumor in the prostate bed or the pelvic lymph nodes was visible on preradiation imaging, it was contoured as gross tumor volume (GTVboost).
CTVPB was expanded isotopically by 5 mm (CTgRT) or 3 mm (MRgRT) to form the corresponding planning target volumes (PTVPB); expansions for CTVN and GTVboost were generally 5 and 3 mm, respectively, regardless of platform. Plans were designed to deliver 30 to 34 Gy, 25 Gy, and 35 to 40 Gy in 5 fractions to PTVPB, PTVN, and PTVboost, respectively, such that 95% of each PTV received prescription dose, unless doing so would lead to violations of OAR constraints (Appendix E1 Trial Protocol). Sample CTgRT and MRgRT plans are shown in Figs. E1 and E2. RT was delivered every other day. For CTgRT volumetric modulated arc therapy was used with a kilovoltage cone beam CT acquired before each treatment to verify anatomy. For MRgRT, 13 to 17 static gantry intensity modulated RT fields were used, and a set-up MRI was acquired before each treatment. During MRgRT delivery, sagittal cine MRI images were acquired at 4 frames per second to track the anterior rectal wall motion in real time. A gating boundary of 3 mm around the anterior rectal wall was set such that if >10% of the volume moved outside this window, beam delivery was automatically paused. Online adaptive RT was delivered in a minority of MRgRT fractions (4 fractions or 2.5%) owing to staffing restrictions during the COVID-19 pandemic in the setting of unclear clinical benefit.
Assessments
Physician-scored toxicity and patient-reported outcomes were collected at baseline, at 1 and 3 months after treatment completion, at every 3 months for the first year after treatment, and then every 6 months for a minimum of 4 years after treatment. PSA was drawn every 3 months for the first year, then every 6 months until 4 years after SBRT. Beyond 4 years, patients were assessed on a yearly basis.
Statistical analysis
Descriptive analysis was conducted for all patients, with medians, interquartile ranges, and ranges calculated for continuous variables and proportions calculated for categorical variables. Patient responses on the EPIC-26 questionnaires were scored as per scoring manual and were summarized and graphed by mean and 95% confidence interval (CI) of change in scores at each follow-up visit compared with the baseline. Minimally clinically important difference values of 6, 5, 4, and 10 points for the urinary incontinence domain, urinary irritative/obstructive domain, bowel domain, and sexual domain, respectively, were used in accordance with prior literature.47 χ2 tests were used to assess the association of SBRT platform and frequency of toxicity by grade. The Mann-Whitney test was used to compare baseline toxicity grade frequencies between the SBRT platforms. Wilcoxon matched-pairs signed rank test was used to compare EPIC-26 subdomain and IPSS scores at various time points to the baseline.
Univariable analyses were performed to evaluate associations between the selected variables (Tables E10–17) and physician-scored GU, GI, and/or sexual toxicity within 6 months of SBRT. Logistic regression was used to develop multivariable prediction models for any-grade GI and GU toxicity and clinically relevant (1 × minimally clinically important difference) change in EPIC-26 bowel domain within the first 6 months. Covariates were selected by a combination of clinical relevance and/or significance on univariable analyses. The analyses reported were specified a priori. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Statistical significance was set at a 2-sided P value of <.05.
Results
Patients
From February 2018 to March 2021, 108 patients were screened and enrolled in the SCIMITAR trial at UCLA (100 patients) and USC (8 patients). Eight patients withdrew from the study before starting treatment, thus leaving 100 patients (median age, 69 years; range, 50-82) who completed the study treatment (Fig. 1). Baseline demographic and clinical characteristics as well as treatment parameters are shown in Table 1. Sixty-nine (69%) patients underwent CTgRT while 31 (31%) received MRgRT. The median pre-SBRT PSA was 0.3 ng/mL (range, 0.0-9.3 ng/mL) and 76% of patients received advanced imaging. The median prostate bed dose was 32 Gy (range, 30-34 Gy). Twenty-seven (27%) patients received a prostate bed boost, 27 (27%) received elective pelvic nodal RT, and 5 (5%) received a boost to gross pelvic nodes. The median follow-up was 29.5 months (33.3 months for CTgRT and 22.6 months for MRgRT). Three patients died during the follow-up period, all unrelated to prostate cancer treatment (n = 1 each from congestive heart failure, myocardial infarction, and intracranial hemorrhage).
Fig. 1.
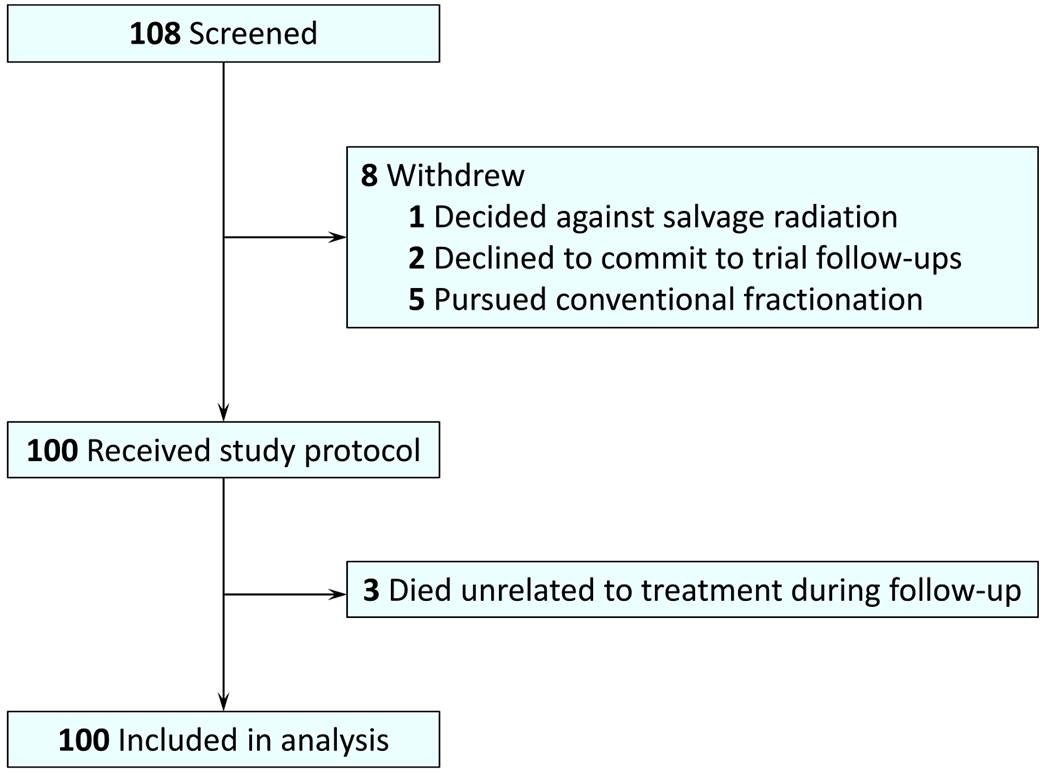
Flow diagram of patient accrual and analysis.
Table 1.
Baseline characteristics and treatment parameters
| Overall cohort (n = 100) | CTgRT (n = 69) | MRgRT (n = 31) | P value | |
|---|---|---|---|---|
| Age (y), median (range) | 69 (50-82) | 69 (52-79) | 68 (50-82) | .8573 |
| Race | .4070 | |||
| Black | 9 (9.0%) | 5 (7.2%) | 4 (12.9%) | |
| White | 85 (85.0%) | 60 (87.0%) | 25 (80.6%) | |
| Asian | 3 (3.0%) | 2 (2.9%) | 1 (3.2%) | |
| Other | 2 (2.0%) | 2 (2.9%) | 0 (0%) | |
| Unknown | 1 (1.0%) | 0 (0%) | 1 (3.2%) | |
| PSA at initial diagnosis (ng/mL), median (range) | 8.6 (2.7-78.9) | 8.0 (2.7-78.3) | 9.8 (3.1-78.9) | .2094 |
| Pathologic Gleason GG | .5138 | |||
| I | 2 (2.0%) | 2 (2.9%) | 0 (0%) | |
| II | 35 (35.0%) | 22 (31.9%) | 13 (41.9%) | |
| III | 32 (32.0%) | 21 (30.4%) | 11 (35.5%) | |
| IV | 15 (15.0%) | 13 (18.8%) | 2 (6.5%) | |
| V | 14 (14.0%) | 10 (14.5%) | 4 (12.9%) | |
| Treatment effect* | 2 (2.0%) | 1 (1.4%) | 1 (3.2%) | |
| Pathologic T stage and adverse pathologic features | ||||
| pT2 | 44 (44.0%) | 33 (47.8%) | 11 (35.5%) | .0130 |
| pT3a (ECE) | 33 (33.0%) | 16 (23.2%) | 17 (54.8%) | |
| pT3b (SVI) | 22 (22.0%) | 19 (27.5%) | 3 (9.7%) | |
| pT4 | 1 (1.0%) | 1 (1.4%) | 0 (0%) | |
| Bladder neck involvement | 10 (10.0%) | 7 (10.1%) | 3 (9.7%) | 1.0 |
| Positive margin | 39 (39.0%) | 26 (37.7%) | 13 (41.9%) | .6867 |
| Tertiary grade 5 | 17 (17%) | 14 (20.2%) | 3 (9.7%) | .2555 |
| Baseline sexual function | .2938 | |||
| Very good/good | 16 (16.0%) | 13 (18.8%) | 3 (9.7%) | |
| Fair | 14 (14.0%) | 11 (15.9%) | 3 (9.7%) | |
| Poor/very poor | 70 (70.0%) | 45 (65.2%) | 25 (80.6%) | |
| Baseline daily pads use | .1669 | |||
| None | 62 (62.0%) | 43 (62.3%) | 19 (61.3%) | |
| 1 | 19 (19.0%) | 16 (23.2%) | 3 (9.7%) | |
| 2 | 11 (11.0%) | 5 (7.2%) | 6 (19.4%) | |
| ≥3 | 8 (8.0%) | 5 (7.2%) | 3 (9.7%) | |
| Advanced imaging | .0006 | |||
| PSMA PET/CT | 44 (44.0%) | 34 (49.3%) | 10 (32.3%) | |
| 18F-Fluciclovine PET/CT | 32 (32.0%) | 14 (20.3%) | 18 (58.1%) | |
| None | 24 (24.0%) | 21 (30.4%) | 3 (9.7%) | |
| Pre-SBRT PSA | ||||
| Mean | 0.8 | 0.7 | 0.8 | .9926 |
| Median (range) | 0.3 (0.0-9.3) | 0.3 (0.0-6.1) | 0.3 (0.1-9.3) | |
| 0-0.2 | 38 (38.0%) | 26 (37.7%) | 12 (38.7%) | |
| 0.21-0.49 | 30 (30.0%) | 18 (26.1%) | 12 (38.7%) | |
| 0.50-1.0 | 13 (13.0%) | 11 (15.9%) | 2 (6.5%) | |
| >1.0 | 19 (19.0%) | 14 (20.3%) | 5 (16.1%) | |
| Time from prostatectomy to SBRT (mo), median (range) | 22.8 (3.8-184.1) | 20.3 (3.9-184.1) | 25.4 (3.8-178.8) | .6050 |
| SBRT parameters | ||||
| Prostate bed dose (Gy), median (range) | 32 (30-34) | 32 (30-34) | 32 (30-34) | .4930 |
| Prostate bed boost use | 27 (27.0%) | 18 (26.1%) | 9 (29.0%) | .7590 |
| Prostate bed boost dose (Gy), median (range) | 40 (36.25-40) | 40 (36.25-40) | 40 (36.25-40) | .0933 |
| Elective nodal RT use | 27 (27.0%) | 24 (34.8%) | 3 (9.7%) | .0136 |
| Elective nodal RT dose (Gy), median (range) | 25 (25-25) | 25 (25-25) | 25 (25-25) | 1.0 |
| Gross node boost use | 5 (5.0%) | 5 (7.2%) | 0 (0%) | .3204 |
| Gross node boost dose (Gy), median (range) | 40 (35-40) | 40 (35-40) | - | - |
| Concurrent ADT | ||||
| ADT use | 41 (41.0%) | 25 (36.2%) | 16 (51.6%) | .1481 |
| Duration (mo), median (range) | 6 (1-8) | 6 (1-6) | 6 (4-8) | .1720 |
Data are presented as n (%) unless otherwise indicated.
Abbreviations: ADT = androgen deprivation therapy; CT = computed tomography; CTgRT = CT-guided radiation therapy; ECE = extraprostatic extension; GG = grade group; MRgRT = MRI-guided radiation therapy; MRI = magnetic resonance imaging; PET = positron emission tomography; PSA = prostate-specific antigen; PSMA = prostate-specific membrane antigen; SBRT = stereotactic body radiation therapy; SVI = seminal vesicle invasion.
No Gleason score was reported in the surgical pathology specimen due to treatment effect from neoadjuvant androgen deprivation therapy. If a patient underwent both a PSMA PET/CT and a fluciclovine F18 PET/CT, only PSMA PET/CT was counted. P values apply to the comparison between CTgRT and MRgRT cohorts, calculated using the Wilcoxon rank sum test for continuous variables and the χ2 test or Fisher exact test when appropriate for categorical variables.
Physician-scored CTCAE toxic effects
Physician-scored acute and late GU and GI toxicities are shown in Fig. 2A–B. Detailed breakdown by time points and symptoms is shown in Tables E1 and E2. The majority of worst acute toxicity events were grade 1 for GU and GI toxicity, at 43% and 57%, respectively. Late grade 1 GU and GI toxicity rates were 40% and 34%, respectively. Worst acute grade 2 GU and GI toxicity rates were 9% and 5%, respectively. The incidences of late grade 2 GU and GI toxicities were 9% and 0%, respectively. No grade ≥2 GI toxicity events were seen in patients treated with MRgRT. Grade 3 toxicity occurred in 3 patients, with one experiencing grade 3 acute and late GU toxicity and one each with grade 3 acute and late GI toxicity, all treated with CTgRT.
Fig. 2.
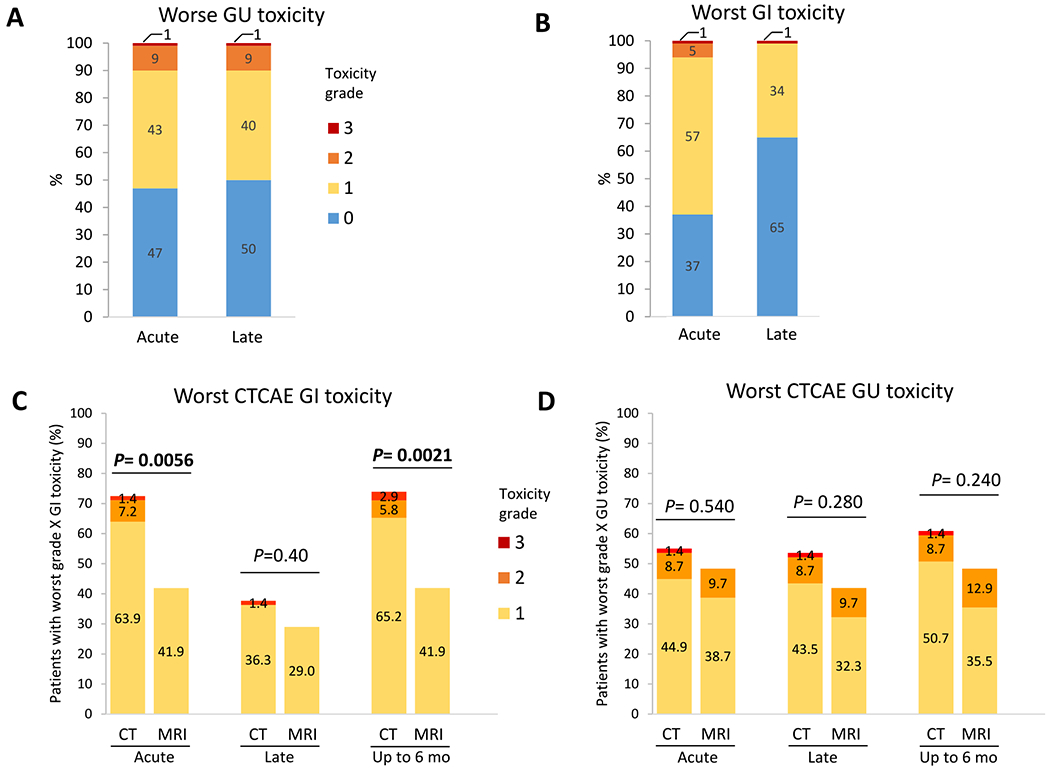
(A-D) Physician-scored acute and late genitourinary (GU) and gastrointestinal (GI) toxicities and breakdown by radiation delivery platforms. Physician-score toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. P values in (C) and (D) apply to between-platform comparisons (computed tomography– [CT] vs magnetic resonance imaging (MRI)–guided stereotactic body radiation therapy [SBRT]) of any-grade toxicities at the specified period. P values were calculated with the χ2 test, and those <.05 are bolded. Acute toxicities are side effects possibly, probably, or definitely related to radiation treatment within 90 days of SBRT, whereas late toxicities are those occurring 90 to 180 days after SBRT.
One patient (1%) developed grade 3 hematuria due to radiation cystitis 1 month after SBRT, which persisted with medical management and was ultimately successfully treated with hyperbaric oxygen therapy. One patient (1%) had severe grade 3 acute GI toxicity (diarrhea) 1 week after the completion of SBRT, which resolved with conservative medical management. A third patient, who was on immunosuppressive medications, developed grade 2 radiation proctitis 6 months after SBRT. A colonoscopy was performed at the 9-month mark, which revealed ulcerations that were aggressively biopsied by a specialist not affiliated with either trial institution. This showed histoplasmosis, rather than radiation effect, and the patient received a diagnosis of disseminated histoplasmosis, which he was estimated to have acquired due to occupational exposure. After extensive multidisciplinary review, this was scored as a grade 3 GI toxicity with respect to SBRT in the interest of conservatively estimating toxicity.
A comparison of the CTgRT and MRgRT with respect to physician-scored toxicity is shown in Fig. 2C–D, Tables E3 to E8, and Fig. E3. Compared with CTgRT, MRgRT was associated with significantly lower rates of any-grade acute GI toxicity (41.9% vs 72.5%, P = .006, or an estimated absolute reduction of 30.5% [95% CI, 11.6%-49.5%]) and significantly lower rates of any-grade GI toxicity of up to 6 months after SBRT (41.9% vs 73.9%, P = .002, or an estimated absolute reduction of 32% [95% CI, 12.9%-51.1%]). Late any-grade GI toxicity was 37.7% for CTgRT and 29.0% for MRgRT (P = .4). Though MRgRT was associated with numerically lower rates of acute (48.4% vs 55.0%, P = .54), late (42.0% vs 53.6%, P = .28) and up to 6 months (48.4% vs 60.8%, P = .24) any-grade GU toxicity, none of these differences were statistically significant.
Patient-reported outcomes and QOL
Figure 3 and Table E9 show changes in EPIC-26 and IPSS scores over time while Fig. E4 shows the proportion of patients with a clinically relevant deterioration in EPIC-26 domains over time. There was a consistent decline in EPIC-26 urinary irritative/obstructive domain score (mean, −2.9; 95% CI, −4.9 to −0.9; P = .049) and increase in IPSS sum scores (IPSS_S) (mean, 1.2; 95% CI, 0.2-2.2; P = .007) 1 month after SBRT; both normalized by 3 months post-SBRT. No statistically significant deterioration in the urinary irritative/obstructive domain was seen at any time in patients treated with MRgRT, and no significant changes in urinary incontinence or the IPSS QOL summary scale were seen with either platform. A statistically significant decline in the EPIC-26 bowel domain score was observed at 1 month (mean, −8.6; 95% CI, −12.4 to −4.8; P < .0001), 3 months (mean, −2.6; 95% CI, −4.8 to −0.4; P = .02) and 6 months (mean, −4.6; 95% CI, −8.4 to −0.8; P = .04). However, the proportion of patients with clinically relevant deterioration improved from 1 month to 6 months, with the clinically relevant deterioration resolving earlier in men receiving MRgRT. EPIC-26 sexual domain scores were unchanged over time in patients without ADT.
Fig. 3.
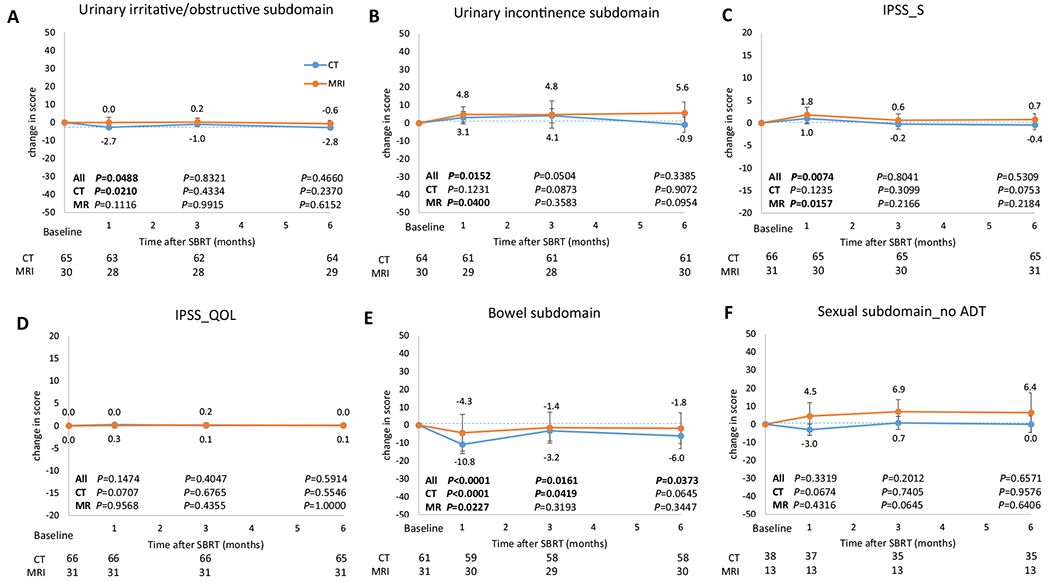
Changes from baseline in Expanded Prostate Cancer Index composite (EPIC-26) subdomains and International Prostate Symptom Score (IPSS). (A, B, E, F) Derived from the EPIC-26 questionnaire. (C, D) Derived from the IPSS questionnaire. Data represent subdomain median scores, with error bars showing 95% confidence intervals. Scores are change from baseline, with 0 representing no change. IPSS_S is the sum score of the first 7 questions (incomplete emptying, frequency, intermittency, urgency, weak stream, and nocturia) in the IPSS questionnaire. Higher numbers denote worse urinary symptoms. IPSS_QOL is the score of the quality-of-life question related to urinary symptoms in the IPSS questionnaire. A score of 0 is “delighted” and 6 is “terrible.” P values comparing the scores at baseline and at corresponding time points (1, 3, and 6 months after stereotactic body radiation therapy [SBRT]) in the overall cohort and computed tomography (CT)–guided (CTgRT) and magnetic resonance imaging (MRI)–guided SBRT (MRgRT) groups are shown inside the graphs. Bolded P values denote statistically significant differences compared with baseline. P values were calculated using the Wilcoxon matched-pairs signed rank test. The number of patients who completed the questionnaire at each time point are recorded below each graph. Abbreviation: ADT = androgen deprivation therapy.
Predictors of physician-scored toxicities and patient-reported outcomes
We performed univariable (Tables E10–E17) and multivariate analyses (Table 2) to evaluate predictors of toxicity and QOL outcomes. In the univariable analysis, baseline IPSS score (P = .010), elective nodal RT (P < .001) and baseline urinary pads use (P = .02) were significantly associated with any-grade GU toxicities within 6 months of SBRT. In the multivariable analysis, elective nodal RT (odds ratio [OR], 10.30; 95% CI, 2.56-41.43; P = .001) and baseline urinary pads use (OR, 2.78; 95% CI, 1.02-7.61; P = .046) continued to be significant predictors. CTgRT (P = .002), elective nodal RT (P = .03), prostate bed boost (P = .04), and baseline EPIC-26 bowel domain score (P = .02) were significantly associated with any-grade GI toxicity within the first 6 months in the univariable analysis while CTgRT was the only significant predictor (OR, 3.71; 95% CI, 1.38-9.99; P = .010) in the multivariable analysis. Similarly, CTgRT (OR, 3.02; 95% CI, 1.12-8.17; P = .03) and baseline EPIC-26 bowel score (OR, 1.08; 95% CI, 1.00-1.16; P = .047) were significant predictors of clinically relevant decline in EPIC-26 bowel scores within 6 months of SBRT.
Table 2.
Multivariable analysis of predictors of any CTCAE grade GU, GI, and clinically relevant deterioration in EPIC-26 bowel domain score within 6 months of SBRT
| Variable | Any CTCAE grade GU toxicity | Any CTCAE grade GI toxicity | 1 × MCID in EPIC-26 bowel score | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| RT platform (CT-versus MRI-guided SBRT) | 1.18 | 0.44-3.16 | .7475 | 3.71 | 1.38-9.99 | 0.0095 | 3.02 | 1.12-8.17 | .0290 |
| Elective nodal RT (yes versus no) | 10.30 | 2.56-41.43 | .0010 | 3.09 | 0.86-11.12 | 0.0840 | 0.65 | 0.23-1.81 | .4077 |
| Prostate bed boost (yes versus no) | - | - | - | 0.37 | 0.12-1.15 | 0.0858 | 1.54 | 0.52-4.58 | .4326 |
| Time from RP to SBRT (1-mo increase) | 1.00 | 0.99-1.01 | .7033 | - | - | - | - | - | - |
| Baseline pad use (yes versus no) | 2.78 | 1.02-7.61 | .0462 | - | - | - | - | - | - |
| Baseline IPSS score (1-unit increase) | 1.12 | 1.00-1.25 | .0544 | - | - | - | - | - | - |
| Baseline EPIC-26 bowel score (1-unit increase) | - | - | - | 0.95 | 0.89-1.02 | 0.1428 | 1.08 | 1.00-1.16 | .0474 |
Values in boldface are statistically significant.
Abbreviations: CI = confidence interval; CTCAE = Common Terminology Criteria for Adverse Events; EPIC-26 = Expanded Prostate Cancer Index-26; GI = gastrointestinal; GU = genitourinary; IPSS = International Prostate Symptom Score; MCID = minimal clinically important difference; OR = odds ratio; RP = radical prostatectomy; RT = radiation therapy; SBRT = stereotactic body RT.
Discussion
The present study, which is the largest prospective study of postprostatectomy SBRT, demonstrates low rates of GU and GI toxicity through 6 months of follow-up and suggests the benefits of MRgRT although the comparison is nonrandomized. Urinary QOL metrics declined at 1 month but were indistinguishable from baseline at 3 months. Whereas a detectable decrease in bowel QOL persisted through 6 months, the proportion of patients with clinically relevant declines improved over time. MRgRT was independently associated with lower any-grade GI toxicity and was less clinically relevant to a decline in bowel QOL on multivariable analysis. Additionally, no grade ≥2 acute GI toxicity or grade 3 acute GU toxicity events were seen in patients treated with MRgRT.
The acute toxicity rates in our study compare favorably to published hypofractionation series,25,28,29,33 which report acute grade 2 GU and GI toxicity rates of CTCAE criteria that are approximately 9% to 13% and 9% to 18%, respectively. These are similar to the grade 2 acute GU and GI toxicity rates of 9% and 5% in the present study. The present results also compare favorably with the acute grade ≥2 GU toxicity rates of 0% to 8% and grade ≥2 GI toxicity rates of 33% to 58% reported for patients receiving similar doses on the 2 prior phase 1 SBRT studies,36,37 although these studies all used smaller (nonconsensus) clinical treatment volumes without nodal RT or boost doses to gross disease.
The apparent benefits of MRgRT in this setting can likely be attributed to narrower PTV margins (3 mm instead of 5 mm with CTgRT). Notably, standard PTV margins in the context of CTgRT (with moderate hypofractionation) are typically up to 7 mm; the 5 mm margins used for CTgRT on the SCIMITAR protocol are thus also narrow. The further drop to 3 mm with MRgRT was considered safe because of 2 key features of the device: improved visualization of soft tissue with MRI for initial treatment alignment, and realtime treatment gating based on tracking of the anterior rectal wall. The latter may effectively avoid overdosing the rectum when it migrates into the higher dose area due to changes in rectal distention during the course of treatment delivery, and may ultimately also avoid underdosing the clinical target volume.
Notably, the SCIMITAR trial was designed before the publication of the SAKK 09/10 trial, which failed to show a benefit in freedom from biochemical progression with dose-escalation in the salvage setting.48,49 Thus, while SCIMITAR was designed to detect a potential improvement in 4-year BCRFS with functional dose-escalation based on ultrahypofractionation, it would no longer be expected to show an oncologic benefit. Nonetheless, we will continue to monitor efficacy and toxicity, with a second planned analysis once the 100th patient has 2-year PRO data available.
Limitations
This study has several limitations. First, the treatment platform was not randomly allocated and the majority of patients treated with CTgRT were treated earlier in the trial, when planning and treatment experience were lower. Second, the study was limited to 2 tertiary centers, limiting generalizability. Third, physicians were not blinded to the assignment of the RT platform when assessing toxicities. It would have been impossible to blind the patient to the intervention. However, significant differences favoring the MRgRT group were also observed in patient-reported outcomes, which provide a bias-free-as-possible glimpse at true toxicity. Fourth, the rate of online adaptive RT was low, mainly due to the COVID-19 pandemic prioritizing short treatment time and skeleton staffing. The benefit of MRgRT may have been more pronounced had adaptive RT been used.50 This concept will be explored in the recently activated phase II EXCALIBUR study (NCT04915508), which will exclusively use MRgRT. Fifth, the follow-up time was short and longer follow-up is needed to truly assess late toxicity. The length of follow-up was also shorter in patients treated with MRgRT compared with those treated with CTgRT. However, all patients in both groups had at least 6 months of follow-up. Finally, as a single arm study, direct comparisons to longer fractionation cannot be made; the randomized phase II SHORTER trial (NCT04422132) is comparing 55 Gy in 20 fractions versus 32.5 Gy in 5 fractions.
Conclusions
In this multicenter phase 2 trial, postprostatectomy SBRT was well tolerated within the first 6 months as evaluated by both physician-scored toxicity and patient-reported outcomes. MRgRT was associated with significantly decreased GI toxicity and better-preserved bowel QOL compared with CTgRT. Long-term follow-up and randomized trials comparing conventional fractionation or moderate hypofractionation to SBRT are needed to further characterize the safety and efficacy of this technique.
Supplementary Material
Disclosures:
A.U.K. reports funding support from grant P50CA09213 from the Prostate Cancer National Institutes of Health Specialized Programs of Research Excellence and grant W81XWH-22-1-0044 from the Department of Defense, as well as grant RSD1836 from the Radiological Society of North America, the STOP Cancer organization, the Jonsson Comprehensive Cancer Center, and the Prostate Cancer Foundation. He also reports consulting fees, research funding, and low-value stock in ViewRay, Inc., as well as consulting fees from Varian Medical Systems, Inc. All other authors have no disclosures to declare.
This trial was partially funded by ViewRay, Inc.
Footnotes
Data sharing statement: Research data are not available at this time.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ijrobp.2022.08.041.
References
- 1.Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: Long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 2012;380:2018–2027. [DOI] [PubMed] [Google Scholar]
- 2.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: Long-term followup of a randomized clinical trial. J Urol 2009;181:956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiegel T, Bartkowiak D, Bottke D, et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol 2014;66:243–250. [DOI] [PubMed] [Google Scholar]
- 4.Pfister D, Bolla M, Briganti A, et al. Early salvage radiotherapy following radical prostatectomy. Eur Urol 2014;65:1034–1043. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer; 2022. Available at: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed June 1, 2022. [Google Scholar]
- 6.Kneebone A, Fraser-Browne C, Duchesne GM, et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): A randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol 2020;21:1331–1340. [DOI] [PubMed] [Google Scholar]
- 7.Parker CC, Clarke NW, Cook AD, et al. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): A randomised, controlled phase 3 trial. Lancet 2020;396:1413–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sargos P, Chabaud S, Latorzeff I, et al. Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with localised prostate cancer after radical prostatectomy (GETUG-AFU 17): A randomised, phase 3 trial. Lancet Oncol 2020;21:1341–1352. [DOI] [PubMed] [Google Scholar]
- 9.Vale CL, Fisher D, Kneebone A, et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: A prospectively planned systematic review and meta-analysis of aggregate data. Lancet 2020;396:1422–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakic N, Sood A, Dalela D, et al. A nationwide persistent underutilization of adjuvant radiotherapy in North American prostate cancer patients. Clin Genitourin Cancer 2020;18:489–499 .e486. [DOI] [PubMed] [Google Scholar]
- 11.Hawken SR, Spratt DE, Qi J, et al. Utilization of salvage radiation therapy for biochemical recurrence after radical prostatectomy. Int J Radiat Oncol Biol Phys 2019;104:1030–1034. [DOI] [PubMed] [Google Scholar]
- 12.Sethukavalan P, Cheung P, Tang CI, et al. Patient costs associated with external beam radiotherapy treatment for localized prostate cancer: the benefits of hypofractionated over conventionally fractionated radiotherapy. Can J Urol 2012;19:6165–6169. [PubMed] [Google Scholar]
- 13.Brand DH, Tree AC, Ostler P, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol 2019;20:1531–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 2019;394:385–395. [DOI] [PubMed] [Google Scholar]
- 15.Fransson P, Nilsson P, Gunnlaugsson A, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer (HYPO-RT-PC): patient-reported quality-of-life outcomes of a randomised, controlled, non-inferiority, phase 3 trial. Lancet Oncol 2021;22:235–245. [DOI] [PubMed] [Google Scholar]
- 16.Kishan AU, Dang A, Katz AJ, et al. Long-term Outcomes of Stereotactic Body Radiotherapy for Low-Risk and Intermediate-Risk Prostate Cancer. JAMA Netw Open 2019;2 e188006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson WC, Silva J, Hartman HE, et al. Stereotactic Body Radiation Therapy for Localized Prostate Cancer: A Systematic Review and Meta-Analysis of Over 6,000 Patients Treated On Prospective Studies. Int J Radiat Oncol Biol Phys 2019;104:778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JZ, Li XA, Yu CX, DiBiase SJ. The low alpha/beta ratio for prostate cancer: What does the clinical outcome of HDR brachytherapy tell us? Int J Radiat Oncol Biol Phys 2003;57:1101–1108. [DOI] [PubMed] [Google Scholar]
- 19.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 1999;43:1095–1101. [DOI] [PubMed] [Google Scholar]
- 20.Katz AJ, Kang J. Quality of life and toxicity after SBRT for organ-confined prostate cancer, a 7-year study. Front Oncol 2014;4:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halpern JA, Sedrakyan A, Dinerman B, Hsu WC, Mao J, Hu JC. Indications, utilization and complications following prostate biopsy: New York State analysis. J Urol 2017;197:1020–1025. [DOI] [PubMed] [Google Scholar]
- 22.Sher DJ, Parikh RB, Mays-Jackson S, Punglia RS. Cost-effectiveness analysis of SBRT versus IMRT for low-risk prostate cancer. Am J Clin Oncol 2014;37:215–221. [DOI] [PubMed] [Google Scholar]
- 23.Muralidhar V, Nguyen PL. Maximizing resources in the local treatment of prostate cancer: A summary of cost-effectiveness studies. Urol Oncol 2017;35:76–85. [DOI] [PubMed] [Google Scholar]
- 24.Wong GW, Palazzi-Churas KL, Jarrard DF, et al. Salvage hypofractionated radiotherapy for biochemically recurrent prostate cancer after radical prostatectomy. Int J Radiat Oncol Biol Phys 2008;70:449–455. [DOI] [PubMed] [Google Scholar]
- 25.Fersino S, Tebano U, Mazzola R, et al. Moderate hypofractionated postprostatectomy volumetric modulated arc therapy with daily image guidance (VMAT-IGRT): A mono-institutional report on feasibility and acute toxicity. Clin Genitourin Cancer 2017;15:e667–e673. [DOI] [PubMed] [Google Scholar]
- 26.Kruser TJ, Jarrard DF, Graf AK, et al. Early hypofractionated salvage radiotherapy for postprostatectomy biochemical recurrence. Cancer 2011;117:2629–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis SL, Patel P, Song H, et al. Image guided hypofractionated postprostatectomy intensity modulated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2016;94:605–611. [DOI] [PubMed] [Google Scholar]
- 28.Franzese C, Badalamenti M, Baldaccini D, et al. Moderate hypofractionated radiotherapy for post-operative treatment of prostate cancer: Long-term outcome and pattern of toxicity. Strahlenther Onkol 2021;197:133–140. [DOI] [PubMed] [Google Scholar]
- 29.Leite ETT, Ramos CCA, Ribeiro VAB, et al. Hypofractionated radiation therapy to the prostate bed with intensity-modulated radiation therapy (IMRT): A phase 2 trial. Int J Radiat Oncol Biol Phys 2021;109:1263–1270. [DOI] [PubMed] [Google Scholar]
- 30.Chin S, Fatimilehin A, Walshaw R, et al. Ten-year outcomes of moderately hypofractionated salvage postprostatectomy radiation therapy and external validation of a contemporary multivariable nomogram for biochemical failure. Int J Radiat Oncol Biol Phys 2020;107:288–296. [DOI] [PubMed] [Google Scholar]
- 31.Cozzarini C, Fiorino C, Deantoni C, et al. Higher-than-expected severe (grade 3-4) late urinary toxicity after postprostatectomy hypofractionated radiotherapy: A single-institution analysis of 1176 patients. Eur Urol 2014;66:1024–1030. [DOI] [PubMed] [Google Scholar]
- 32.Ippolito E, Cellini N, Digesù C, et al. Postoperative intensity-modulated radiotherapy with simultaneous integrated boost in prostate cancer: A dose-escalation trial. Urol Oncol 2013;31:87–92. [DOI] [PubMed] [Google Scholar]
- 33.Katayama S, Striecker T, Kessel K, et al. Hypofractionated IMRT of the prostate bed after radical prostatectomy: Acute toxicity in the PRIAMOS-1 trial. Int J Radiat Oncol Biol Phys 2014;90:926–933. [DOI] [PubMed] [Google Scholar]
- 34.Barra S, Belgioia L, Marcenaro M, et al. Moderate hypofractionated radiotherapy after prostatectomy for cancer patients: Toxicity and clinical outcome. Cancer Manag Res 2018;10:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buyyounouski MK, Pugh S, Chen RC, et al. Primary endpoint analysis of a randomized phase III trial of hypofractionated vs conventional post-prostatectomy radiotherapy: NRG Oncology GU003. Int J Radiat Oncol Biol Phys 2021;111:S2–S3. [Google Scholar]
- 36.Ballas LK, Luo C, Chung E, et al. Phase 1 trial of SBRT to the prostate fossa after prostatectomy. Int J Radiat Oncol Biol Phys 2019;104:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sampath S, Frankel P, Vecchio BD, et al. Stereotactic body radiation therapy to the prostate bed: Results of a phase 1 dose-escalation trial. Int J Radiat Oncol Biol Phys 2020;106:537–545. [DOI] [PubMed] [Google Scholar]
- 38.Tocco BR, Kishan AU, Ma TM, Kerkmeijer LGW, Tree AC. MR-guided radiotherapy for prostate cancer. Front Oncol 2020;10 616291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013;310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 40.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 2000;56:899–905. [DOI] [PubMed] [Google Scholar]
- 41.Barry MJ, Fowler FJ Jr., O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992;148:1549–1557 discussion 1564. [DOI] [PubMed] [Google Scholar]
- 42.Spratt DE, Yousefi K, Deheshi S, et al. Individual patient-level meta-analysis of the performance of the decipher genomic classifier in high-risk men after prostatectomy to predict development of metastatic disease. J Clin Oncol 2017;35:1991–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kishan AU, Lamb JM, Jani SS, Kang JJ, Steinberg ML, King CR. Pelvic nodal dosing with registration to the prostate: Implications for high-risk prostate cancer patients receiving stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2015;91:832–839. [DOI] [PubMed] [Google Scholar]
- 44.Ma TM, Lamb JM, Casado M, et al. Magnetic resonance imaging-guided stereotactic body radiotherapy for prostate cancer (mirage): A phase III randomized trial. BMC Cancer 2021;21:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma TM, Neylon J, Casado M, et al. Dosimetric impact of interfraction prostate and seminal vesicle volume changes and rotation: A post hoc analysis of a phase III randomized trial of MRI-guided versus CT-guided stereotactic body radiotherapy. Radiother Oncol 2022;167:203–210. [DOI] [PubMed] [Google Scholar]
- 46.Michalski JM, Lawton C, El Naqa I, et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2010;76:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skolarus TA, Dunn RL, Sanda MG, et al. Minimally important difference for the Expanded Prostate Cancer Index Composite Short Form. Urology 2015;85:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghadjar P, Hayoz S, Bernhard J, et al. Dose-intensified versus conventional-dose salvage radiotherapy for biochemically recurrent prostate cancer after prostatectomy: The SAKK 09/10 randomized phase 3 trial. Eur Urol 2021;80:306–315. [DOI] [PubMed] [Google Scholar]
- 49.Ghadjar P, Hayoz S, Bernhard J, et al. Acute toxicity and quality of life after dose-intensified salvage radiation therapy for biochemically recurrent prostate cancer after prostatectomy: First results of the randomized trial SAKK 09/10. J Clin Oncol 2015;33:4158–4166. [DOI] [PubMed] [Google Scholar]
- 50.Cao M, Gao Y, Yoon SM, et al. Interfractional geometric variations and dosimetric benefits of stereotactic MRI guided online adaptive radiotherapy (SMART) of prostate bed after radical prostatectomy: Post hoc analysis of a phase II trial. Cancers (Basel) 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


