Summary
Background
Transthoracic echocardiography (TTE) has traditionally been the primary method for coronary imaging in children with Kawasaki disease (KD). We aimed to evaluate coronary artery lesions (CALs) of the left circumflex artery (LCx) in KD on computed tomography coronary angiography (CTCA).
Methods
Over a 9-year period (November 2013–December 2022), 225 children with KD underwent radiation-optimized CTCA on a 128-slice dual-source platform. TTE was performed on the same day, or a day prior or after CTCA.
Findings
On CTCA, LCx CALs were seen in 41/225 (18.2%) patients. However, TTE detected CALs in only one third of these patients [15/41 (36.6%)]. CTCA showed 47 LCx CALs in 41 patients–aneurysms in 39 patients (40 fusiform, 2 saccular; 7 giant aneurysms), stenoses in 3, and thrombosis in 2. Thromboses and stenoses were both missed on TTE. Proximal LCx aneurysms were seen in 39 patients–of these, 12 had distal extension. Six patients had distal LCx aneurysms without proximal involvement and 2 non-contiguous multiple aneurysms. Four (9.75%) patients had isolated LCx involvement. Based on CTCA findings, treatment protocols had to be modified in 3/41 (7.3%) patients.
Interpretation
This study highlights anatomical findings of LCx involvement in KD. Isolated LCx CALs were noted in 4/41 (9.75%) patients. TTE alone proved inadequate for LCx assessment in children with KD. With abnormalities detected in 18.2% of cases, including those missed by TTE, CTCA emerges as an essential imaging modality. The findings have implications for treatment planning and follow-up strategies in children with KD.
Funding
None.
Keywords: Kawasaki disease, Left circumflex coronary artery, Computed tomography coronary angiography, Transthoracic echocardiography, Coronary artery lesions, Aneurysms, Stenosis, Thrombosis, Thrombotic occlusion
Research in context.
Evidence before this study
Kawasaki Disease (KD) primarily affects young children and can lead to coronary artery lesions (CALs) in 15–25% of cases due to diagnostic and treatment delays. A PubMed search on CALs in KD shows that the existing literature has mainly focused on involvement of left main, left anterior descending, and right coronary arteries. Information about involvement of left circumflex coronary artery (LCx) remains limited. Two-dimensional transthoracic echocardiography (TTE) has hitherto been considered to be the preferred imaging modality for the evaluation of CALs at presentation, and on follow-up. However, assessment of the left circumflex (LCx) coronary artery is difficult on TTE due to its anatomy and limited accessibility to an appropriate acoustic window. Due to these reasons, normative data for LCx have also not been given in most published nomograms on Z-scores of coronary arteries.
Added value of this study
This is the first study focusing on the involvement of LCx in children with KD. As transthoracic echocardiography (TTE) alone is inadequate for the evaluation of LCx, computed tomography coronary angiography (CTCA) should be considered an essential imaging modality in KD. We present a CTCA series on LCx involvement in KD highlighting novel anatomical findings. LCx CALs were detected on CTCA in 41/225 (18.2%) cases. TTE detected LCx CALs in only 15 of these 41 patients. CTCA emerges as an essential imaging modality for LCx evaluation. Based on CTCA findings, treatment protocols had to be modified in 3/41 (7.3%) patients.
Implications of all the available evidence
Coronary artery lesions (CALs) in LCx are seen in 18.2% of children with KD on CTCA. TTE alone is inadequate for the evaluation of LCx in children with KD. CTCA emerges as the preferred imaging modality for LCx. Based on CTCA findings, treatment protocols had to be modified in 7.3% patients. These findings have significant implications for treatment strategies and follow-up in KD.
Introduction
Kawasaki disease (KD) is a common childhood vasculitis that preferentially affects coronary arteries. KD cases are being reported worldwide.1 The three countries that have the highest reported incidence of KD are Japan, Korea, and Taiwan.1 Before the COVID-19 pandemic, Japan had a KD incidence of 371/100,000 children under 5.2 Korea and Taiwan report incidence rates of 194.7 and 69.5, respectively, whereas in the United States and Europe, the rates range from 5 to 30/100,000 children under 5.3, 4, 5 There are no nationwide data on the incidence of KD in India, but city-specific data from Chandigarh have been published in 2010 and 2015.6,7 We have reported an increase in KD incidence from 0.51/100,000 in 1994 to 4.54/100,000 in 2008 among children below 15 years.6 A subsequent study for 2009–2014 reported an incidence of 5.35/100,000 for children below 5 years.7
Timely detection of coronary artery lesions (CALs) is important for appropriate treatment planning and follow-up in KD.8, 9, 10 Two-dimensional transthoracic echocardiography (TTE) has hitherto been considered to be the preferred imaging modality for the evaluation of CALs at presentation, and on follow-up. However, assessment of the left circumflex (LCx) coronary artery is difficult on TTE due to its anatomy and limited accessibility to an appropriate acoustic window.11,12 Due to these reasons, normative data for LCx have also not been given in most published nomograms on Z-scores of coronary arteries.8,13,14 There is a paucity of published work focusing on the involvement of LCx in KD.11,12,15,16
Invasive coronary angiography (ICA) is considered to be the standard of reference for the diagnosis of coronary lesions. Still, its application in children is limited due to its invasive nature and inordinate radiation exposure.17 There have been several recent advances in computed tomography (CT) technologies.12,18 These have led to CT coronary angiography (CTCA) being increasingly used for coronary artery assessment in KD.12,19 We, and others, have shown that it is now possible to carry out CTCA in this condition at sub-millisievert radiation exposure.12,20,21 In this study, we evaluated the involvement of LCx in KD using radiation-optimized CTCA.
Methods
Our centre is a tertiary care teaching hospital in North India. It is a federally funded, not-for-profit institute. Our unit has more than 29 years of experience in managing children with KD. We follow the largest single centre cohort of KD in India. During the period January 1994–December 2022, 1230 patients have been diagnosed to have KD at our centre. We started using CTCA as a diagnostic imaging modality in KD in October 2013, and have so far carried out this investigation in 225 patients with KD.
Diagnosis of KD was based on guidelines given by the American Heart Association (AHA).8,22,23 Treatment for children with KD consisted of intravenous immunoglobulin (IVIg; 2 g/kg) along with aspirin (initially in high doses, and later in anti-platelet [3–5 mg/kg/day] doses).8,22,23 Children with IVIg resistant KD, and those with CALs at presentation, were also given adjunctive therapies (second dose IVIg; steroids; infliximab; cyclosporine). Children with KD having large coronary aneurysms were also put on anticoagulation (heparin; warfarin).
Data on patients with KD who presented to our centre and underwent both TTE and CTCA, were analysed. CTCA was carried out in patients with KD who had (i) CALs on TTE at presentation (ii) IVIg resistance (iii) presentation in infancy (iv) myocarditis (v) macrophage activation syndrome or, (vi) KD shock syndrome. Patients underwent CTCA either at presentation, or on follow-up. Those with involvement of LCx coronary artery were analysed in detail. Corresponding findings on TTE were retrieved from patient records.
The study protocol was approved by the Institute Ethics Committee (IEC No. NK/1837/Res/2890). Informed consent was obtained from the parents prior to the procedure. The manuscript has been approved by the Departmental Review Board. No funding was involved in the study.
CTCA
CTCA was performed using a 128-slice dual-source CT scanner (Somatom Definition Flash, Siemens Healthineers, Erlangen, Germany). Non-ionic contrast agent (Omnipaque 350, GE Healthcare, Ireland) was administered for imaging. To stabilize the heart rate, children received a single oral dose of metoprolol (2 mg/kg), 1 h prior to CTCA. Sedation, involving oral triclofos (50 mg/kg/dose) and/or intravenous midazolam (0.1 mg/kg/dose), was administered to children below 5 years. CTCA does not require general anesthesia or breath holding and can be carried out without sedation in older children. Efforts were put in to minimize the radiation exposure using automated tube current modulation (Care Dose 4D, Siemens Healthineers), lower kilovoltage settings (fixed at 80 kVp), adaptive prospective electrocardiography-gated sequencing, and iterative image reconstruction algorithms (Safire, Siemens Healthineers). Additionally, ECG-gated calcium scoring (Agaston score) was performed. Radiation exposure median dose length product (DLP) and median radiation exposure was 21.0 mGy cm, IQR (13, 28), and 0.83 mSv, IQR (0.68, 1.01), respectively.24 Post-processing analysis was carried out on SyngoVia (Siemens Healthineers) workstation for coronary artery reconstruction. Interpretation and analysis of all CTCA scans were carried out by a single experienced cardiac radiologist (MS). Side branches of coronary arteries were evaluated when visualized, and CALs, including aneurysms (saccular/fusiform) and dilations, were assessed in terms of location (proximal/distal), number, and morphology. An ‘aneurysm’ was defined as a coronary artery segment with an internal diameter ≥1.5 times that of an adjacent segment,25,26 while ‘dilation’ referred to an increase in internal diameter that was less than 1.5 times that of an adjacent segment.25,26 The presence of luminal narrowing in coronary arteries was classified as ‘stenosis’. Furthermore, complications such as intraluminal thrombosis (indicated by a hypodense filling defect within the arterial lumen) and mural calcification were noted.
TTE
Transthoracic echocardiography (TTE) was performed following standard protocols. TTE was conducted during the acute stage of illness and during follow-up evaluations for all children. Since 2015, we have an in-house TTE machine for ‘Point of Care Echocardiography’. TTE during this period was usually carried out by fellows trained in cardiovascular assessment of KD under the supervision of faculty members in the unit. TTE was performed on the Esaote machine (MyLab30Gold) prior to 2019. Since March 2019, TTE has been performed on Philips EPIQ7G machine (model number US 51881625). The recorded results of CALs on TTE were compared with the findings from CTCA scans.
Role of the funding source
There was no funding source for this study.
Results
CTCA was performed in 225 patients with KD in this study. Median age at diagnosis of KD was 3 years (range 2 months–12 years). LCx involvement was noted in 41 children (35 boys; 6 girls) — in 30/41, CTCA had been performed during the acute phase, and in 11/41, CTCA was performed in convalescent phase (follow-up at a median interval of 5 years after diagnosis; range: 1–10 years). TTE was able to detect CALs in LCx in only 15/41 (36.6%) patients.
CTCA showed 47 CALs in LCx in 41 patients (Table 1). Aneurysms (40 fusiform; 2 saccular) were seen in 39 patients; stenoses in 3; thrombosis in 2. One patient had an aneurysm and a stenosis, while 2 patients had only stenoses. Details of CALs in LCx were as follows (Table 1) -
-
1.
Morphology of LCx aneurysms: 42 aneurysms (40 fusiform; 2 saccular) were identified in the LCx coronary artery. Of the 2 patients (Patient. Nos. 2 and 6; Table 1) with saccular aneurysms, 1 had involvement of the distal segment of the obtuse marginal (OM) branch of LCx resembling a bunch of grapes appearance (Fig. 1).
-
2.
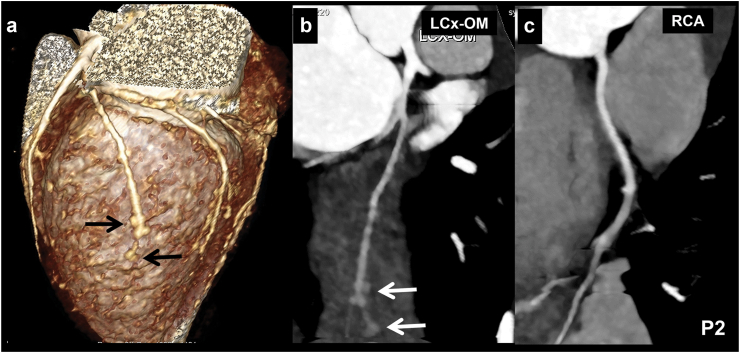
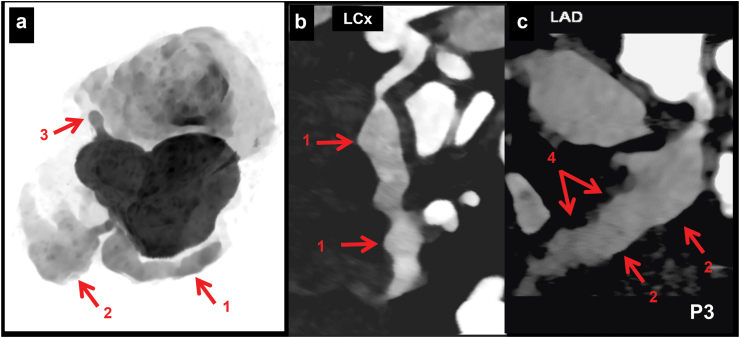
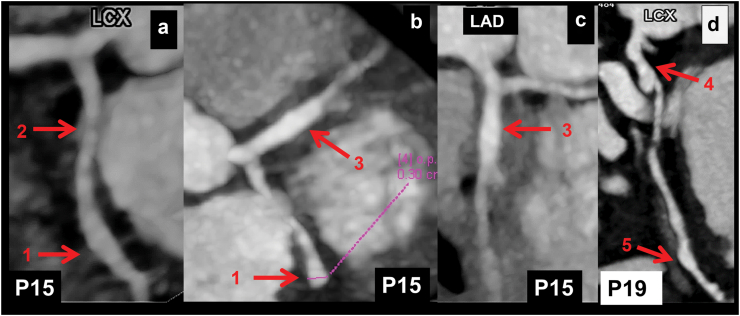
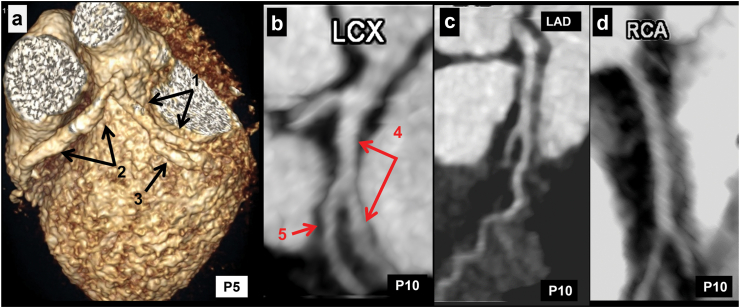
Location of CALs in LCx: 39 patients had proximal LCx involvement—of these, 12 also had distal extension of proximal aneurysm (Fig. 2). Distal LCx involvement, in absence of proximal involvement, was seen in 6 patients (Fig. 3 a-c). Two patients had non-contiguous aneurysms (Fig. 3d). Four patients also had branch vessel involvement (Fig. 1), while 2 had aneurysms at bifurcation (involvement of OM branch) of LCx (Fig. 4 a, b).
-
3.
LCx involvement with CALs in other coronary arteries: 37/41 (90.24%) patients had concomitant involvement of other coronary arteries (LCA in 26, LAD in 35, and RCA in 29 patients) (Fig. 2, Fig. 3, Fig. 4).
-
4.
Isolated LCx involvement without involvement of other coronary arteries: Isolated LCx involvement was seen in 4 patients (Patient Nos. 1, 2, 10, 26; Table 1)—in 1 amongst these (Patient No. 10; Table 1), CTCA had been carried out during acute stage; in the remaining 3, CTCA had been carried out on follow-up at intervals of 4, 2.5, and 6 years respectively after the acute episode. Baseline TTE showed normal coronaries in 1 patient (Patient No. 1; Table 1) and LCA abnormality in 2 patients (Patient Nos. 2, 26; Table 1). One patient (Patient No. 10; Table 1) showed LCx diameter of 1.6 mm with loss of tapering. Two (Patient No. 1, 26; Table 1) amongst these 4 patients had stenosis of LCx on CTCA (that had been carried out 4 and 6 years, respectively after the acute episode) (Fig. 5, Patient No. 26). Patient no. 2 had multiple distal segment fusiform aneurysms (bunch of grapes appearance) (Fig. 1). CTCA in Patient no. 10 showed fusiform aneurysm of proximal (4.5 mm) and mid (4.2 mm) with extnesion into its OM1 branch (2.4 mm) extending over 7 mm (Fig. 4b). In this patient, TTE has also demonstrated an LCx diameter of 1.6 mm with loss of tapering. However, the distal extent of abnormality could not be delineated on TTE. In 3 of the 4 patients, aspirin had been discontinued in view of normal coronary artery assessment on TTE. However, after the results of CTCA came in, aspirin had to be reinitiated.
-
5.
Giant LCx CALs: Giant aneurysms with diameter of more than 8 mm (or a Z score ≥10) were seen in 7 (17.07%) patients—these were fusiform in 6, and saccular in 1 patient (Fig. 6). Six of these 7 patients also had concomitant involvement of other coronary arteries. However, one patient (Patient No. 10; Table 1) had isolated giant aneurysm in LCx. Of these 7 patients, LCx could be visualized on TTE in only 4. TTE showed LCx aneurysms in 3 patients while in 1, only loss of tapering could be visualized.
-
6.
Complications: Two patients had LCx thrombosis (Fig. 7), and 3 had stenosis (Fig. 5). The latter had undergone the procedure on follow-up at 4–6 years after the acute episode (Table 1). TTE had missed thrombosis as well as stenosis in all 5 patients.
Table 1.
Detailed findings of children with KD and left circumflex coronary artery involvement.
| Patient No. | Age at diagnosis/Sex | Interval between diagnosis and CTCA (in years) | Transthoracic echocardiography (TTE) findings | CT coronary angiography |
|
|---|---|---|---|---|---|
| Findings in left circumflex coronary artery | Findings in other coronary arteries (LCA, LAD, RCA) | ||||
| 1. | 4 Y/M | CTCA carried out on follow-up at 4 years | Baseline: LCA: 2.8 (+1.57Z) LAD: 1.8 (+0.30) RCA: 1.8 (−0.33) LCx: NV Follow-upa: Normal |
9.8 mm long segment stenosis with 30–40% luminal diameter reduction. Distal LCx normal | Normal |
| 2. | 9 Y/F (Fig. 1) | CTCA carried out on follow-up at 2.5 years | Baseline: LCA mild dilation, other coronaries normal Follow-upa: Normal |
Multiple saccular aneurysms in distal most OM1 branch give a bunch of grapes appearance. | Normal |
| 3. | 7 months/M (Fig. 2) | CTCA carried out at presentation | LCA: 3.2 mm (+3.7Z) LAD: 12 mm (thrombosis) RCA: 3.5 mm (+7Z) LCx: NV |
LCx 7.2 mm fusiform giant aneurysm starting at proximal segments extending to the distal segment | LAD 18 mm (proximal and mid-segment) giant fusiform aneurysm along with thrombosis measuring 9 mm. RCA 4.5 mm fusiform aneurysm. LCA fusiform aneurysm 3 mm. |
| 4. | 4Y/M | CTCA carried out on follow-up at 5 years | Baseline: LCA: 3.2 mm fusiform aneurysm (Z score NA) LAD: 4.4 mm fusiform aneurysm (Z score NA) RCA: 2.2 mm LCx: NV Follow-upa: LCA 4.2 mm dilated and LAD: 2.5 mm dilated, LCx: NV |
Proximal segment fusiform aneurysm for 2.6–2.8 cm segment | Fusiform aneurysm in LCA and LAD |
| 5. | 12 Y/M (Fig. 4a) | CTCA carried out at presentation | LAD: 6.8 mm (+9.79Z) RCA: 6.4 mm (+8.09Z) LCx: NV |
Fusiform aneurysm of proximal LCx with extension to OM1 branch | Fusiform aneurysm of entire LAD and RCA |
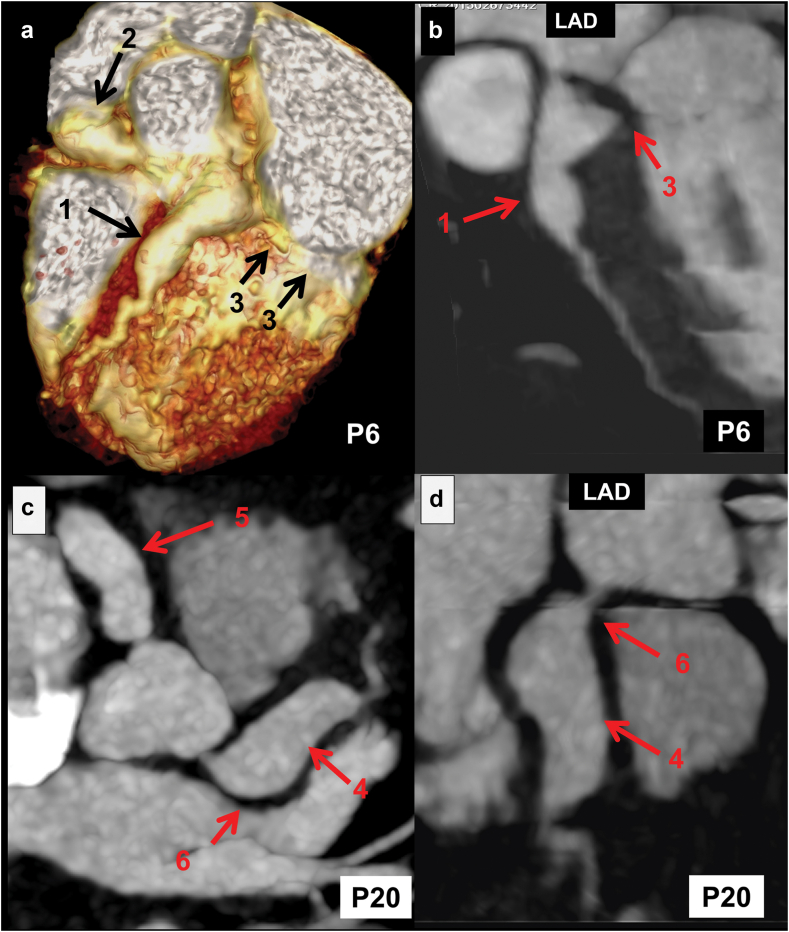
| 6. | 8 months/M (Fig. 7a and b) | CTCA carried out at presentation | LCA: 6.0 mm (+12.5Z) LAD: 6.8 mm (+17.06Z) RCA: 7 mm (+17.0Z) LCx: NV |
Saccular aneurysm at origin with non-opacified LCx in further course suggestive of thrombosis (8 mm) | Distal LCA saccular aneurysm (8 mm), proximal LAD saccualr aneurysm (7 mm), proximal RCA fusiform (6.4 mm) and distal RCA saccualr aneurysm (2.7 mm) |
| 7. | 7.5 months/M | CTCA carried out on follow-up at 10 years | Baseline: LCA: 3.2 mm fusiform aneurysm (Z score NA) RCA: 2.2 mm fusiform aneurysm (Z score NA) LAD: 1.5 mm dilated LCx: NV Follow-upa: normal |
LCx fusiform aneurysm (4.7 mm; length 1.4 cm) | Fusiform dilatation in LCA with extension into LCx. Saccular aneurysm in RCA |
| 8. | 7 months/M | CTCA carried out at presentation | LCA: 5 mm (+10.3Z) LAD: 4.7 mm (+11.3Z) RCA: 4.2 mm (+8.5Z) LCx: 1.7 mm (+1.7Z) |
Proximal and mid LCx fusiform giant aneurysm (3.2 mm) | LCA giant aneurysm (5.2 mm), proximal and mid LAD fusiform aneurysm (3.5 mm), proximal RCA fusiform aneurysm and fusiform aneurysm (3.5 × 5 mm) at bifurcation of RCA |
| 9. | 2 Y/M | CTCA carried out on follow-up at 8 years | LCA: 3.3 mm (+3.56Z) LAD: 10 mm (+24.81Z) RCA: 5.8 mm (+11.75Z) LCx normal |
LCx just beyond its origin fusiform aneurysm till bifurcation (3.4 mm and 13 mm length) | LCA dilated, LAD long segment (2.2 cm) dilatation, and RCA fusiform aneurysm (4.3 mm). Mid segment RCA thickly calcified aneurysm (5.3 mm). |
| 10. | 11 months/M (Fig. 4b) | CTCA carried out at presentation | LCA: 2.4 mm (+0.77Z) LAD: 1.7 mm (+0.16Z) RCA: 1.2 mm (−1.4Z) LCx 1.6 mm (+0.93Z) with loss of tapering |
Fusiform aneurysm of proximal (4.5 mm) and mid (4.2 mm) and its OM1 branch involvement (2.4 mm; length 6–7 mm) | Normal |
| 11. | 5 months/M | CTCA carried out at presentation | LCA: 2.3 mm (+2.62Z) LAD: 2.2 mm (+3.61Z) RCA: 1.3 mm (+0.19Z) LCx: NV |
Proximal LCx fusiform aneurysmal dilatation | Proximal and mid LAD dilatation and fusiform aneurysm in distal LAD |
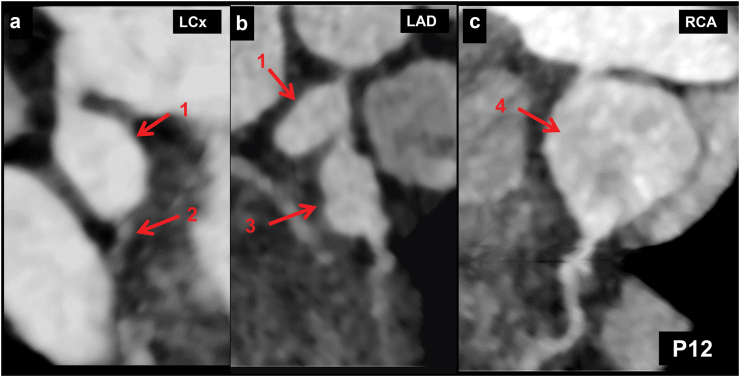
| 12. | 1.5 Y/F (Fig. 6) | CTCA carried out at presentation | LCA: 5.9 mm (+12.0Z) LAD: 5.1 mm (+11.9 Z) RCA: 3.2 mm (+5.0Z) LCx: 3.4 mm (+6.5Z) |
Proximal LCx giant fusiform aneurysm (5.8 mm) | LCA, LAD and RCA (14 mm × 1.7 cm) giant aneurysms |
| 13. | 3 Y/M | CTCA carried out at presentation | LCA: 4.7 mm (+6.32Z) LAD: 5.3 mm (+12.79Z) RCA: 5.8 mm (+11.6Z) LCx: 2.3 mm (+3.3Z) |
Proximal LCx fusiform giant aneurysm (4 mm × 25 mm) | Proximal LAD fusiform aneurysm, proximal RCA giant aneurysm |
| 14. | 6 Y/F | CTCA carried out on follow-up at 9 years | Baseline: LCA: 6 mm (+8.79Z) LAD: 8 mm (+16.30Z) RCA: 4 mm (+4.39Z) LCx: NV Follow-upa: LCA: 3 mm (−1.11Z) LAD: 9.4 mm (+14.31Z) with calcification RCA: 3.9 mm (+1.28Z) LCx: NV |
Fusiform aneurysm in LCx in its proximal and mid segment (4 mm) | Distal LCA and proximal segment LAD shows densely calcified giant fusiform aneurysm (8.4 mm × 20 mm). RCA densely calcified fusiform aneurysm (5.5mm × 17.5 mm) in RCA in proximal segment and distal RCA (4.2 mm) aneurysm |
| 15. | 4 Y/M (Fig. 3) | CTCA carried out at presentation | LCA: 3 mm (+2.17Z) LAD: 3.85 mm (+6.13Z) RCA: NA LCx: NV |
Distal LCx fusiform aneurysm (3 mm) | LAD fusiform aneurysm (3.5 mm) |
| 16. | 10 months/M | CTCA carried out at presentation | LCA: 3.8 mm (+5.83 Z) LAD: 4.5 mm (+9.70) RCA: 3.2 mm (+4.71Z) LCx: 2.5 mm (+3.53) |
Proximal Lcx fusiform aneurysm | LAD saccular giant aneurysm (6.5 mm), RCA fusiform aneurysm |
| 17. | 9 months/M | CTCA carried out on follow-up at 5 years | Baseline: LCA: 4.8 mm (+8.9Z) LAD: NA RCA: 5 mm (+11.08Z) LCx: NV Follow-upa: LCA: 3.4 (+2.1Z) LAD: 3.1 mm (+2.9Z) RCA: 2.9 mm (+1.5Z) LCx: NV |
Proximal and mid LCx fusiform aneurysm | LCA, LAD and RCA fusiform aneurysms |
| 18. | 4 Y/M | CTCA carried out on follow-up at 1 year | Baseline: LCA: 7.0 mm (+12.78) LAD: 5 mm (+9.55) RCA: 2.2 mm (+0.83) LCx: NV Follow-upa: LCA: 6.7 mm (+11.98) LAD: 5 mm (+9.55) RCA: 2.7 mm (+2.10) LCx: NV |
LCx arising from aneurysmal segment followed marked narrowing | Distal LCA fusiform aneurysm, LAD fusiform aneurysm (4.5 mm) |
| 19. | 5 Y/M | CTCA carried out at presentation | LCA: 3.5 mm (+2.23 Z) LAD: 6.8 mm (+16.05Z) RCA: 5.7 mm (+10.14Z) LCx: 4.3 mm (+6.43Z) |
Proximal fusiform aneurysm (3.9 mm). Another fusiform aneurysm in the distal LCx segment (3.6 mm). Intervening mid-segment is normal. | LAD and RCA show fusiform aneurysm in proximal segments LAD (7.7 mm); RCA (6.0 MM) with multiple skip aneurysms giving a beaded appearance. |
| 20. | 8 months/M (Fig. 7c and d) | CTCA carried out at presentation | LAD: 9.2 mm (+16Z) RCA: 7 mm (+13Z) LCx: NV |
LCx nonopacified (suggestive of thrombosis) | Large fusiform aneurysm LAD (7.0 mm), RCA (5.0 mm) diffusely dilated in entire course. |
| 21. | 11 Y/M | CTCA carried out at presentation | LCA: 5.1 mm (+5.2Z) LAD: 5.6 mm (8.4Z) RCA: NA LCx: NV |
Proximal and mid LCx fusiform giant aneurysm (5.1 mm) | LCA dilated, multiple fusiform aneurysms throughout RCA and proximal RCA giant aneurysm |
| 22. | 3 Y/M | CTCA carried out at presentation | LCA: 3.2 mm (+2.95Z) LAD: 3.5 mm (+5.42 Z) RCA: 2.6 mm (+1.98Z) LCx: NV |
Proximal LCx fusiform aneurysm (3.6 mm) | Fusiform dilatation of LCA with extending into proximal LAD |
| 23. | 2 Y/M | CTCA carried out at presentation | LCA: (3 mm; +2.74Z) LAD: (8 mm; +18.96Z) LCx: NV |
Proximal LCx fusiform aneurysm (3.5 mm) | LCA dilated (3.5 mm), distal LAD giant fusiform aneurysm (6.7 mm), and RCA dilatation |
| 24. | 5 months/M | CTCA carried out on follow-up at 2 years | Baseline: LCA: 4 mm (+7.11Z) LAD: 6.6 mm (+17.11Z) RCA: 4.6 mm (+10.54Z) LCx: NV Follow-upa: LCA: 3.6 mm (+1.38Z) LAD: 3.0 mm (+1.68Z) RCA: 2.1 mm (−1.19Z) LCx: 2 mm (−0.54Z) |
Proximal LCx fusiform aneurysm | LCA (3.6 mm), proximal and mid LAD (3.1 mm) fusiform aneurysm along with discontinuous fusiform aneurysm in distal LAD. Normal RCA. |
| 25. | 3 Y/F | CTCA carried out at presentation | LCA: 3.56 mm (+3.85Z) LAD: 2.5 mm (+2.5Z) RCA: 4.02 mm (+6.43Z) LCx: NV |
Proximal LCx fusiform aneurysm (2.7 mm) | Proximal and mid LAD aneurysm (3.2 mm × 15 mm), RCA (4.4 mm) |
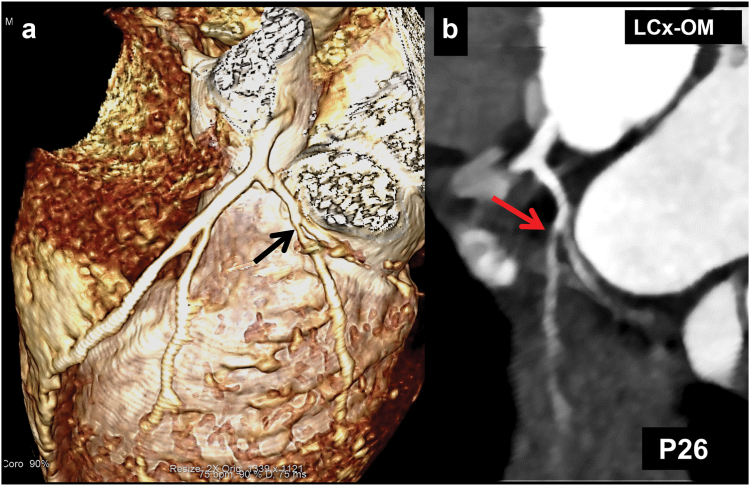
| 26. | 1.5 Y/M (Fig. 5) | CTCA carried out on follow-up at 6 years | Baseline: LCA: 3 mm (+3.49Z) LAD: 1.4 mm (+0.09) RCA: 2.2 mm (+1.85Z) LCx: NV Follow-upa: Normal |
OM1 branch of LCx shows stenosis causing 30–50% luminal narrowing | Normal |
| 27. | 6 Y/M | CTCA carried out at presentation | LCA: 3.1 mm (+4.6Z) LAD: 6.4 mm (+16.8Z) RCA: 9.4 mm (+24Z) LCX: 3.2 mm (+6.4Z) |
Proximal LCx fusiform giant aneurysm (4.1 mm × 10.7 mm) | LCA dilated (3.8 mm), LAD fusiform aneurysm (6.6 mm × 16.3 mm), RCA fusiform aneurysm (prox-8 mm, mid-5.2 mm, distal −4.2 mm) |
| 28. | 3 Y/M | CTCA carried out at presentation | LCA: 5.2 mm (+7.6Z) LAD: 3.2 mm (+4.1Z) RCA: 3.7 mm (+4.3Z) LCx: 2.8 mm (+2.9Z) |
Fusiform aneurysm in (2.4 mm) in entire course of LCx | LCA fusiform aneurysm, LAD fusiform aneurysm (3.5 mm) and RCA fusiform aneurysm (2.4 mm) |
| 29. | 9 Y/M | CTCA carried out at presentation | LCA: 4.5 mm (+3.87Z) LAD: 4.3 mm (+5.78Z) RCA: 4.6 mm (+5.11Z) LCx: 3 mm (+2.06Z) |
Fusiform aneurysm in (3.3 mm) in entire course of LCx | LCA dilated, LAD dilated (4.2 mm) and RCA diffusely dilated in entire course (5.6 mm) |
| 30. | 1.9 Y/M | CTCA carried out at presentation | LCA: 3.7 mm (+4.92Z) LAD: 6.5 mm (+14.96Z) RCA: 4.3 mm (+7.11Z) LCx: 4.3 mm (+8.01Z) |
Proximal LCx fusiform giant aneurysm (6.0 mm) | LCA and LAD fusiform giant aneurysm (8 mm) and RCA fusiform dilatation (4.6 mm) |
| 31. | 10 Y/M | CTCA carried out at presentation | LCA: 4.5 mm (+2.49Z) LAD: 4.1 mm (+3.49Z) RCA: 3.8 mm (+1.6Z) LCX: 3.9 mm (+2.6Z) |
Proximal and mid segment fusiform dilation (2.7 mm) | LCA and LAD diffuse dilated in entire course (2.7 mm), RCA dilated proximal and mid segment (1.8 mm) |
| 32. | 1 Y/F | CTCA carried out at presentation | LCA: 4.9 mm (+9.9Z) LAD: 2.5 mm (+4Z) RCA: 1.4 mm (+2.7Z) LCX: 2.0 mm (+2.7Z) |
Proximal LCx fusiform aneurysm (2.0 mm) | LCA saccular aneurysm (6.4 mm), LAD fusiform aneurysm proximal segment (3.6 mm) |
| 33. | 2 months/F | CTCA carried out at presentation | LCA: 2.9 mm (+5.31Z) LAD: 3.3 mm (+8.27Z) RCA: 2.9 mm (+6.1Z) LCx: 1.9 mm (+3.74Z) |
Proximal LCx fusiform aneurysm (2.4 mm) | LCA dilated (2.5 mm), LAD fusiform aneurysm (3.8 mm) and RCA fusiform aneurysm (2.7 mm) |
| 34. | 6 Y/M | CTCA carried out at presentation | LCA: 3 mm (+1.3Z) LAD: 6.6 mm (+12.1Z) RCA: 6.3 mm (+9.43Z) LCX: 5.4 mm (+8.1Z) |
Proximal LCx fusiform large aneurysm (4.1 mm) | LAD fusiform aneurysm (6.4 mm) and RCA discontinuous fusiform aneurysms RCA (5.1 mm, 6.3 mm. 3.4 mm). |
| 35. | 7 months/M | CTCA carried out at presentation | LCA: 3.29 mm (+4.28Z) LAD: 3.22 mm (+6.59Z) RCA: 4.9 mm (+11.58Z) LCx: 1.8 mm (+2.13Z) |
Two discontinuous fusiform aneurysms in mid LCX (2.5 mm, 3.3 mm) | LAD fusiform aneurysm (4.2 mm), RCA fusiform aneurysms (4.9 mm, 5.8 mm) |
| 36. | 3.5 Y/M | CTCA carried out on follow-up at 4.5 years | Baseline: LCA: 6.5 mm (+11.3Z) LAD: 2.2 mm (+1.5Z) RCA: 4 mm (+5.3Z) LCx: NV Follow-upa: LCA: 6.17 mm (+6.71Z) LAD: 10.3 mm (+18.6Z) RCA: 2.98 mm (+0.46Z) LCx: NV |
Proximal LCx giant fusiform aneurysm (8.4 mm) | Fusiform aneurysm in distal LCA extending in LCx. LAD thrombotic occlusion beyond its origin, thickly calcified thrombosed saccular aneurysm LAD (12.6 mm). RCA dilated in proximal segment (2.8 mm) with fusiform aneurysms in mid and distal RCA (3.5 mm and 3.2 mm) |
| 37. | 6 Y/M | CTCA carried out at presentation | LCA: 4 mm (+3.96Z) LAD: 4.2 mm (+6Z) RCA: 5.6 mm (+7.98Z) LCx: 2 mm (+0.31Z) |
Fusiform aneurysm in (3.5 mm) in entire course of LCx | Fusiform aneurysms in LCA (5.9 mm), LAD (3.9 mm) and RCA (7.3 mm) |
| 38. | 9 Y/M | CTCA carried out at presentation | LCA: 6.8 mm (+9.46Z) LAD: 5 mm (+8.9Z) RCA: 3.2 mm (+2.7Z) LCx: 4.87 mm (+6.52Z) |
Fusiform aneurysm in (3.7 mm) in entire course of LCx | Fusiform aneurysms in LCA (4.5 mm), LAD (4.4 mm) and RCA (2.5 mm) |
| 39. | 5 Y/M | CTCA carried out at presentation | LCA: 3.7 mm (+3.25Z) LAD: 4.1 mm (+6.08Z) RCA: 4.6 mm (+6.82Z) LCX: NV |
Proximal LCx fusiform aneurysm (2.6 mm) | LCA bulbous aneurysmal bifurcation (3.6 mm), LAD (2.5 mm) with filling defect and RCA (1.7 mm) |
| 40. | 13 Y/M | CTCA carried out at presentation | LCA: 6.4 mm (+8.14Z) LAD: 4.7 (+6.3Z) RCA: 5.4 mm (+5.98Z) LCx: NV |
Proximal LCx fusiform dilatation and fusiform aneurysm (4.6 mm) at bifurcation involving OM1 branch | Fusiform aneurysms in LCA (6.3 mm), LAD (5.0 mm) and RCA (6.6 mm) |
| 41. | 8 Y/M | CTCA carried out at presentation | LCA: 3 mm (+0.8Z) LAD: 3 mm (+2.43Z) RCA: 3 mm (+1.45Z) LCx: 2 mm (−0.1Z) Diffuse dilation of LCA, LAD and RCA |
Distal LCx fusiform dilatation (3 mm) | Fusiform aneurysms in RCA (5.3 mm, 4.2 mm) and LAD (3.4 mm, 8.4 mm) |
CTCA: Computed tomography coronary angiography; LCA: Left main coronary artery; LAD: Left anterior descending coronary artery; LCx: Left circumflex coronary artery; OM: Osteoproximal; RCA: Right coronary artery.
TTE findings in follow-up at time of CTCA; NV: Not visualized on TTE; NA: values not available.
Fig. 1.
(Patient No. 2): CT coronary angiography (CTCA) volume rendered (VR) (a), and, curved planar reformatted (CPR) images of obtuse marginal branch of left circumflex coronary artery (LCx) (b) and right coronary artery (RCA) (c) in an 11.5 years girl with KD (diagnosed at 9 years). Multiple aneurysms (bunch of grapes appearance) are seen (arrows in a and b). Transthoracic echocardiography (TTE) at presentation showed dilated proximal RCA, which on follow-up CTCA shows normalization (c). TTE, however, did not demonstrate aneurysms in LCx either at presentation, or on follow-up.
Fig. 2.
(Patient No. 3): CT coronary angiography (CTCA) grey-scaled volume rendered (VR) (a), and curved planar reformatted (CPR) (b and c) images in a 7 months infant with KD at presentation show fusiform aneurysm involving entire course of left circumflex coronary artery (LCx) (1 in a and b). A giant aneurysm is seen in proximal left anterior descending coronary artery (LAD) (2 in a and c) and saccular aneurysm in proximal right coronary artery (RCA) (3 in a). RCA beyond aneurysm is not opacified and was interpreted as thrombosed. Giant saccular aneurysm involving proximal LAD (2 in a and c) shows filling defect consistent with thrombus (4 in 2c). Transthoracic echocardiography demonstrated aneurysmal dilatation of proximal segments of RCA and LAD with thrombus, but failed to demonstrate LCx aneurysm.
Fig. 3.
a–c (Patient No. 15): CT coronary angiography (CTCA) curved planar reformatted (CPR) (a and c) and axial (b) images in a 4 years boy with KD at presentation show a fusiform aneurysm in distal left circumflex coronary artery (LCx) (1 in a and b) with normal calibre of proximal LCx (2 in a). There seems in a stenotic lesion in the osteo-proximal segment of LCx in figure a, however, this is due to an off-centre centreline. Fusiform aneurysm is also seen in proximal left anterior descending coronary artery (LAD) (3 in b and c). Transthoracic echocardiography (TTE) demonstrated aneurysmal dilatation of proximal LAD; LCx, however, could not be evaluated. d (Patient No. 19): CTCA CPR image of LCx (d) in a 5 years boy with KD at presentation shows fusiform aneurysm of proximal (4 in d) and distal (5 in d) LCx with normal intervening segment. Although TTE showed a aneurysm of proximal LCx, it failed to demonstrate involvement of distal LCx.
Fig. 4.
a (Patient No. 5): CT coronary angiography (CTCA) volume rendered (VR) (a) image in a 2 years boy with KD at presentation shows fusiform aneurysm in proximal left circumflex coronary artery (LCx) (1 in a), and left anterior descending coronary artery (LAD) (2 in a). There is extension of aneurysm in obtuse marginal (OM) branch of LCx (3 in a). Transthoracic echocardiography (TTE) demonstrated aneurysmal dilatation of proximal segments of coronary arteries but failed to demonstrate the involvement of OM branch and distal LCx. b–d (Patient No. 10): CTCA curved planar reformatted (CPR) images of LCx (b), LAD (c), and right coronary artery (RCA) (d) in an 11 months boy with KD at presentation show fusiform aneurysm involving proximal and mid segments of LCx (4 in b) and dilated OM branch (5 in b). LAD (c) and RCA (d) are unremarkable with normal distal tapering.
Fig. 5.
(Patient No. 26): CT coronary angiography (CTCA) volume rendered (VR) (a) and curved planar reformatted (CPR) images of left circumflex coronary artery (LCx) (b) carried out during follow-up in a 7.5 years boy with KD (diagnosed at 1.5 years of age) shows stenosis in the osteo-proximal segment of the obtuse marginal branch of LCx (arrows in a and b). Transthoracic echocardiography, however, did not demonstrate CALs in LCx either at presentation, or on follow-up.
Fig. 6.
(Patient No. 12): CT coronary angiography (CTCA) curved planar reformatted (CPR) images of left circumflex artery (LCx) (a), left anterior descending artery (LAD) (b), and right coronary artery (RCA) (c) in a 4-year girl with KD at presentation shows giant aneurysm in proximal LCx (1 in a and b) with normal mid and distal LCx (2 in a). In addition, there is a giant fusiform aneurysm in proximal LAD (3 in b) and a giant saccular aneurysm in proximal RCA (4 in c). Transthoracic echocardiography (TTE) demonstrated aneurysms of proximal coronary arteries. Distal anatomy of coronaries, however, could not be ascertained on TTE.
Fig. 7.
CT coronary angiography (CTCA) images of two patients of Kawasaki disease at presentation: Upper panel of images (Patient No. 6)–volume rendered (VR) (a) and curved planar reformatted (CPR) images of left anterior descending coronary artery (LAD) (b) in a 7 months boy with KD at presentation show saccular aneurysms in proximal LAD (1 in a and b) and proximal right coronary artery (RCA) (2 in a). The left circumflex coronary artery (LCx) is not opacified in the expected course (left atrioventricular groove), suggesting thrombotic occlusion (3 in a and b). Lower panel of images (Patient No. 20)–axial (c) and CPR image of LAD (d) of an 8 months boy with KD at presentation shows fusiform aneurysms in proximal LAD (4 in c and d), and RCA (5 in c). LCx is not opacified in the expected course (left atrioventricular groove), suggesting thrombotic occlusion (6 in c and d).
Discussion
KD is a vasculitis of medium-sized blood vessels that usually affects young children. CALs can develop in 15–25% of patients who are not given treatment during the acute stage. Most investigators have focused on the involvement of LMCA, LAD, and RCA in KD,8 and there is paucity of literature on LCx involvement in this condition.11,12,15,16
We have shown that LCx coronary artery involvement was seen in 18.2% (41/225) of children with KD on CTCA—4 amongst these had isolated LCx involvement. Our findings are in concordance with those reported by van Stijn et al.,12 who have also previously demonstrated the limitations of TTE in detecting CALs in LCx. The authors had carried out CTCA in 71 children with KD. CALs in LCx were found in 6 patients (8.6%)–these had been completely missed on TTE. Therefore, LCx involvement in KD is often missed or underestimated on TTE. CTCA is easy to perform, safe, well tolerated, requires minimal sedation, and has a radiation exposure less than 1 mSv in most patients.24 In present study, we have also shown that CTCA can clearly delineate (i) abnormalities of LCx in distal segments (ii) distal extension of proximal CALs (iii) multiple skip lesions (iv) thrombotic lesions and (v) stenotic lesions. These are crucial determinants of the long-term prognosis of children with KD. We have also shown that these abnormalities are either missed or underestimated on TTE.
TTE has hitherto been considered the preferred imaging modality for coronary artery assessment in KD.8 TTE, however, has several drawbacks. These include inter-observer variability, difficulty in delineating distal segments of coronary arteries, and difficulty in identifying segmental branches and the LCx due to its anatomical course.8,11 We have previously shown that CALs can manifest solely in distal segments of coronary arteries or in conjunction with, or as an extension of, proximal CALs. CTCA has proven to be a valuable tool in detecting CALs in distal coronary artery segments, including their branches. Such distal CALs are frequently missed on TTE.21 The present study has similarly shown that involvement of LCx can be underestimated, if one is using TTE alone as an imaging modality.
Although the standard guidelines for KD do not provide precise indications for CTCA, it is clear that this imaging modality adds value and impact on treatment, clinical care, and follow-up.8,27 CTCA is also indicated in all children with KD who have CALs on TTE at the presentation.12,21,28 CTCA may also be beneficial in children with IVIg-resistant KD, and in situations wherein the risk of coronary artery involvement is higher (e.g., IVIg resistance, presentation in infancy, associated myocarditis, and macrophage activation syndrome). In this study, we have shown that based on the findings of CTCA, treatment protocols had to be modified in 7.3% of patients with KD.
Accurate assessment of LCx may not always be possible on TTE due to its anatomical course and difficulty in obtaining an appropriate acoustic window. The LCx artery branches off the LCA, travels in the left atrioventricular groove, and then curves to the left within the coronary sulcus.29 LCx is known to have several anatomical variations including aberrant origin from the right coronary cusp, or directly from the left coronary cusp and from the LAD.30,31 As the origin of LCx cannot always be localized, assessment of LCx on TTE becomes difficult even in experienced hands.29,32 Due to these reasons, there is a paucity of Z-score based published nomograms for LCx.8,13,14
ICA has been considered to be the standard of reference for coronary artery assessment in KD. The main limitations are invasive technique, high radiation exposure, and inability to detect intramural changes. Further, ICA may lead to false negative results in the event of a largely thrombosed aneurysm yielding a pesudonormal sized coronary artery segment.17,33 Being an invasive procedure, it cannot be repeated often. CTCA is a non-invasive imaging modality that explicitly demonstrates abnormalities in both proximal and distal segments of coronary arteries. It can clearly delineate both intra-luminal and mural abnormalities.12,16,19,26 The main drawback of CTCA was considered to be radiation exposure. This has been largely addressed by current generation scanners with radiation optimization strategies that have enabled CTCA to be carried out with radiation exposure less than 1 mSv in most patients, and without compromising image quality.12,21,25,34,35 This has been made possible using strategies like ECG-controlled CTCA, child size bowtie filter, low tube voltage technique, increase in number of slices and iterative image reconstruction algorithms.18,25,36 Magnetic resonance coronary angiography (MRCA) is another imaging modality that has been used for the assessment of CALs but it has the disadvantage of long acquisition times and lower spatial and temporal resolution.37,38 Cardiac MRI, however, is better for assessment of myocardial perfusion, fibrosis, and intramural calcification.39
The main strength of this study is that it is the largest series on CTCA in KD that has been reported so far in the published literature. CTCA was carried out by the same radiologist during the entire study period (2013–2022). The radiation exposure (median dose 0.83 mSv) has been the lowest recorded so far. We report several novel anatomical findings involving the LCx on CTCA. We have shown that imaging diagnosis of KD may be underestimated when TTE is used versus CTCA, if the affectation is on the LCx. However, we identify several limitations as well. This study is based on a review of patient records. TTE had been carried out by different fellows during the study period (i.e., 2013–2022) as part of patient care. However, this is inevitable in a clinical setting such as ours.
To summarize, CTCA is the preferred imaging modality for LCx in KD. We have shown that abnormalities in LCx coronary artery are seen in 18.2% of children with KD on CTCA, and majority of these (i.e., 26/41; 63.4%) were missed on TTE. This study shows that TTE alone under-estimates coronary artery involvement in KD. Several abnormalities in LCx (as for instance CALs in mid and distal segments, distal extension of proximal CALs, multiple skip lesions, thrombotic or stenotic lesions) may be completely missed or under-estimated on TTE, and these can only be identified on CTCA. This modality also enables one to obtain coronary artery calcium scores on follow-up of children with KD. It also provides an opportunity to do survey scans for detection of aneurysms in systemic (e.g., axillary, brachial, iliac, femoral) arteries in patients with KD who have developed giant coronary aneurysms—this would, however, be at the cost of additional radiation exposure.40 The results of our study have important implications for prognostication of children with KD.
Contributors
MS, RKP: Conceptualization, study design, data curation, formal analysis, investigation, methodology, supervision, validation, figures, writing—original draft, and writing–review & editing. MS and RKP contributed equally, and share the first authorship and corresponding authorship. AT, HS, AG, SL, AG, SB, AKJ, PV, DS: Literature search, data collection, editing of the manuscript. RS, MSS: Figures, radiology data interpretation, literature search, manuscript editing. SS: conceptualization, study design, overall supervision, critical editing of the manuscript at each step.
Data sharing statement
The data underlying this article are available in the manuscript, online supplementary material, and our records. These data can be shared on request.
Declaration of interests
None.
Acknowledgements
None.
Contributor Information
Manphool Singhal, Email: drmsinghal74@gmail.com.
Rakesh Kumar Pilania, Email: kumarpilania007@gmail.com.
References
- 1.Watts R.A., Hatemi G., Burns J.C., Mohammad A.J. Global epidemiology of vasculitis. Nat Rev Rheumatol. 2022;18(1):22–34. doi: 10.1038/s41584-021-00718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ae R., Makino N., Kuwabara M., et al. Incidence of Kawasaki disease before and after the COVID-19 pandemic in Japan: results of the 26th nationwide survey, 2019 to 2020. JAMA Pediatr. 2022;176(12):1217–1224. doi: 10.1001/jamapediatrics.2022.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Y.H., Lin K.M., Ho S.C., Yan J.H., Lo M.H., Kuo H.C. Increased incidence of Kawasaki disease in Taiwan in recent years: a 15 Years nationwide population-based cohort study. Front Pediatr. 2019;7:121. doi: 10.3389/fped.2019.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim G.B. Reality of Kawasaki disease epidemiology. Korean J Pediatr. 2019;62(8):292–296. doi: 10.3345/kjp.2019.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manlhiot C., Mueller B., O’Shea S., et al. Environmental epidemiology of Kawasaki disease: linking disease etiology, pathogenesis and global distribution. PLoS One. 2018;13(2) doi: 10.1371/journal.pone.0191087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh S., Aulakh R., Bhalla A.K., et al. Is Kawasaki disease incidence rising in Chandigarh, North India? Arch Dis Child. 2011;96(2):137–140. doi: 10.1136/adc.2010.194001. [DOI] [PubMed] [Google Scholar]
- 7.Singh S., Bhattad S. Kawasaki disease incidence at Chandigarh, North India, during 2009-2014. Rheumatol Int. 2016;36(10):1391–1397. doi: 10.1007/s00296-016-3543-y. [DOI] [PubMed] [Google Scholar]
- 8.McCrindle B.W., Rowley A.H., Newburger J.W., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation. 2017;135(17):e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 9.Burns J.C., Matsubara T. New insights into cardiovascular disease in patients with Kawasaki disease. Curr Opin Pediatr. 2018;30(5):623–627. doi: 10.1097/MOP.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 10.Brogan P., Burns J.C., Cornish J., et al. Lifetime cardiovascular management of patients with previous Kawasaki disease. Heart Br Card Soc. 2020;106(6):411–420. doi: 10.1136/heartjnl-2019-315925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margossian R., Lu M., Minich L.L., et al. Predictors of coronary artery visualization in Kawasaki disease. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2011;24(1):53–59. doi: 10.1016/j.echo.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Stijn-Bringas Dimitriades D., Planken R.N., Groenink M., Streekstra G.J., Kuijpers T.W., Kuipers I.M. Coronary artery assessment in Kawasaki disease with dual-source CT angiography to uncover vascular pathology. Eur Radiol. 2020;30(1):432–441. doi: 10.1007/s00330-019-06367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi T., Fuse S., Sakamoto N., et al. A new Z score curve of the coronary arterial internal diameter using the lambda-mu-sigma method in a pediatric population. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2016;29(8):794–801.e29. doi: 10.1016/j.echo.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Dallaire F., Dahdah N. New equations and a critical appraisal of coronary artery Z scores in healthy children. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2011;24(1):60–74. doi: 10.1016/j.echo.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Goo H.W. Quantitative evaluation of coronary artery visibility on CT angiography in Kawasaki disease: young vs. old children. Int J Cardiovasc Imaging. 2021;37(3):1085–1092. doi: 10.1007/s10554-020-02054-6. [DOI] [PubMed] [Google Scholar]
- 16.Singhal M., Gupta P., Singh S., Khandelwal N. Computed tomography coronary angiography is the way forward for evaluation of children with Kawasaki disease. Glob Cardiol Sci Pract. 2017;2017(3) doi: 10.21542/gcsp.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuda E., Singhal M. Role of imaging studies in Kawasaki disease. Int J Rheum Dis. 2018;21(1):56–63. doi: 10.1111/1756-185X.13210. [DOI] [PubMed] [Google Scholar]
- 18.Singhal M., Pilania R.K., Gupta P., Johnson N., Singh S. Emerging role of computed tomography coronary angiography in evaluation of children with Kawasaki disease. World J Clin Pediatr. 2023;12(3):97–106. doi: 10.5409/wjcp.v12.i3.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thangathurai J., Kalashnikova M., Takahashi M., Shinbane J.S. Coronary artery aneurysm in Kawasaki disease: coronary CT angiography through the lens of pathophysiology and differential diagnosis. Radiol Cardiothorac Imaging. 2021;3(5) doi: 10.1148/ryct.2021200550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dietz S.M., Tacke C.E., Kuipers I.M., et al. Cardiovascular imaging in children and adults following Kawasaki disease. Insights Imaging. 2015;6(6):697–705. doi: 10.1007/s13244-015-0422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singhal M., Pilania R.K., Jindal A.K., et al. Distal coronary artery abnormalities in Kawasaki disease: experience on CT coronary angiography in 176 children. Rheumatol Oxf Engl. 2023;62(2):815–823. doi: 10.1093/rheumatology/keac217. [DOI] [PubMed] [Google Scholar]
- 22.Newburger J.W., Takahashi M., Gerber M.A., et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the committee on rheumatic fever, endocarditis and Kawasaki disease, council on cardiovascular disease in the young, American heart association. Circulation. 2004;110(17):2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 23.Management of Kawasaki syndrome: a consensus statement prepared by North American participants of the third international Kawasaki disease symposium, Tokyo, Japan, December, 1988. Pediatr Infect Dis J. 1989;8(10):663–667. [PubMed] [Google Scholar]
- 24.Bhatt M.C., Singhal M., Pilania R.K., et al. Radiation dose analysis of computed tomography coronary angiography in children with Kawasaki disease. World J Clin Pediatr. 2023;12(4):230–236. doi: 10.5409/wjcp.v12.i4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duan Y., Wang X., Cheng Z., Wu D., Wu L. Application of prospective ECG-triggered dual-source CT coronary angiography for infants and children with coronary artery aneurysms due to Kawasaki disease. Br J Radiol. 2012;85(1020):e1190–e1197. doi: 10.1259/bjr/18174517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singhal M., Singh S., Gupta P., Sharma A., Khandelwal N., Burns J.C. Computed tomography coronary angiography for evaluation of children with Kawasaki disease. Curr Probl Diagn Radiol. 2018;47(4):238–244. doi: 10.1067/j.cpradiol.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Fukazawa R., Kobayashi J., Ayusawa M., et al. JCS/JSCS 2020 guideline on diagnosis and management of cardiovascular sequelae in Kawasaki disease. Circ J Off J Jpn Circ Soc. 2020;84(8):1348–1407. doi: 10.1253/circj.CJ-19-1094. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y.M., Chu Y.Q., Li X.M., et al. The complementary relationship between echocardiography and multi-slice spiral CT coronary angiography in the diagnosis of coronary artery thrombosis in children with Kawasaki disease. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.670887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krzanowski M., Bodzoń W., Dimitrow P.P. Imaging of all three coronary arteries by transthoracic echocardiography. An illustrated guide. Cardiovasc Ultrasound. 2003;1:16. doi: 10.1186/1476-7120-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujimoto S., Kondo T., Orihara T., et al. Prevalence of anomalous origin of coronary artery detected by multi-detector computed tomography at one center. J Cardiol. 2011;57(1):69–76. doi: 10.1016/j.jjcc.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Sohrabi B., Habibzadeh A., Abbasov E. The incidence and pattern of coronary artery anomalies in the north-west of Iran: a coronary arteriographic study. Korean Circ J. 2012;42(11):753–760. doi: 10.4070/kcj.2012.42.11.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jrad M., Ben Salem F., Barhoumi C., et al. The role of computed tomography coronary angiography in Kawasaki disease: comparison with transthoracic echocardiography in a 25-case retrospective study. Pediatr Cardiol. 2019;40(2):265–275. doi: 10.1007/s00246-018-2044-z. [DOI] [PubMed] [Google Scholar]
- 33.Crean A., Benson L., Shah A., Han K., Lesser J., McCrindle B.W. Imaging the delayed complications of childhood Kawasaki disease. F1000Research. 2022;11:147. doi: 10.12688/f1000research.73097.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Secinaro A., Curione D., Mortensen K.H., et al. Dual-source computed tomography coronary artery imaging in children. Pediatr Radiol. 2019;49(13):1823–1839. doi: 10.1007/s00247-019-04494-2. [DOI] [PubMed] [Google Scholar]
- 35.Ghoshhajra B.B., Lee A.M., Engel L.C., et al. Radiation dose reduction in pediatric cardiac computed tomography: experience from a tertiary medical center. Pediatr Cardiol. 2014;35(1):171–179. doi: 10.1007/s00246-013-0758-5. [DOI] [PubMed] [Google Scholar]
- 36.Sun Z. Multislice CT angiography in coronary artery disease: technical developments, radiation dose and diagnostic value. World J Cardiol. 2010;2(10):333–343. doi: 10.4330/wjc.v2.i10.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Stijn D., Planken N., Kuipers I., Kuijpers T. CT angiography or cardiac MRI for detection of coronary artery aneurysms in Kawasaki disease. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.630462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J.W., Goo H.W. Coronary artery abnormalities in Kawasaki disease: comparison between CT and MR coronary angiography. Acta Radiol. 2013;54(2):156–163. doi: 10.1258/ar.2012.120484. [DOI] [PubMed] [Google Scholar]
- 39.Muthusami P., Luining W., McCrindle B., et al. Myocardial perfusion, fibrosis, and contractility in children with Kawasaki disease. JACC Cardiovasc Imaging. 2018;11(12):1922–1924. doi: 10.1016/j.jcmg.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Sudhakar M., Singhal M., Anjani G., et al. Kawasaki disease does not affect coronaries alone: large vessels can be involved as well. Clin Rheumatol. 2022;41(6):1927–1928. doi: 10.1007/s10067-022-06118-x. [DOI] [PubMed] [Google Scholar]