Abstract
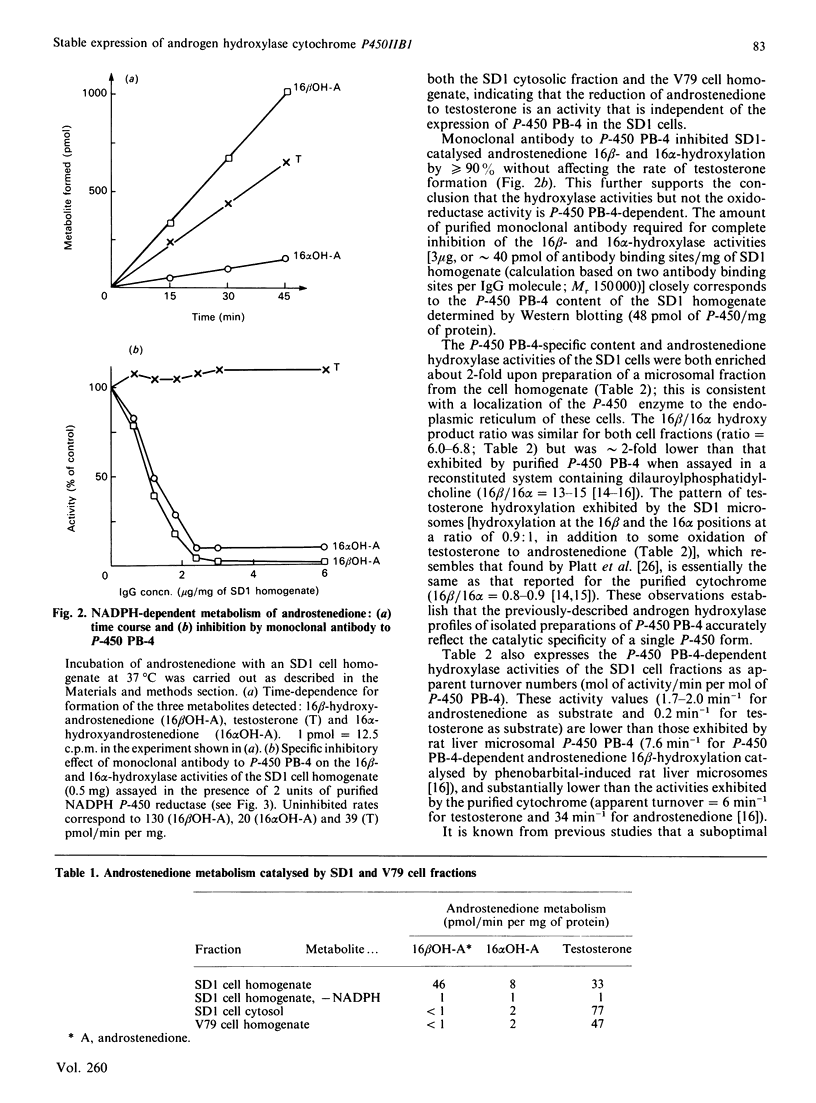
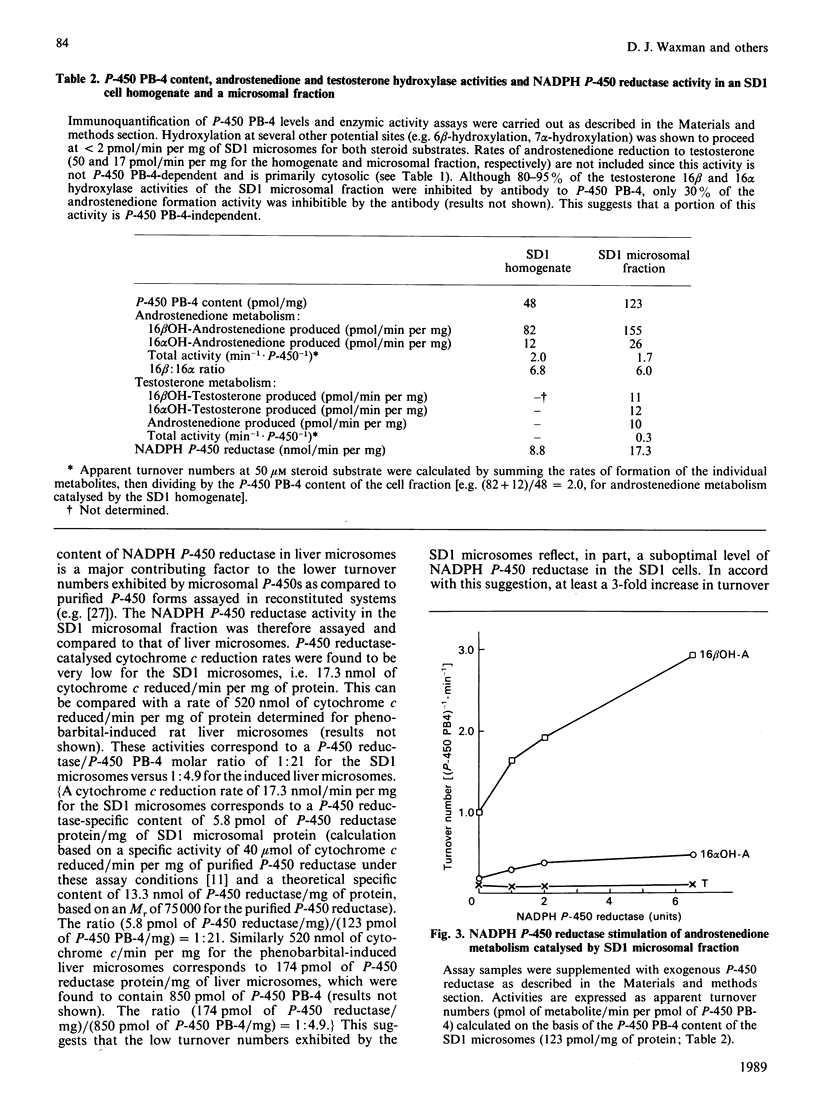
Androgen hydroxylation catalysed by Chinese hamster fibroblast SD1 cells, which stably express cytochrome P-450 form PB-4, the rat P450IIB1 gene product, was assessed and compared to that catalysed by purified cytochrome P-450 PB-4 isolated from rat liver. SD1 cell homogenates catalysed the NADPH-dependent hydroxylation of androstenedione and testosterone with a regioselectivity very similar to that purified by P-450 PB-4 (16 beta-hydroxylation/16 alpha-hydroxylation = 6.0-6.8 for androstenedione; 16 beta/16 alpha = 0.9 for testosterone). Homogenates prepared from the parental cell line V79, which does not express detectable levels of P-450 PB-4 or any other cytochrome P-450, exhibited no androgen 16 beta- or 16 alpha-hydroxylase activity. The hydroxylase activities catalysed by the SD1 cell homogenate were selectively and quantitatively inhibited (greater than 90%) by a monoclonal antibody to P-450 PB-4 at a level of antibody (40 pmol of antibody binding sites/mg of SD1 homogenate) that closely corresponds to the P-450 PB-4 content of the cells (48 pmol of PB-4/mg of SD1 homogenate). Fractionation of cell homogenates into cytosol and microsomes revealed that the P-450 PB-4-mediated activities are associated with the membrane fraction. Although the P-450 PB-4-specific content of the SD1 microsomes was 15% of that present in phenobarbital-induced rat liver microsomes, the P-450 PB-4-dependent androstenedione 16 beta-hydroxylase activity of the SD1 membrane fraction was only 2-3% of that present in the liver microsomes. This activity could be stimulated several-fold, however, by supplementation of SD1 microsomes with purified rat NADPH P-450 reductase. These studies establish that a single P-450 gene product (IIB1) can account for the hydroxylation of androgen substrates at multiple sites, and suggest that SD1 cells can be used to assess the catalytic specificity of P-450 PB-4 with other substrates as well.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backes W. L., Jansson I., Mole J. E., Gibson G. G., Schenkman J. B. Isolation and comparison of four cytochrome P-450 enzymes from phenobarbital-induced rat liver: three forms possessing identical NH2-terminal sequences. Pharmacology. 1985;31(3):155–169. doi: 10.1159/000138110. [DOI] [PubMed] [Google Scholar]
- Battula N., Sagara J., Gelboin H. V. Expression of P1-450 and P3-450 DNA coding sequences as enzymatically active cytochromes P-450 in mammalian cells. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4073–4077. doi: 10.1073/pnas.84.12.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehmer J., Dogra S., Friedberg T., Monier S., Adesnik M., Glatt H., Oesch F. Stable expression of rat cytochrome P-450IIB1 cDNA in Chinese hamster cells (V79) and metabolic activation of aflatoxin B1. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5769–5773. doi: 10.1073/pnas.85.16.5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. J., Schmid B. J., Umeno M., Mcbride O. W., Hardwick J. P., Meyer U. A., Gelboin H. V., Idle J. R. Human P450PCN1: sequence, chromosome localization, and direct evidence through cDNA expression that P450PCN1 is nifedipine oxidase. DNA. 1988 Mar;7(2):79–86. doi: 10.1089/dna.1988.7.79. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Dannan G. A., Wright S. T., Martin M. V., Kaminsky L. S. Purification and characterization of liver microsomal cytochromes p-450: electrophoretic, spectral, catalytic, and immunochemical properties and inducibility of eight isozymes isolated from rats treated with phenobarbital or beta-naphthoflavone. Biochemistry. 1982 Nov 9;21(23):6019–6030. doi: 10.1021/bi00266a045. [DOI] [PubMed] [Google Scholar]
- Imai Y. Characterization of rabbit liver cytochrome P-450 (laurate omega-1 hydroxylase) synthesized in transformed yeast cells. J Biochem. 1988 Jan;103(1):143–148. doi: 10.1093/oxfordjournals.jbchem.a122220. [DOI] [PubMed] [Google Scholar]
- Imai Y. Cytochrome P-450 related to P-4504 from phenobarbital-treated rabbit liver: molecular cloning of cDNA and characterization of cytochrome P-450 obtained by its expression in yeast cells. J Biochem. 1987 May;101(5):1129–1139. doi: 10.1093/oxfordjournals.jbchem.a121977. [DOI] [PubMed] [Google Scholar]
- Kaminsky L. S., Guengerich F. P. Cytochrome P-450 isozyme/isozyme functional interactions and NADPH-cytochrome P-450 reductase concentrations as factors in microsomal metabolism of warfarin. Eur J Biochem. 1985 Jun 18;149(3):479–489. doi: 10.1111/j.1432-1033.1985.tb08950.x. [DOI] [PubMed] [Google Scholar]
- LeBlanc G. A., Waxman D. J. Feminization of rat hepatic P-450 expression by cisplatin. Evidence for perturbations in the hormonal regulation of steroid-metabolizing enzymes. J Biol Chem. 1988 Oct 25;263(30):15732–15739. [PubMed] [Google Scholar]
- McKinney M. M., Parkinson A. A simple, non-chromatographic procedure to purify immunoglobulins from serum and ascites fluid. J Immunol Methods. 1987 Feb 11;96(2):271–278. doi: 10.1016/0022-1759(87)90324-3. [DOI] [PubMed] [Google Scholar]
- Nagata K., Matsunaga T., Gillette J., Gelboin H. V., Gonzalez F. J. Rat testosterone 7 alpha-hydroxylase. Isolation, sequence, and expression of cDNA and its developmental regulation and induction by 3-methylcholanthrene. J Biol Chem. 1987 Feb 25;262(6):2787–2793. [PubMed] [Google Scholar]
- Oeda K., Sakaki T., Ohkawa H. Expression of rat liver cytochrome P-450MC cDNA in Saccharomyces cerevisiae. DNA. 1985 Jun;4(3):203–210. doi: 10.1089/dna.1985.4.203. [DOI] [PubMed] [Google Scholar]
- Park S. S., Fujino T., Miller H., Guengerich F. P., Gelboin H. V. Monoclonal antibodies to phenobarbital-induced rat liver cytochrome P-450. Biochem Pharmacol. 1984 Jul 1;33(13):2071–2081. doi: 10.1016/0006-2952(84)90576-8. [DOI] [PubMed] [Google Scholar]
- Porter T. D., Kasper C. B. Coding nucleotide sequence of rat NADPH-cytochrome P-450 oxidoreductase cDNA and identification of flavin-binding domains. Proc Natl Acad Sci U S A. 1985 Feb;82(4):973–977. doi: 10.1073/pnas.82.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampersaud A., Walz F. G., Jr At least six forms of extremely homologous cytochromes P-450 in rat liver are encoded at two closely linked genetic loci. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6542–6546. doi: 10.1073/pnas.80.21.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D. E., Wood A. W., Thomas P. E., Walz F. G., Jr, Yuan P. M., Shively J. E., Levin W. Comparisons of highly purified hepatic microsomal cytochromes P-450 from Holtzman and Long-Evans rats. Biochim Biophys Acta. 1982 Dec 20;709(2):273–283. doi: 10.1016/0167-4838(82)90470-8. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Hirano K., Takahashi M., Hatano M., Fujii-Kuriyama Y. Site-directed mutageneses of rat liver cytochrome P-450d: axial ligand and heme incorporation. Biochemistry. 1988 May 31;27(11):4138–4141. doi: 10.1021/bi00411a035. [DOI] [PubMed] [Google Scholar]
- Sonderfan A. J., Arlotto M. P., Dutton D. R., McMillen S. K., Parkinson A. Regulation of testosterone hydroxylation by rat liver microsomal cytochrome P-450. Arch Biochem Biophys. 1987 May 15;255(1):27–41. doi: 10.1016/0003-9861(87)90291-8. [DOI] [PubMed] [Google Scholar]
- Stevens J. C., Halpert J. Selective inactivation of four rat liver microsomal androstenedione hydroxylases by chloramphenicol analogs. Mol Pharmacol. 1988 Jan;33(1):103–110. [PubMed] [Google Scholar]
- Waxman D. J. Interactions of hepatic cytochromes P-450 with steroid hormones. Regioselectivity and stereospecificity of steroid metabolism and hormonal regulation of rat P-450 enzyme expression. Biochem Pharmacol. 1988 Jan 1;37(1):71–84. doi: 10.1016/0006-2952(88)90756-3. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Ko A., Walsh C. Regioselectivity and stereoselectivity of androgen hydroxylations catalyzed by cytochrome P-450 isozymes purified from phenobarbital-induced rat liver. J Biol Chem. 1983 Oct 10;258(19):11937–11947. [PubMed] [Google Scholar]
- Waxman D. J., Lapenson D. P., Park S. S., Attisano C., Gelboin H. V. Monoclonal antibodies inhibitory to rat hepatic cytochromes P-450: P-450 form specificities and use as probes for cytochrome P-450-dependent steroid hydroxylations. Mol Pharmacol. 1987 Nov;32(5):615–624. [PubMed] [Google Scholar]
- Waxman D. J. Rat hepatic cytochrome P-450 isoenzyme 2c. Identification as a male-specific, developmentally induced steroid 16 alpha-hydroxylase and comparison to a female-specific cytochrome P-450 isoenzyme. J Biol Chem. 1984 Dec 25;259(24):15481–15490. [PubMed] [Google Scholar]
- Waxman D. J., Walsh C. Phenobarbital-induced rat liver cytochrome P-450. Purification and characterization of two closely related isozymic forms. J Biol Chem. 1982 Sep 10;257(17):10446–10457. [PubMed] [Google Scholar]
- Wood A. W., Ryan D. E., Thomas P. E., Levin W. Regio- and stereoselective metabolism of two C19 steroids by five highly purified and reconstituted rat hepatic cytochrome P-450 isozymes. J Biol Chem. 1983 Jul 25;258(14):8839–8847. [PubMed] [Google Scholar]
- Zuber M. X., Mason J. I., Simpson E. R., Waterman M. R. Simultaneous transfection of COS-1 cells with mitochondrial and microsomal steroid hydroxylases: incorporation of a steroidogenic pathway into nonsteroidogenic cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):699–703. doi: 10.1073/pnas.85.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber M. X., Simpson E. R., Waterman M. R. Expression of bovine 17 alpha-hydroxylase cytochrome P-450 cDNA in nonsteroidogenic (COS 1) cells. Science. 1986 Dec 5;234(4781):1258–1261. doi: 10.1126/science.3535074. [DOI] [PubMed] [Google Scholar]



