Abstract
Particle therapy (PT) represents a significant advancement in cancer treatment, precisely targeting tumor cells while sparing surrounding healthy tissues thanks to the unique depth-dose profiles of the charged particles. Furthermore, their linear energy transfer and relative biological effectiveness enhance their capability to treat radioresistant tumors, including hypoxic ones. Over the years, extensive research has paved the way for PT's clinical application, and current efforts aim to refine its efficacy and precision, minimizing the toxicities. In this regard, radiobiology research is evolving toward integrating biotechnology to advance drug discovery and radiation therapy optimization. This shift from basic radiobiology to understanding the molecular mechanisms of PT aims to expand the therapeutic window through innovative dose delivery regimens and combined therapy approaches. This review, written by over 30 contributors from various countries, provides a comprehensive look at key research areas and new developments in PT radiobiology, emphasizing the innovations and techniques transforming the field, ranging from the radiobiology of new irradiation modalities to multimodal radiation therapy and modeling efforts. We highlight both advancements and knowledge gaps, with the aim of improving the understanding and application of PT in oncology.
Keywords: Particle therapy, Bragg peak, Radiobiology
Introduction
Particle therapy (PT) has emerged as a revolutionary tool in cancer treatment, offering precision in targeting tumor cells while minimizing damage to surrounding healthy tissues. Originating from the concept of Robert R. Wilson at Lawrence Berkeley National Laboratory, the primary objective has always been to harness the unique properties of ions to optimize radiation dose delivery.1, 2 Charged particles exhibit a characteristic dose distribution (Bragg peak) depositing most of their energy at the end of their track. This energy deposition can be directed to the tumor site, minimizing damage to critical organs beyond it.2, 3 Apart from the favorable dose distribution, the relative biological effectiveness (RBE) of PT is larger than that of x-rays due to greater ionization density.3, 4, 5, 6 Collectively, this allows for the destruction of particularly radioresistant diseases, including hypoxic tumors. Studies suggest that concentrating high linear energy transfer (LET) particles into a hypoxic volume within a tumor or into a region containing tumor stem cells should lead to greater biological efficiency of PT and improvements in the therapeutic ratio.7, 8
Over many years, essential physical and radiobiological studies to enable patients to be treated with PT have been performed. Nowadays, in physics, current efforts focus on refining PT, emphasizing precision, efficiency, and cost-efficacy. Strategies include developing novel detectors, targeting moving tumors, adapting treatments to daily patient variations, reducing accelerator size, and exploring innovative approaches like gantry-less treatments, spatial irradiations (grid or minibeams), or UHDRs. For radiobiology, recent research underscores the point that this discipline can leverage the latest advancements in biotechnology to herald a new era of drug discovery and radiation therapy (RT) optimization in PT. Biological studies in PT must evolve from basic radiobiology to a deeper investigation of the mechanisms underlying its efficacy, aiming to expand the therapeutic window with new approaches in dose delivery regimens and evidence-based combined therapy approaches. This emerging advancement within radiobiology is poised to synergize with novel discoveries in oncology, such as immune system activation, radiosensitization/radioprotection, radiomics, biomarker discovery, genomically adjusted RT, and other innovative strategies.
A comprehensive exploration of the biological fundamentals of PT seeks to enhance comprehension and position PT as a frontrunner in oncology. This review delineates the areas of intense research in modern PT radiobiology, summarized in the Table, highlighting both advancements and knowledge gaps, covering methodologies, modeling, and clinical contexts to catalyze further exploration and discovery.
Table.
Topics of current investigation in particle radiobiology.
| Aspect | Concept |
|---|---|
| Beam delivery | |
| Ultrahigh dose rates | Short exposure times (∼ps-ms) result in higher normal tissue tolerance |
| Spatial fractionation | Higher doses can be better tolerated by normal tissues when delivered in a spatially fractionated manner (µm-mm exposure lengths) |
| Hypofractionation | Exploit good dose conformity to deliver ablative or immunogenic doses |
| New ions | High-LET ions (O, Ne) to target radioresistant (eg, hypoxic) volumes. |
| NTCP modeling and analysis | Understand the impact of dose distribution on normal tissue reactions (volume effects) for different endpoints, including second cancer induction |
| RBE in clinical endpoints | Optimize exploitation of RBE by modeling and translation between dose systems |
| Combined approaches | |
| Radioimmunotherapy | Radiation doses stimulate immune response, which can be converted into a strong antitumoral response by immune checkpoint inhibitors, eventually exhibiting abscopal response |
| Radiosensitizers | Enhance the DNA damage inflicted to DNA on the subcellular level, for example, by Auger electron emission of metallic nanoparticles, target specific DNA repair pathways to enhance tumor response without eliciting a normal tissue penalty |
| BNCT | Neutron irradiation of boron substances selectively targeted to tumor cells releases local alpha decay |
| Radiopharmaceuticals | Targeting residual tumor and metastases following the initial RT treatment of primary tumor |
| Accounting for individual radiation sensitivity | |
| Biomarkers and liquid biopsies | Monitor radiation action, for example, by considering DNA damage response markers; identify mutations in key genes that suggest conditional vulnerabilities to PT as opposed to conventional x-ray therapy |
| GARD | Consider an established genomic pattern to assess individual radiosensitivity |
Abbreviations: BNCT, boron neutron capture therapy; GARD, genomic-adjusted radiation dose; LET, linear energy transfer; NTCP, Normal Tissue Complication Probability; PT, particle therapy; RBE, relative biological effectiveness; and RT, radiation therapy.
Radiobiology of new irradiation modalities
FLASH
Ultrahigh dose rate (UHDR, “FLASH”) RT is a breakthrough in cancer treatment with the potential to widen the therapeutic window.9 In preclinical models, the application of UHDRs (>40 Gy/s) of radiation has, in some cases, demonstrated a substantial reduction in normal tissue toxicity while maintaining tumor control. Recent advances in experimental capabilities have enabled FLASH studies with radiation types spanning from x-rays,10 electrons,11 clinical12 and low-energy protons,13 helium,14 and carbon ions.15, 16, 17, 18 The use of charged particles might enable the attainment of high dose rates more readily in clinical settings as compared to photons, and ongoing clinical trials are investigating the effectiveness of FLASH with protons and electrons.19, 20 While electrons offer a larger flexibility in terms of achievable dose rates, protons and heavier ions are seen as the more direct way toward clinical translation for deep-seated tumors.
FLASH experiments with heavier ions can now be performed inside the spread-out Bragg peak via the application of 3D range modulators, leading to studies assessing the maintenance of FLASH effects with high LET particles. Although the biophysical models attempting to explain the FLASH effect were predicting a loss of the sparing effect at high LET, recent experiments demonstrated persistent normal tissue-sparing effects in both in vitro and in vivo models.14, 17, 18 In addition, a study with 12C FLASH irradiations revealed a unique feature, notably the apparent suppression of distant metastases.18 These recent experiments with UHDR 12C beams have laid the groundwork for preclinical tests with 16O, or even 20Ne ions.
As the interest in FLASH radiobiology surged, it became clear that the existing understanding of tissue responses to radiation fails to explain the benefits of FLASH. Furthermore, although there is substantial evidence supporting the normal tissue-sparing effects of UHDR treatments, some irradiations with electrons and protons have demonstrated no such benefit.21, 22 The traditional understanding of dose rate effects, primarily based on chronic low-dose-rate exposures, was challenged by FLASH-RT's unique outcomes,23 suggesting a departure from reliance on DNA double-strand break (DSB) repair kinetics. Hypotheses such as increased free radical recombination/diffusion24 and oxygen depletion initially gained traction25 but were later countered by evidence showing insufficient oxygen reduction to confer biological benefits.26, 27 Alternative hypotheses, including protection of stem cell niches,28 dose-rate dependent changes in lipid peroxidation29 or other dose-rate-responsive molecules,30 and the impact on blood volume irradiation,31 which may be due to sparing some immune cells, have emerged but lack conclusive experimental validation.
Although the underlying mechanisms that drive the FLASH effect have not been fully explained, we believe the prospects of this novel technique as a common RT modality will be moved forward as the biology is elucidated and clinical trials are completed. To advance the understanding of the FLASH effect, additional experimental data are needed, both in vitro and in vivo, including data examining dose rate and other treatment parameters relevant to each radiation modality.
Spatially fractionated radiation therapy
In spatially fractionated radiation therapy (SFRT), a heterogeneous pattern of radiation is delivered to tissues by creating regions with high (peaks) and low (valleys) doses, resulting in reduced normal tissue toxicities.32 The beam size is inversely correlated with the maximal doses tolerated by normal tissues.33, 34 In this context, minibeam radiation therapy (MBRT) utilizes planar beamlets with widths ranging from 0.3 to 1 mm. Compared to clinical SFRT techniques like grid and lattice RT (hot spots of 1-2 cm2), MBRT allows for smaller beam sizes that allow for increased doses to be delivered to a tumor.
The superior tissue-sparing capacities of proton minibeam radiation therapy (pMBRT) were demonstrated in preclinical studies with peak doses as high as 100 Gy, with no significant neurotoxicity in cranial irradiation on evaluation of memory impairment and histopathology,35, 36, 37 skin toxicity,38, 39 and, in thoracic irradiation, lung fibrosis.40 X-ray MBRT has shown a higher therapeutic index compared to that seen when an equivalent homogeneous dose is used against a rat glioblastoma model41, 42, 43 and de novo brain tumors in canine patients.44 The biological mechanisms underlying SFRT are under study. Activation of bystander cell-to-cell communication45 and an antitumor immune response seem key for tumor eradication with MBRT.46
Recently, pMBRT was proposed as a method to enable the delivery of MBRT to deep-seated tumors.47 Importantly, pMBRT has shown a similar tumor control capacity in high-grade glioblastoma orthotopic models in rats to highly toxic curative broad-beam doses.37, 48, 49 Integrating pMBRT with temporal fractionation in a crossed-beam approach appears to be the most effective approach to date.50
These promising in vivo results have led to the exploration of heavy-ion MBRT, including C, Ne, and Ar. While the latter ions are particularly efficient in treating hypoxic tumors, they also induce normal tissue toxicities,51 which may be mitigated by MBRT. Additionally, their physical scattering properties are ideal for maintaining the minibeam spatial dose pattern at greater depths in tissues than possible with protons.52 Neon MBRT has shown substantially lower skin tissue toxicities53 than Ne-broad beam RT, with significant tumor growth delay in a mouse sarcoma model despite 75% of the tumor receiving <2 Gy (forthcoming data). A similar sparing of skin toxicity was also shown using Li-7 ions.54 MBRT with high-LET charged particles offers significant promise for improving the therapeutic index in PT, especially for tumors near organs at risk. More preclinical data are necessary to unravel the mechanism underlying MBRT and to explore potential synergies with PT, including the hypothesis of enhanced immune activation. Clinical translation of MBRT in the modern era is in its early stages; the authors are aware of efforts in photon and proton modalities, although currently there are no published results.
Boron neutron capture therapy
The principle of boron neutron capture therapy (BNCT) lies in the capture reaction of a thermal neutron by the boron 10B isotope, resulting in the production of a high-LET alpha particle (4He) and a recoiling lithium (7Li) nucleus. Due to the small tissue range of alpha particles (5-9 µm), the damage is primarily restricted to cancer cells where 10B atoms are preferentially delivered.55, 56 Clinical interest in BNCT spans a number of malignancies as high-grade gliomas,57, 58 cerebral metastases,59 primary cutaneous melanomas,60 and recently expanding also to liver and head and neck cancers.61, 62
Due to its complex radiation composition, BNCT's biological effects cannot be solely explained by absorbed dose. A better understanding of BNCT biological effects necessitates research using biological models and clinical trials integrating responses with known radiobiology. The conventional procedure for computing photon-equivalent doses in BNCT consists of adding the contributions of the individual radiation types to the total absorbed dose, each one weighed by a fixed factor, independent of dose and dose-rate.63 The currently used "weights" are RBE and compound biological effectiveness, obtained from reference cell survival experiments with γ-rays or x-rays. Patient treatment planning, despite the selected model, depends on reliable radiobiological data. It is thus crucial to report not only the photon-equivalent but also the total absorbed doses, including its components for the neutron dose used, the neutron source's spectrum, as well as the values of boron concentration in blood and tissues.
Boron neutron capture therapy efficacy strongly depends on the selective delivery and accumulation of 10B in tumors, achieved through boron carriers characterized by high tumor uptake and rapid clearance from blood and healthy tissues.61, 64 There are currently 3 constantly refined generations of boron compounds. The first generation includes boric acid and its derivatives; the second consists of boronophenylalanine and sodium mercaptan decahydro-closo-dodecaborate; and in the last decade, boron carrier development has taken principally 2 directions: small boron molecules and boron-conjugated biological complexes that represent the third generation.62
Studies pertaining to the use of fast neutron RT have contributed to a better understanding of the improved biological efficiency of particles in comparison to x-rays.64 With the emergence of accelerator-based BNCT, which will facilitate clinical trials in the traditional hospital setting, and improved drug delivery approaches under investigation, BNCT is primed to emerge as an important area of preclinical and clinical PT research in the years ahead.
Radiopharmaceutical therapy
Radiopharmaceutical therapy (RPT) uses radionuclides emitting α-particle or β-particle, Auger electrons, and γ-rays,65 in combination with targeting vectors (including peptides, antibodies or their fragments, scaffolds, and small molecules) against a range of tissue-specific tumor biomarkers that maximize the localization of these radionuclides at the site of disease, while sparing normal tissues.66, 67
There are several critical differences between RPT and external beam PT.68 Radiopharmaceutical therapy dosimetry is less well-defined69 due to a heterogeneous dose distribution in tissues and organs. While in RT absorbed doses may be directly measured or calculated with knowledge of the particle field and patient anatomy, in RPT both radiation transport calculations and the pharmacokinetics of the administered radiopharmaceutical are required.65 The Medical Internal Radiation Dose Committee formalism for estimating absorbed doses in nuclear medicine imaging66 has been adapted for alpha-particle emitters in RPT.67 Standardization, advancements in imaging techniques, and rigorous dosimetry, along with clinical reporting, are crucial for assessing the RBE for different tissues and agents used in RPT and for facilitating its use alone or in combination with other modalities.68 Second, RPT typical dose rates (<0.5 Gy/h) are nearly 2 orders of magnitude lower than conventional PT, influencing the biological response to radiation and the potential for bystander effects.70
Moreover, the radiation penetration depth differs between radiopharmaceuticals and PT. Both α-particles and Auger electrons have a high LET and consequently deposit their energy over micrometer (28-100 µm) and nanometer (<500 nm) ranges, respectively, compared to the millimeter (0.5-10 mm) range associated with β-particles.71, 72 This impacts the microscale distribution of radiation dose within the target volume and surrounding tissues. Lastly, RPT involves continuous radiation exposure with an exponential decay over hours to days/weeks, whereas therapy with particles is typically delivered over a shorter period, which may result in distinct radiobiological effects.
Several studies have investigated the combination of external RT with RPT.73, 74, 75 This approach aims to deliver precise high doses to the primary tumor using external RT while also targeting residual tumor and micrometastases with systemic RPT. By combining these modalities, the protection of normal tissues surrounding tumors during external RT and dose-limiting organs such as kidneys and bone marrow during RPT can be better achieved. Novel therapeutic modalities like carbon ion RT (CIRT) are being explored in combination with RPT in tumor cells.76 Carbon ion RT has been shown to uniquely activate immune responses, DNA damage pathways, and cell-cycle control mechanisms, enhancing the effects of RPT agents.75
Despite early forays, the translation of combination of RPT and external R into clinical practice has not gained significant momentum. This may be attributed to the complexity of dose estimations, as well as legal and administrative challenges in certain countries, particularly in the context of radiation treatment. However, with recent advancements in RPT and improved dosimetry protocols, further exploration of combining RPT with external RT is warranted, offering the potential for improved treatment outcomes and enhanced patient care.
Particle stereotactic irradiation
Stereotactic body radiation therapy (SBRT), also known as stereotactic ablative radiation therapy or radiosurgery when delivered in a single fraction, endeavors to deliver a wholly tumoricidal, ablative dose to a tumor target. The radiobiology associated with doses per fraction of >8 to 10 Gy differs significantly from conventional fractionation, with tumoricidal and normal tissue effects unexplained by the linear-quadratic model.77, 78 With conventional RT, it is generally accepted that most of the effects arise from unrepaired radiation-induced DNA damage, which results in mitotic death. In contrast, SBRT effects appear to be mediated by multifactorial indirect cell killing.79 First, vascular effects may drive excess tumor cell death as data have shown a differential effect on dysfunctional tumor vessels with endothelial apoptosis becoming significant above a ∼8 to 10 Gy single dose threshold (microvascular disruption resulting in death of the tissue supplied by that vasculature).80, 81 Second, large fractional doses with hypofractionation to some degree overcome hypoxia and radioresistance.81 Third, high fractional doses of radiation also seem to play a role in stimulating an immune response by releasing tumor antigens and inducing specific tumor responses.82 Finally, radiation-induced stem cell depletion is also likely important as stem cells can migrate into the radioablated tissue from neighboring undamaged tissue.83
Hypofractionation with Charged Particle Therapy (CPT) is promising.84 Based upon the seminal findings of Ando et al,85 who described a distinct advantage in treating a rat tumor with limited fraction numbers and high dose per fraction in the high LET region of a carbon ion beam compared to the limited adverse normal tissue response of the skin over the tumor site led to a number of clinical trials in different tumor types. For a review of those results, see.84, 86 An evaluating panel of Quantum Science and Technology clinical trials suggested that hypofractionation be accelerated because of the favorable outcomes in radioresistant tumors as well as providing greater access to the technology.86
Of concern, though, is the penumbra effects of smaller particles, such as proton RT, which may result in reduced low-dose delivery to surrounding healthy tissue but less sharp ablative dose fall-off outside the target tumor. Whether additional radiobiological mechanisms are involved is an area of investigation, particularly regarding LET deposition within the tumor target. Conventional conformal SBRT techniques may result in an LET distribution disproportionately deposited in tissues distal to the tumor along the beam path if LET-painting methods are not adequately applied.8 Limitations to wider implementation of particle stereotactic irradiation87 include uncertainties about dose computation, measurement, and radiobiological effects. Experience with protons is paradoxical: Once a classical/historical indication for pituitary tumors and vascular disorders (arteriovenuos malfromation) in the 1950s, the dosimetric limitations of small beams have slowed the implementation of modern particle SBRT for field sizes below 3 cm in the smallest axis. Detector design and algorithmic dose computational tools are currently an area of active investigation with promising proton stereotactic irradiation data.
New ions
While most radiobiological studies in PT focus on proton or CIRT treatments, ongoing research aims to explore alternative radiation modalities, building on efforts initiated in the 1970s during trials at Lawrence Berkeley National Laboratory and followed by decades of advancements in both physics and biology. To this end, ions heavier than carbon, particularly 16O,88 have been considered appealing for targeting radioresistant and hypoxic tumors. Their increased oxygen enhancement ratio, associated with increased LET values in the target, makes them less sensitive to the presence of hypoxic regions within the tumor. However, the use of these ions remains hampered by the increased risk of normal tissue toxicities associated with their high RBE.
Combinations with lighter ion beams, such as protons or 4He, where different ions, based on their physical and biological properties, are directed to certain parts of the tumors, may mitigate these risks.89 However, the efficacy of such approaches remains uncertain due to a lack of clinical and preclinical data, partly attributable to the absence of animal models suitable for testing field redistributions. Additionally, high-LET particle oxygen enhancement ratio (OER) models heavily rely on in vitro data, introducing uncertainties to in vivo effect estimation. Further experimental efforts expanding to preclinical models are thus crucial to refine OER modeling and understand the biological, chemical, and physical aspects of hypoxia radioresistance.
Heavy-ion facilities are cost-prohibitive in comparison with conventional or proton therapy, hampering their clinical adoption. Light ions, such as 4He or 7Li, may represent a good compromise between proton and CIRT in terms of cost-effectiveness. Furthermore, while 4He beams have already been recently integrated into clinical practice,90, 91 Monte Carlo models suggest that their radioactive isotopes can offer higher Bragg peak doses without an increase of the dose in the plateau region,92 though the clinical translation of helium monotherapy has not been verified to date. Although their production at sufficient intensities remains challenging in clinical settings, modern high-intensity accelerator facilities already offer the possibility of first pilot studies.
Multimodal radiation therapy
Numerous novelties within systemic therapy and photon RT may provide robust translation opportunities for combinatorial effect with PT, above and beyond what is possible with conventional RT.
Charged particle therapy in combination with immunotherapies
Immunotherapies, mainly immune checkpoint inhibitors (ICI), are currently a component of standard of care in several cancer types and are often included in cancer therapy regimens in combination with other systemic and local therapies, including RT. The combination of immune and RT has shown promising results in patients who did not respond to other therapies.93, 94 The mechanistic basis for such a combination is RT immunogenicity, that is, the creation of de novo antigens (referred to as antigenicity) and the release of factors attracting and activating immune cells (ie, adjuvanticity).95 The induced immunogenicity is then boosted with (neo)adjuvant immunotherapies, mostly ICI.
However, only a fraction of patients responds to such combinations, and recent studies combining photon RT with ICI have failed to meet their primary endpoints.96 The design of these studies has been criticized, showing the importance of clinical trial design for combined treatment with immunotherapies, and leaving significant room for improvement. From a biological perspective, the lack of signal from these trials could be influenced by the decision to irradiate lymph nodes. While they are intentionally irradiated during therapy to eradicate putative metastases, recent experimental data have shown that sparing lymph nodes is pivotal for an efficient immune response following RT.97 From this perspective, PT may provide beneficial properties for combining radiation with immunotherapy due to the physical and biological features of particles.98, 99 The high precision of beam delivery allows for improved sparing of circulating lymphocytes (and other lymphoid organs-at-risk like bone marrow, thymus, or spleen), and hence immune cells are available for an immune response. This is supported by evidence showing a lower degree of lymphopenia following PT.99 The level of immunogenicity of the cellular response to RT is crucial and mainly depends on 2 factors, that is, antigenicity (neoantigen repertoire triggered by radiation exposure) and adjuvanticity (release of immunogenic danger signals during cell death or stress response).100 In this light, charged particles, especially CIRT, are discussed to be of advantage due to an increased RBE, different cell death patterns, and clustered and more complex DNA damage. Ultimately, the question is whether immune “cold” tumors can better be turned into “hot” tumors by PT as compared to conventional RT.101, 102 While generally the mechanisms of RT on immunogenicity are not well understood (eg, with respect to dose or fractionation schemes), this is particularly true for PT. Other open questions are related to the sequence of administration of immunotherapies relative to RT.103
Two interesting new approaches in RT regimens propose to bootstrap immune-related features of tumors via the host or the treatment, by changing patient fractionation schemes either temporally or spatially. In preclinical animal models, the personalized ultrafractionated stereotactic adaptive radiation therapy (PULSAR) regimen separated fractions by several days, thereby allowing time for adaptation within tumor tissue as well as with respect to the immune response.104 In human patients, this gap between fractions can be weeks. This follows the hypothesis that repeated longitudinal exposure to tumor antigens may amplify the adaptive immune response and thereby improve immune control of metastatic cancer disease.105 Personalized ultrafractionated stereotactic adaptive radiation therapy has not been used with PT yet, but the ultrafractionation and a hypothesized improved immune response render PT attractive for implementation in PULSAR.
One spatial fractionation approach is called stereotactic body RT-based partial tumor irradiation targeting hypoxic segments of bulky tumors (SBRT-PATHY), demonstrating promising initial outcomes. The authors are investigating apparently improved exploitation of bystander and abscopal effects, aiming to specifically target the hypoxic and immunosuppressive parts in the tumor microenvironment while sparing the peritumoral immunological microenvironment, including nearby tissues, blood-lymphatic vessels, and lymph nodes.106, 107 This is hypothesized to enhance immune response. Based upon these promising results, carbon-PATHY takes advantage of the proposed mechanisms above along with the advantages of carbon ions, including a reduced OER. Indeed, the approach is being tested with CIRT in clinics with promising results.108, 109
In summary, the combination of charged particles and immunotherapy may be powerful but many questions remain to be answered. It is pivotal that particle radiobiologists work closely together with immunologists to shed light on the immune-related mechanisms of PT. Initial forays into clinical translation have begun, but poor access to PT centers limits robust study of these observed effects.
Radiosensitizers
Radiosensitizers are compounds that augment the potency of ionizing radiation in eradicating tumor cells, typically measured by their enhancement ratio.110, 111, 112 The interest in applying radiosensitizers in combination with particles is growing due to the hypothetical ability to reduce total patient dose while increasing tumor control probability.113 Considering the basic biological mechanisms of action, radiosensitizers can be classified into 5 categories: (1) suppression of intracellular thiols or other endogenous radioprotective substances, (2) formation of cytotoxic substances by radiolysis of the radiosensitizer, (3) inhibitors of DNA repair, (4) thymine analogs that can incorporate into DNA, and (5) oxygen mimetics that have electrophilic activity.114, 115 Regarding their different structures, radiosensitizers can be classified as small molecules, macromolecules, or nanomaterials.116, 117 Some small molecules that are currently being investigated include peptides, miRNAs, siRNAs, and oligonucleotides.114, 117 One strategy for radiosensitizers has been to repurpose drugs with approved noncancer indications and review their efficacy in combination with radiation or chemoradiation through clinical trials, allowing rapid trial evaluation without the need for early-phase, regulatory-approval studies.118 Other radiosensitizers, such as naturally occurring products (phytocompounds), are also undergoing clinical trials.119 Examples include curcumin, resveratrol, dihydroartemisinin, and paclitaxel.114, 117
Furthermore, the use of nanoparticles (NPs) has drawn attention in recent years.120, 121, 122 Technological advances in NP synthesis/functionalization have led to significant advances in molecular detection, imaging, targeting, multifunctional therapeutics, and prevention and control of diseases.121 In particular, the application of metallic NP has received growing attention as a radiosensitizer in PT.113, 114, 120 Some NPs with proven radiosensitizing effects are made of noble metals (eg, gold, silver, and platinum) or heavy metals (eg, gadolinium, hafnium, tantalum, tungsten, and bismuth).114, 122 The radiosensitization potential depends on numerous factors: cell line, NP type and size, concentration, coating, intracellular localization, and energy and nature of radiation.122 Different methodologies have been proposed to study the radiobiological effect of these materials.123 Noble metal nanomaterials have been extensively studied using x-rays.113, 114, 124 However, the mechanistic explanation for the local dose enhancement provided for photons is different from that for ions, where the dose is already highly localized along the tracks, and an extremely high local dose is required to increase the damage further, without even accounting for overkill effects. In this case, the enhancement of the radiation effects is not yet fully understood.113 Similar mechanisms have been reported, such as an increase in secondary electrons together with the increase in reactive oxygen species formation, oxidative stress, inhibition of DNA repair, changes in the cell cycle and organelle function that increase cytotoxicity, inhibition of the expression of radiation resistance genes, or the promotion of expression of radiation-sensitive genes.114, 124 Considering that NPs induce oxidative stress and inflammation, an evaluation of ion release and subsequent biological responses, oxidative stress, and inflammation is important for nanotoxicity.125 Also, the selective delivery of NPs could be passive or via delivery systems with tumor-specific agents.113, 126 Moreover, antisense oligonucleotide genetically loaded NPs can also be designed for use via gene radiosensitization.126
Despite the advantages of nanomaterials, few have been translated into clinical trials.127, 128 To confirm the uptake in correct locations, NPs that have translated to clinical trials have tended to be “theranostic“ agents, that is, visible on diagnostic images prior to irradiation.129, 130, 131, 132, 133 Although there has been great interest in the use of noble metal NPs, investigation is still needed to control and optimize their effect before translation into clinical trials. Furthermore, other NPs were found to have radioprotector effects instead,134, 135, 136, 137 which is a potentially alternative approach. In addition, while the oxygenation of the tissues may play a significant role, the OER effect associated with the presence of NP has not been considered.113 Despite that, some compounds that mimic oxygen capabilities are being investigated.114 Moreover, hypoxia-specific cytotoxins could be used for overcoming the radioresistance of hypoxia tumors.114
Prediction and prognosis in radiation oncology
In medicine, prediction can be directed at 3 aspects of an individual’s health status. One can predict the risk for a given cancer, one can predict the response to a given therapy and one can predict the risk for disease recurrence. Prognosis, on the other hand, speaks only to the overall outcome regardless of therapy or perhaps to standard therapy and does not rely on the data one might use for prediction. Prediction relies on biomarkers, a term often used casually and which is defined by the FDA as a validated characteristic that is objectively measured as indicators of health, disease, or a response to an exposure or intervention, including therapeutic interventions.
Precision medicine in radiation oncology can be broadly divided into efforts by the physical sciences, that is, not only the application of imaging-based physical mapping and precise localization of target tissue but also the heterogeneous and unique features derived from image analysis, so-called radiomics. Biological approaches that quantify the entire collection of specific categories of biological molecules that can translate into the dynamic function of a cell, tissue, or organism are called radiogenomics.
Omics can be broadly classified at the whole genome level, where single nucleotide polymorphisms (SNPs) are examined, and by copy number variation, where chromosome structure is examined to identify gene copy number, inversions, deletions, and other genomic rearrangements. Just below the genome level is transcriptomics—the characterization of all transcribed RNA species whether translated into protein or not; proteomics—the characterization of proteins; epigenomics—the evaluation of the locations and functions of chemical tags, DNA methylation as an example, along the genome; and metabolomics, where the metabolites generated by cellular function are characterized. In radiation oncology, most effort in omics analysis has focused on tumor transcriptomics; however, unlike other disciplines in medicine, radiation oncology has also examined the responses of normal tissue given that treatment outcomes are not only based upon tumor control probability but also the probability of normal tissue complications. Furthermore, assay development besides following more traditional approaches to omics analysis focused on direct analysis of tumor or normal tissues, new less invasive approaches that examine circulating factors such as circulating fragmented tumor DNA (ctDNA), circulating miRNA, or circulating exosomal cargo (DNA, RNA, and proteins) are gaining favor based upon increased computational power for next generation sequencing and the limited invasiveness of fluid (blood, urine, sputum)-based assays.
Indicators of individual radiosensitivity
Individual radiation sensitivity (iRS) characterizes the specific tissue/cellular response to ionizing radiation and has a significant influence on the variables affecting late RT toxicity. Like other biological processes, iRS is represented as a Gaussian curve, with patients with very severe tissue responses but with a broadly normal phenotype at the left of this curve.138 At the molecular level, damage to DNA and biomolecules, DNA repair pathways, cell death, as well as oxidative stress strongly impact the RT toxicities and the sensitivity to specific DNA damaging systemic therapies. As a possible biomarker for radiosensitivity, the relationship between genes, their products, or regulators has been largely investigated.139 However, implementing these potential biomarkers in the RT workflow remains challenging. Several approaches have been developed to identify biomarkers for patients undergoing RT, including preclinical and clinical studies, agnostic approaches like high-throughput proteomics or genome wide CRISPR screenings or genomic analysis of resistant cellular models.140, 141, 142
Most known biomarkers are related to the Hallmarks of Radiobiology: DNA damage repair, tumor cell redistribution in the cell cycle, repopulation, reoxygenation, and radiosensitivity. For instance, the functional analysis of subnuclear DNA damage response (DDR) foci in tumor tissues, peripheral blood lymphocytes,143 or circulating tumor cells (CTCs) might predict iRS. These foci are dynamic multiprotein complexes centered around a DSB. They appear as "dots" in cells or tissues under immunofluorescence and have a structure related to the entity of DNA damage, the cell cycle stage, and local chromatin structure. These sites represent local spreading and intensification of signal after DNA damage and function as a "toolbox" to support downstream function of the DDR including DNA repair and cell cycle checkpoint responses.144, 145 The primary advantage of these DDR foci as an iRS functional biomarker is their ability to disclose the presence of repair deficiencies, including defects from changes in signal transduction pathways,146 epigenetic events, or gene mutations.138, 147, 148, 149 These foci offer a comprehensive evaluation of DDR network performance without requiring knowledge of every network component—many of which are currently unknown.145 It is possible to imagine creating mechanism-based "DDR foci signatures" where network nodes are represented by the presence of key proteins such as γ-H2AX, 53BP1, BRCA1/2, RAD51, FANCD2, or others. Indeed, there is a linear correlation between cell survival after irradiation and residual γ-H2AX, making them a surrogate of radiosensitivity,150, 151, 152 as well as tumor bioptic predictive biomarkers of radiosensitization caused by a molecular targeted agent.153, 154 Compared to γ-H2AX, 53BP1 seems to be more accurate in identifying DSBs making it an alternative biomarker.155, 156 Other mentioned proteins are employed as a surrogate of homologous recombination repair and nonhomologous end joining activity and might contribute to the additive toxicity in the combination approaches, also after CIRT.2, 157
It is also noteworthy that cell lines with homologous recombination repair mutations have decreased OERs compared to normoxic scenarios, with irradiation in hypoxia leading to the creation of more DNA interstrand cross-link formation. Effects of HR on DNA-protein cross-links are still a possibility, as there is strong evidence of higher yields in hypoxic environments. Because of hypoxic dependency and proliferation, targeting HR to promote radiosensitivity might provide for partial tumor selectivity.158 Moreover, hypoxia-induced changes in DNA mismatch and base excision repair lead to chromosomal instability, providing the basis for a “mutator” phenotype.158 The variation in hypoxia might be traceable in gene expression signatures (ie, HIF-1)159, 160 and in the meantime through the analysis of radiomics and radiogenomic features,161, 162 serving as promising noninvasive/minimal-invasive predictive biomarkers of radiosensitivity/resistance.
Fluid-based biomarker development
Single nucleotide polymorphisms in DDR genes identified in peripheral blood cells have been shown to be associated with iRS, with a tissue specificity for each genetic determinant and “linkage disequilibrium” for which some SNPs can catch most of a regional genetic variation.138 One of the most analyzed is the missense ATM SNP rs1801516 that has shown to correlate with high risk of post-RT fibrosis, especially in breast and prostate cancers.163, 164 Moreover, for some SNPs,165, 166 only the heterozygous state confers their respective radioprotective and sensitizing effects. The combination of polymorphisms in different alleles seems to be a feasible approach to assess the iRS.167 Although encouraging, the SNPs model was rarely applied to the validation cohorts, and it is difficult to obtain significant statistical power to assess isolated SNPs. Through the European REQUITE project, most advancements have been made in identifying SNPs linked to late toxicities in breast and prostate cancer.168 The combination of SNPs with the dosimetric parameters (Normal Tissue Complication Probability (NTCP) and LET distributions), clinical risk factors, and comorbidities linked to high intrinsic radiosensitivity (ie, radiosensitive syndromes) might be helpful in defining patient-tailored iRS.
Besides following more traditional approaches to omics analysis focused on direct analysis of tumor or normal tissues, new less invasive approaches that examine circulating factors such as ctDNA, circulating miRNA, or circulating exosomal cargo (DNA, RNA, and proteins) are gaining favor based upon increased computational power for next generation sequencing necessary to analyze ctDNA from circulating free DNA and the limited invasiveness of fluid (blood, urine, sputum)-based assays.
The assessment of ctDNA found in blood and urine can also take a different strategy from the approaches described above in that the analysis is designed to detect cancers earlier169, 170 and predict therapeutic response but also to detect minimal residual disease (MRD).171, 172, 173 By tracking specific biomarkers, the overall response to therapy and, subsequently, MRD can be followed, and, most importantly, there is the potential to identify disease recurrence well before pathophysiologic indicators of recurrence. Exosomal cargo (DNA, RNA protein, miRNA)174, 175, 176 and circulating miRNAs177, 178 are also advancing in the search for fluid-based biomarkers of radiotherapeutic response and MRD.
Circulating tumor cells
Isolating CTCs from patients' blood has been a challenge for researchers, which has led to only a few studies to detect the effects of radiation on the CTCs. However, being able to isolate and culture these cells in vitro could provide enormous benefits and is worth exploring. Monitoring CTCs in cancer patients undergoing RT could provide new insights into how metastatic spread is influenced by radiation and vice versa. Additionally, CTCs could become a valuable source of biomarkers, a liquid biopsy, used to study treatment response. A relapse during RT has been associated with an increase in the number of CTCs and thus, their count could be used as predictive biomarker for clinical trials.179
Conventional radiation has been shown to disrupt the primary tumor vasculature, potentially increasing the dissemination of CTCs. For instance, during early-phase RT an increase in CTC number in non–small cell lung cancer (NSCLC) was observed.180 Photon radiation can also induce epithelial-mesenchymal transition, which could potentially lead to dormant CTCs awakening, fostering proliferation, resistance, and metastasis. The shedding of mesenchymal marker-expressing CTCs was observed during NSCLC RT, potentially informing new strategies to monitor metastatic spread post treatment.180, 181 In a study of a large cohort of patients with early-stage breast cancer, it was reported that CTC-positive patients that received RT had a longer survival rate compared with nontreated patients.181 Radiation therapy in combination with chemotherapy has been shown to reduce the number of head and neck squamous cell carcinoma and prostate CTCs.182, 183, 184 The number of CTCs, indeed, is a suitable marker for the response to x-rays RT,185, 186 but there is a lack of publications that examine in detail the influence of RT on CTC genotype. Furthermore, it should be noted that almost all previous studies on RT and CTCs were focused on x-rays with some exceptions dealing with protons and carbon ions. Exploring the quantity and nurturing of CTCs in vitro postparticle irradiation could unveil novel markers for probing therapy development. This exploration may also illuminate diverse mechanisms underlying metastasis formation in both conventional and particle RT.
Besides CTCs or other genomic content in blood, blood chemistry is also of increased interest for stratifying patients and predicting treatment response.187 In this scenario, the blood cell count is an easily and often available parameter that might reflect the inflammatory response188, 189 as well as the oxygenation status.190 Recent literature showed their prognostic role in several tumors and in different RT settings, including high LET.191 Routinely available in this context is also the blood glycemic status that, when increased, seems related to more aggressive tumor phenotype.192, 193
Genomic-adjusted radiation dose
The concept of genomic-adjusted radiation dose (GARD)194 proposes to exploit information on genomic expression obtained from a tumor biopsy or a liquid biopsy, like the CTCs extracted from a patients' blood, to estimate the radioresistance of the malignancy affecting a specific patient. In this framework, it is then theoretically possible to set up a personalized dose prescription for each single patient for whom the genomic expression test is available. This would comply with the ideal need to deliver a treatment that is as far as possible tailored to tumor biology, thus aiming at higher effectiveness. An approach of this type could be pan-cancer, that is, tumor site-agnostic or tumor site-specific, where the genomic panel used to interrogate the tumor is unique to the tumor site.
A pan-cancer approach was developed by ultimately combining the surviving fraction at 2 Gy for 48 cancer cell lines from the NCI-60 panel with a biomarker discovery platform that, starting from an initial set of 500 genes, identified 10 hub genes whose expression could be used to estimate surviving fraction at 2 Gy.195 This Radiosensitivity Index (RSI) appeared to be strongly related to radiosensitivity. Subsequently, the RSI algorithm was then tested using 3 gene expression data sets of patients treated with chemoradiation to predict tumor response and prognosis,196 followed by examination of other tumor sites treated with, or not, radiation including breast,197 endometrial,198 and pancreatic cancers.199
To develop the concept of a genomic-adjusted radiation dose, the RSI was combined with the linear-quadratic model for cell survival, where the RSI value contributes to the α term, which includes the patient-specific treatment response. The GARD determined for a given patient is calculated as nd(α + βd), with n number of fractions and d dose per fraction. The GARD was tested on several cohorts of patients previously receiving RT.194, 200 It was shown that, when accounting for the RSI, the variability in the GARD values determined was much larger than that due to the physical dose received. Namely, where a dose stratification was performed, there could be patients originally assigned to the low-dose group, who due to their intrinsic radiosensitivity might be better served in a higher dose group based upon their calculated GARD value, and vice versa. This was also corroborated by a later study, where GARD was found to be associated significantly with both time to first recurrence and overall survival.200
The proposal of GARD elicited positive feedback as well as criticism such as the patient populations used for analysis and criteria for defining recurrence, among others. The application of GARD in an era of increasing use of stereotactic ablative radiation therapy was also noted which would likely apply to hypofractionated CPT. Furthermore, a recent study based on a reanalysis of the original publication as well as on additional data raised skepticism on the possibility to use RSI as a robust tool to adjust dose prescriptions in RT,201 which was defended through correspondence with the original GARD proponents. Obviously, the limited data currently available do not allow drawing final conclusions of the clinical potential of the RSI and, therefore, of GARD as an effective tool for RT individualization. Importantly, there is at least 1 clinical trial (ie, NCT05528133 focused on triple-negative breast cancer) currently recruiting patients. The outcomes of this and similar studies will shed light on this promising as well as debated topic.
The RSI tool and the GARD approach were developed in the framework of photon RT using conventional fractionation. It is possible that gene expression is differentially modulated by different types of radiation such that extending GARD to CPT would require additional investigation. At the same time, it could be hypothesized that the RSI is associated with smaller interindividual variations in RBE. Nevertheless, it could be intriguing to investigate the possibility of combining the high spatial selectivity of particle beams with both their higher RBE and a genomic-based personalized dose prescription.
Besides GARD other biomarkers within a specific disease site have been developed and validated. This includes the radiation sensitivity signature for breast cancer.202 This 51 gene signature was independent of disease subclassification and outperformed all other clinicopathologic predictors of treatment response. The radiation sensitivity signature signature is strongly linked to cell cycle and DNA repair pathways. Similarly, the RadR signature, developed in HPV-head and neck squamous cell carcinoma was unique for the use of tumor and paired normal mucosal samples, cell lines, and genes associated with disease-free status after surgery and radiation.203 The resulting 13 gene signature was used in an integrated analysis along with genomic alterations, protein expression, and drug sensitivity. While the RadR score was associated with molecular classification, the median RadR score was capable of segregating patients treated by surgery plus radiochemotherapy based upon recurrence-free survival, but not those treated by surgery and chemotherapy, thus validating the specificity of RadR for radiation response. Neither of these signatures has been applied to PT, although there is potential to determine if these signatures are pan-RT, that is, apply to CPT as well as to x-rays.
Confounding the development of radiation response biomarkers via omics analysis are dosimetric and volumetric considerations that are rarely, if at all, accounted for. Hypofractionated schedules, like those often used with 12C or stereotactic or ultrafractionated schedules, may negatively impact the accuracy of some algorithms that were developed from information gleaned from patient populations treated with conventional 2 Gy/fraction exposures. There may be archived tissues from CPT trials with dosimetric and volumetric information along with clinical and pathologic information, where putative biomarkers could be tested for application in CPT, be it normal tissue or tumor response. Not addressed at all is the contention that age (cancer patients are generally older) and sex may bias biomarker screening.204
Radiomics
Genomic-adjusted radiation dose and RSI represent a possibility for biologically motivated patient stratification; however, they are associated with additional time, effort, and costs for the requisite biological analyses. Radiomics endeavors to utilize the diagnostic images gathered as part of routine clinical practice, such as computer tomography, magnetic resonance imaging, positron emission tomography, x-ray, and ultrasound, extract objective, quantitative image features from the image, and then analyze these features for correlation or prediction of clinical features, generally using machine learning.205, 206 As early as 2014, a clustering between certain image features and the tumor stage and histology was predicted for NSCLC tumors,206 paving the road for radiomics as potential biomarkers for diagnosis, prognosis, and prediction. To establish such a computerized model, 2 data sets are required: a training data set and a data set for validation. The more heterogeneous the data sets are (different clinics, countries, devices, etc), the more robust the model becomes, but consequently, more data sets (ie, patient images/data) are needed for model development. Particle Therapy Cooperative Group may be one such platform for the development of such databases of particle-treated patients.
Dosiomics extends radiomics by extracting useful features from 3-dimensional RT dose distributions for the prediction of treatment outcomes207, 208 or normal tissue responses.209 Indeed, dosiomics applied to skull base chordomas treated with CIRT revealed the association of these features with adverse outcomes.210 In addition, the dose-averaged LET in CIRT has been found to correlate with local recurrence in chondrosarcomas211 and sacral chordomas,212 leading to an increased interest in a combined RBE-based and LET-based treatment optimization. In this regard, a retrospective analysis213 using dosimetric-identified features to identify possible quantitative prognostic factors to predict local control in sacral chordomas suggested that features extracted from LETd maps can be employed for patient stratification into high-risk or low-risk groups for disease recurrence.
Of particular interest for RT are delta-radiomics, where a longitudinal image sequence is analyzed for signature change. Longitudinal image sequences are continuously generated in RT through imaging diagnostics, during treatment, and as a control in the follow-up. Without additional burden for the patient, a prognosis for the treatment response could already be made during treatment and, if necessary, the treatment could be adjusted. To date, radiomic analyses have not been robustly linkable to biomedical processes, and efforts are underway to develop quality standards for model reporting, such as Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis214 and the Radiomics Quality Score—RQS 2.0.215 Nevertheless, there is great potential for translational biomedical studies that attribute changes in image signatures to biological processes.
Recent research approaches have adapted classical imaging formats such as histological staining of tumor tissue by hematoxylin & eosin staining, used in routine diagnostics with radiomics.162 These histo-radiomics studies are predestined for preclinical trials with orthotopic and heterotopic tumor models or replacement methods such as the chorioallantoic membrane assay, which also allows xenotransplantation of primary and established cell and tissue cultures that grow into well-vascularized tumors for tumor microenvironment studies.216 The tumors can be sampled at different times after irradiation and examined classically by histology or with spatial omics such as Matrix-Assisted Laser Desorption Ionization imaging217 providing insights into biological processes. Combined radiochemotherapy is the standard of care for most tumors, meaning that not only RT alone but also combined approaches with established chemotherapies and new substances such as targeted drugs should be investigated. Here too, preclinical tumor models have an advantage over the clinic in terms of sample throughput and substance spectrum. Overall, (histo)-radiomics has great long-term potential to stratify patients and to make recommendations for dose, fractionation, radiation quality, and systemic therapies.
Radiobiology model systems
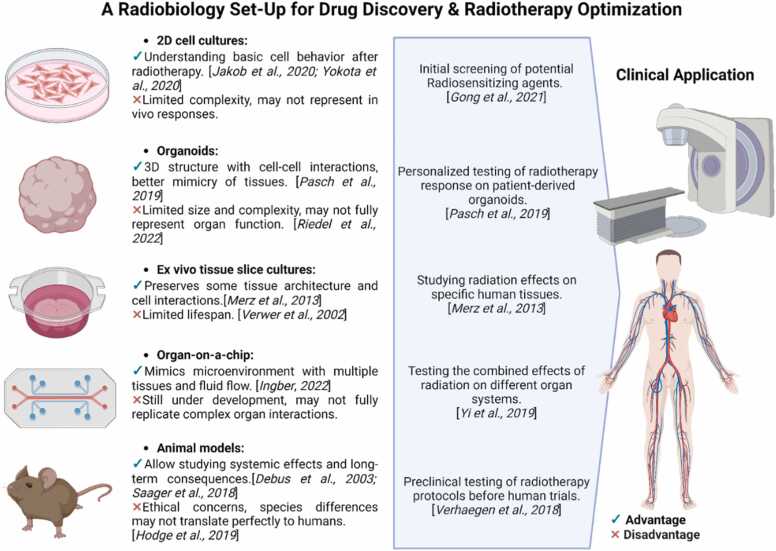
Biological studies often rely on adherent cell monolayers or 2D cell cultures for their convenience and ease of maintenance. Such models have advanced the understanding of basic of cellular mechanisms post RT, including DNA damage and repair, survival, and cell death.218, 219 They also serve as effective tools for drug screening, particularly for evaluating radiosensitizing agents.114 Alternatively, ex vivo tissue slice cultures or patient-derived explants can be relatively easily prepared in replicates to study the effects of radiation in the same sample in an organotypic environment with preserved original tissue architecture. While long-term (several months) culture of rodent tissue slices has been reported, human glioblastoma slices could only be maintained for several days or weeks in some cases220, 221 with the quality of patient material being the limiting factor.
Other conventional models that have advanced cancer radiobiology research include animals and small animal RT research platforms.222, 223 Mouse models, for example, allow for mimicking systemic radiation effects in order to observe and evaluate the impact of acute and late normal tissue effects at the organismal level. Studies in mice have for instance shown that radiation does not only affects synaptic function and behavior224 but also causes a decrease in dendritic complexity, reduction in dendritic spines and synaptic plasticity in the brain, leading to cognitive dysfunction.225 Long-term radiation studies in rodents also showed increased vascular perforation leading to radiation myelopathy.226, 227 Unfortunately, animal findings may not fully translate to human clinical settings due to differences in genetics, morphology, anatomy, and metabolism,228 all of which are factors in the response to therapeutic treatments. In addition, animal studies also bring ethical considerations.
Recent advances in stem cell research and 3D cell/tissue technologies may circumvent these issues. Unlike spheroids, generated from immortalized cell lines or primary cells, organoids are derived from stem cells or primary tissue (ie, patient-derived organoids [PDOs]).229, 230, 231 The use of PDOs, which truly resemble the original tissue or tumor with its specific set of mutations and/or inflammatory characteristics, allows for predicting the efficacy of RT and optimizing personalized treatment strategies.232 While PDOs represent a patient-specific disease state and are generated in a short time, organoids are derived from induced pluripotent or embryonic stem cells. This circumvents the problem of limited availability of human samples and allows the generation of multiple organ-specific cell types and subtypes from the same genetic background as well as easier gene editing. Their organ-like properties bridge the gap between cell culture studies and clinical approaches. A recent study using human brain organoids showed that postradiation image changes, that is, contrast-enhancing lesions, can be attributed to the formation of aberrant blood-cerebrospinal fluid barrier or choroid plexus in response to altered NOTCH and WNT signaling involved in cell differentiation.233 Furthermore, human organoids can be maintained in long-term culture as organoid slices cultured at the air-liquid interface,234 allowing for studies of long-term radiation effects.
To support the biological application, engineers, together with biologists, are developing an organ-on-a-chip, microfluidics-based approach, which may open up even more possibilities for Tumor MicroEnvironment modeling235, 236, 237 and other applications within radiobiological research.238, 239, 240
While these model systems, summarized in the Figure, offer significant advantages in terms of controlled experimental conditions and the ability to mimic specific aspects of the tumor microenvironment, they may not fully replicate the intricate interactions and complexities found within living organisms.241 Furthermore, the dose of radiation applied can have different outcomes depending on the dynamic structure and function of the organs. However, despite these limitations, continued advancements in technology and methodology hold great promise for enhancing our understanding of radiation-induced effects and developing novel treatment strategies for conventional x-ray therapy as well as CPT.
Figure.
Different biological models according to their level of complexity, from cellular to tissue/organ to organism level, with their advantages and disadvantages and the possibilities they offer in drug discovery and radiation therapy optimization. Created with BioRender.com.
Modeling
Mathematical models in radiation biology allow us to challenge underpinning mechanistic hypotheses which, once validated, can be used as tools to assess radiation effectiveness for treatment planning or other endpoints related to CPT. Here, we summarize selected fields of current interest in effects modeling for protons and heavier ions.
Relative biological effectiveness modeling for treatment planning
The RBE depends on both physical (LET, energy, dose, and fraction number) and biological factors (α/β ratio, ratio of α’s), requiring a consistent description of these dependencies for complex radiation fields in a therapeutic setting. There are 2 opposing strategies to model RBE: (1) mechanistic models predicting RBE values by simulating selected underlying physical, chemical, and biological processes, while (2) empirical models fit analytical functions to experimental results and extrapolate them to new scenarios. In fact, most models exploit elements of both strategies.
A feature of ion radiation is that RBE increases with LET depending on ion type and α/β ratio before this trend is inverted due to overkill.242 Two mechanistic models are used in treatment planning: the local effect model (LEM I)243 and the modified microdosimetric kinetic model in Japan.244, 245 Model variants have been suggested (eg, LEM IV,246, 247 Mayo Clinic Florida-Microdosimetric Kinetic Model (MKM)247). An assessment of the MKM and other models248 inspired the creation of GSM2.249 Still other improvements are under discussion. Further mechanistic models also exist, for example, in nanodosimetry250,251 and machine learning-driven modeling.252, 253, 254 The advent of new heavy ion centers underlines a need for comparison of treatment plans optimized with different RBE models.255, 256 Issues in dose reporting have been addressed,257 but given the scope of possible future model diversification, discussing model-induced uncertainty in carbon ion therapy is of high relevance. The advent of new heavy ion centers underlines a need for comparison of treatment plans optimized with different RBE models.255, 256 Issues in dose reporting were addressed,257 but in the scope of possible future model diversification, discussing model-induced uncertainty in CIRT is critical.
In proton therapy, an RBE of 1.1 is usually employed. However, there is strong evidence from cell experiments and recent clinical indications for an increase in RBE with LET.258 A family of empirical models attempts to describe the LQ parameter dependence on LET and α/β.259, 260 The models differ in the expressions for the fitting parameters and the data used to obtain them, reflecting uncertainties in RBE determination. Additionally, there have been attempts to apply the LEM and MKM to proton therapy.261, 262, 263
Several pools of experimental RBE collections are becoming available, for example, the particle irradiation data ensemble264, 265, 266 and large single sets of experiments,267, 268 reflecting the richness of RBE systematics. These data sets are fundamental for tests of the models, which should be fitted to specific data and tested independently on other portions of the database (eg, different ions or LET regimes). Dedicated experiments can also be performed to confirm the model predictions, in particular when applying them to multiple situations (eg, varying dose rates and different endpoints).
NTCP modeling
The differences in dose deposition patterns between photon and proton therapy are exploited in the Netherlands in the “model-based approach.”269 Here, NTCP dose-response curves are parameterized for specific therapy-related side effects, and the NTCP for each modality is compared prospectively. The results allow stratification of patients who benefit most from proton therapy in terms of reduced normal tissue effects. Similar concepts have been explored elsewhere.270 While a direct NTCP-driven optimization has not been applied to high LET particles, similar approaches introduced an objective based on the equivalent uniform dose271, 272 or its generalized form.273 Artificial inteligence-driven optimization is currently used in some general treatment planning solutions272 but still not used in dedicated biological dose optimization methods. A new frontier is undoubtedly in this direction.
Modeling immunologic radiation action
Immune-modulating effects are increasingly exploited clinically in combination with immune checkpoint blockers. Model attempts are still rare, mainly because the immune system works as a complicated network of cells and signals, and their interplay with radiation burden is not sufficiently understood. While existing models are mostly tailored for photon radiation,274 some models consider application to particle radiation.275, 276 Particle radiation may offer enhanced effectiveness in achieving a systemic antitumoral response, including abscopal effects and sparing of lymphocytes, which are key players in the immune response.277
Modeling spatial fractionation
Current modeling efforts focus on SFRT mechanisms related to cell-to-cell signaling and the immune system. Free radicals and reactive oxygen species are the first chemicals generated and transported between cells. Numerous studies have investigated their production and diffusion in silico.278, 279, 280 Experimental assays have proven the involvement of these and other later generated species (Ca2+, NO, exosomes, cytokines, and other proteins) in bystander-like effects,281, 282 but there are few experimental studies quantifying their production and cell survival models reflect that, for example.283 In van Luijk et al284 and Asperud et al,285 it was found that the inclusion of a nonlocal repair factor in their NTCP empirical model was key, supporting the idea of the immune system playing a role in SFRT. More recent analytical studies285 managed to model tumor volume growth by considering the activation of cytotoxic T-lymphocytes after partial and full irradiation of the tumor volume.
Modeling FLASH effects
While the dose rate effect for protracted irradiation has been adequately described by current models,286 it appeared very hard to reconcile the observed protective and differential effects in the high dose rate range. Most research was concentrated on the chemical stages. Previous research in spatiotemporal effects of tracks287 was exploited, which contributed to determining the implausibility of some of the initially proposed hypotheses, such as transient hypoxia for oxygen depletion26 and the early intertrack recombination.288 In this context, a clear LET dependence was emphasized289, 290 and led to the hypothesis that high LET should either correlate with a strong reduction of the protective effect15 or several mechanisms should be discarded, focusing on others,291 or there is a different type of FLASH effect, specific for ions.292 Besides mechanistic models, a successful phenomenological description of the FLASH effect has been proposed.293
Closing remarks
This review provided a panoramic overview of critical research topics and emerging new frontiers in radiobiology, highlighting the transformative innovations and techniques reshaping the field. Particle therapy should have distinct clinical advantages over conventional RT, particularly for heavy-ion and multi-ion approaches, and a robust understanding of the underlying radiobiology is critical to exploiting these benefits to enhance patient care. However, to date, we have effectively only scratched the surface of what appears possible.
We have entered a transformative era in radiobiology, one that transcends the traditional boundaries defined by physics and treatment planning verification. Historically, radiobiology was predominantly the domain of physicists, focusing primarily on the physical aspects of radiation and its application in treatment planning. This foundational work was crucial in ensuring the safety and efficacy of RT. However, we have now moved into a new era of radiobiology, where the focus has shifted toward understanding the molecular and systemic mechanisms of radiation response, and the differing effects noted on a per-ion, per-treatment, per-method basis. This new approach is not just about delivering radiation to a target; it is about comprehending the biological responses from the organism itself down to the molecular drivers elicited by different irradiation modalities.
However, for further advancements, a robust funding infrastructure is needed, beginning with a framework for simpler and faster access to particle facilities. New experimental rooms should be dedicated to radiobiology in existing PT centers, and new centers should be developed with a robust study of the underlying radiobiology in mind. Today, securing an hour or 2 of beamtime may require over 6 months of applications, paperwork, and planning. A network is necessary that allows those working in this sector to know the facilities that enable in vitro and in vivo experiments and to understand the procedures for accessing these facilities in a rapid manner. In the meantime, radiobiologists should explore new ways to perform experiments, investigating novel radiobiology model systems and allowing for detailed explorations of cellular and molecular mechanisms.
Traditional radiobiology requires advanced modeling techniques, including RBE modeling for treatment planning, NTCP modeling, and others, but we must recognize that today’s radiobiology requires understanding and as a discipline needs new and advanced models for immunologic radiation action, spatial fractionation, and FLASH effects, among others, in order to provide deeper insights into the complex interactions between radiation and biological systems.
This new era of radiobiology is characterized by a comprehensive understanding of the molecular underpinnings of RT. It is an exciting time where interdisciplinary collaboration is essential, combining the expertise of physicists, biologists, and clinicians to develop innovative treatments that are both effective and safe.
Author Contributions
All the authors contributed equally to the writing.
Declaration of Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The publication is funded by the Open Access Publishing Fund of GSI Helmholtzzentrum fuer Schwerionenforschung.
References
- 1.Wilson R.R. Radiological use of fast protons. Radiology. 1946;47:487–491. doi: 10.1148/47.5.487. [DOI] [PubMed] [Google Scholar]
- 2.Tinganelli W., Durante M. Cabon ion radiobiology. Cancers. 2020;12:3022. doi: 10.3390/cancers12103022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paganetti H., Niemierko A., Ancukiewicz M., et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53:407–421. doi: 10.1016/S0360-3016(02)02754-2. [DOI] [PubMed] [Google Scholar]
- 4.Jones B. Why RBE must be a variable and not a constant in proton therapy. Br J Radiol. 2016;89:20160116. doi: 10.1259/bjr.20160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sørensen B.S., Pawelke J., Bauer J., et al. Does the uncertainty in relative biological effectiveness affect patient treatment in proton therapy? Radiother Oncol. 2021;163:177–184. doi: 10.1016/j.radonc.2021.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Heuchel L., Hahn C., Pawelke J., Sørensen B.S., Dosanjh M., Lühr A. Clinical use and future requirements of relative biological effectiveness: survey among all European proton therapy centres. Radiother Oncol. 2022;172:134–139. doi: 10.1016/j.radonc.2022.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Freyer J.P., Jarrett K., Carpenter S., Raju M.R. Oxygen enhancement ratio as a function of dose and cell cycle phase for radiation-resistant and sensitive CHO cells. Radiat Res. 1991;127:297–307. [PubMed] [Google Scholar]
- 8.Tinganelli W., Durante M., Hirayama R., et al. Kill-painting of hypoxic tumours in charged particle therapy. Sci Rep. 2015;5:1–13. doi: 10.1038/srep17016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vozenin M.-C., Bourhis J., Durante M. Towards clinical translation of FLASH radiotherapy. Nat Rev Clin Oncol. 2022;19:791–803. doi: 10.1038/s41571-022-00697-z. [DOI] [PubMed] [Google Scholar]
- 10.Espinosa-Rodriguez A., Villa-Abaunza A., Díaz N., et al. Design of an X-ray irradiator based on a standard imaging X-ray tube with FLASH dose-rate capabilities for preclinical research. Radiat Phys Chem. 2023;206 doi: 10.1016/j.radphyschem.2023.110760. [DOI] [Google Scholar]
- 11.Moeckli R., Gonçalves Jorge P., Grilj V., et al. Commissioning of an ultra-high dose rate pulsed electron beam medical LINAC for FLASH RT preclinical animal experiments and future clinical human protocols. Med Phys. 2021;48:3134–3142. doi: 10.1002/mp.14885. [DOI] [PubMed] [Google Scholar]
- 12.Diffenderfer E.S., Verginadis I.I., Kim M.M., et al. Design, implementation, and in vivo validation of a novel proton FLASH radiation therapy system. Int J Radiat Oncol Biol Phys. 2020;106:440–448. doi: 10.1016/J.IJROBP.2019.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grilj V., Buonanno M., Welch D., Brenner D.J. Proton irradiation platforms for preclinical studies of high-dose-rate (FLASH) effects at RARAF. Radiat Res. 2020;194:646–655. doi: 10.1667/RADE-20-00062.1. [DOI] [PubMed] [Google Scholar]
- 14.Tessonnier T, Mein S, Walsh D FLASH dose-rate helium ion beams: first in vitro investigations. 2021;111(4):1011–1022. doi: 10.1016/j.ijrobp.2021.07.1703. [DOI] [PubMed]
- 15.Weber U.A., Scifoni E., Durante M. FLASH radiotherapy with carbon ion beams. Med Phys. 2022;49:1974–1992. doi: 10.1002/mp.15135. [DOI] [PubMed] [Google Scholar]
- 16.Tashiro M., Yoshida Y., Oike T., et al. First human cell experiments with FLASH carbon ions. Anticancer Res. 2022;42:2469–2477. doi: 10.21873/anticanres.15725. [DOI] [PubMed] [Google Scholar]
- 17.Tinganelli W., Sokol O., Quartieri M., et al. Ultra-high dose rate (FLASH) carbon ion irradiation: dosimetry and first cell experiments. Int J Radiat Oncol Biol Phys. 2022;112:1012–1022. doi: 10.1016/j.ijrobp.2021.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Tinganelli W., Weber U., Puspitasari A., et al. FLASH with carbon ions: tumor control, normal tissue sparing, and distal metastasis in a mouse osteosarcoma model. Radiother Oncol. 2022;175:185–190. doi: 10.1016/j.radonc.2022.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Mascia A.E., Daugherty E.C., Zhang Y., et al. Proton FLASH radiotherapy for the treatment of symptomatic bone metastases: the FAST-01 nonrandomized trial. JAMA Oncol. 2023;9:62–69. doi: 10.1001/jamaoncol.2022.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinj R., Gaide O., Jeanneret-Sozzi W., et al. Randomized phase II selection trial of FLASH and conventional radiotherapy for patients with localized cutaneous squamous cell carcinoma or basal cell carcinoma: a study protocol. Clin Transl Radiat Oncol. 2024;45 doi: 10.1016/j.ctro.2024.100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaide O., Herrera F., Jeanneret Sozzi W., et al. Comparison of ultra-high versus conventional dose rate radiotherapy in a patient with cutaneous lymphoma. Radiother Oncol. 2022;174:87–91. doi: 10.1016/j.radonc.2021.12.045. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q., Gerweck L.E., Cascio E., et al. Absence of tissue-sparing effects in partial proton FLASH irradiation in murine intestine. Cancers. 2023;15 doi: 10.3390/cancers15082269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buonanno M., Grilj V., Brenner D.J. Biological effects in normal cells exposed to FLASH dose rate protons. Radiother Oncol. 2019;139:51–55. doi: 10.1016/j.radonc.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labarbe R., Hotoiu L., Barbier J., Favaudon V. A physicochemical model of reaction kinetics supports peroxyl radical recombination as the main determinant of the FLASH effect. Radiother Oncol. 2020;153:303–310. doi: 10.1016/j.radonc.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Montay-Gruel P., Acharya M.M., Petersson K., et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc Natl Acad Sci USA. 2019;166:10943–10951. doi: 10.1073/pnas.1901777116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boscolo D., Scifoni E., Durante M., Krämer M., Fuss M.C. May oxygen depletion explain the FLASH effect? A chemical track structure analysis. Radiother Oncol. 2021;162:68–75. doi: 10.1016/j.radonc.2021.06.031. [DOI] [PubMed] [Google Scholar]
- 27.Jansen J., Knoll J., Beyreuther E., et al. Does FLASH deplete oxygen? Experimental evaluation for photons, protons, and carbon ions. Med Phys. 2021;48:3982–3990. doi: 10.1002/mp.14917. [DOI] [PubMed] [Google Scholar]
- 28.Pratx G., Kapp D.S. A computational model of radiolytic oxygen depletion during FLASH irradiation and its effect on the oxygen enhancement ratio. Phys Med Biol. 2019;64 doi: 10.1088/1361-6560/ab3769. [DOI] [PubMed] [Google Scholar]
- 29.Froidevaux P., Grilj V., Bailat C., Geyer W.R., Bochud F., Vozenin M.-C. FLASH irradiation does not induce lipid peroxidation in lipids micelles and liposomes. Radiat Phys Chem. 2023;205 doi: 10.1016/j.radphyschem.2022.110733. [DOI] [Google Scholar]
- 30.Martínez-Rovira I., Montay-Gruel P., Petit B., et al. Infrared microspectroscopy to elucidate the underlying biomolecular mechanisms of FLASH radiotherapy. Radiother Oncol. 2024;196 doi: 10.1016/j.radonc.2024.110238. [DOI] [PubMed] [Google Scholar]
- 31.Jin J.-Y., Gu A., Wang W., Oleinick N.L., Machtay M., Spring Kong F.-M. Ultra-high dose rate effect on circulating immune cells: a potential mechanism for FLASH effect? Radiother Oncol. 2020;149:55–62. doi: 10.1016/j.radonc.2020.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prezado Y. Divide and conquer: spatially fractionated radiation therapy. Expert Rev Mol Med. 2022;24 doi: 10.1017/erm.2021.34. [DOI] [Google Scholar]
- 33.Zeman W., Curtis H.J., Baker C.P. Histopathologic effect of high-energy-particle microbeams on the visual cortex of the mouse brain. Radiat Res. 1961;15:496–514. [PubMed] [Google Scholar]
- 34.Curtis H.J. The use of deuteron microbeam for simulating the biological effects of heavy cosmic-ray particles. Radiat Res Suppl. 1967;7:250–257. [PubMed] [Google Scholar]
- 35.Lamirault C., Doyère V., Juchaux M., et al. Short and long-term evaluation of the impact of proton minibeam radiation therapy on motor, emotional and cognitive functions. Sci Rep. 2020;10 doi: 10.1038/s41598-020-70371-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prezado Y., Jouvion G., Hardy D., et al. Proton minibeam radiation therapy spares normal rat brain: long-term clinical, radiological and histopathological analysis. Sci Rep. 2017;7 doi: 10.1038/s41598-017-14786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prezado Y., Jouvion G., Patriarca A., et al. Proton minibeam radiation therapy widens the therapeutic index for high-grade gliomas. Sci Rep. 2018;8 doi: 10.1038/s41598-018-34796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Girst S., Greubel C., Reindl J., et al. Proton minibeam radiation therapy reduces side effects in an in vivo mouse ear model. Int J Radiat Oncol Biol Phys. 2016;95:234–241. doi: 10.1016/j.ijrobp.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 39.Sammer M., Zahnbrecher E., Dobiasch S., et al. Proton pencil minibeam irradiation of an in-vivo mouse ear model spares healthy tissue dependent on beam size. PLoS One. 2019;14 doi: 10.1371/journal.pone.0224873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertho A., Ortiz R., Maurin M.M., et al. Thoracic proton minibeam radiation therapy: tissue preservation and survival advantage over conventional proton therapy. Int J Radiat Oncol Biol Phys. 2024 doi: 10.1016/j.ijrobp.2024.04.011. S0360-3016(24)00510-8. [DOI] [PubMed] [Google Scholar]
- 41.Prezado Y., Sarun S., Gil S., Deman P., Bouchet A., Le Duc G. Increase of lifespan for glioma-bearing rats by using minibeam radiation therapy. J Synchrotron Radiat. 2012;19:60–65. doi: 10.1107/S0909049511047042. [DOI] [PubMed] [Google Scholar]
- 42.Bazyar S., Inscoe C.R., O’Brian E.T., Zhou O., Lee Y.Z. Minibeam radiotherapy with small animal irradiators; in vitro and in vivo feasibility studies. Phys Med Biol. 2017;62:8924–8942. doi: 10.1088/1361-6560/aa926b. [DOI] [PubMed] [Google Scholar]
- 43.Sotiropoulos M., Brisebard E., Le Dudal M., et al. X-rays minibeam radiation therapy at a conventional irradiator: pilot evaluation in F98-glioma bearing rats and dose calculations in a human phantom. Clin Transl Radiat Oncol. 2021;27:44–49. doi: 10.1016/j.ctro.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kundapur V., Mayer M., Auer R.N., et al. Is mini beam ready for human trials? Results of randomized study of treating de-novo brain tumors in canines using linear accelerator generated mini beams. Radiat Res. 2022;198:162–171. doi: 10.1667/RADE-21-00093.1. [DOI] [PubMed] [Google Scholar]
- 45.Asur R., Butterworth K.T., Penagaricano J.A., Prise K.M., Griffin R.J. High dose bystander effects in spatially fractionated radiation therapy. Cancer Lett. 2015;356:52–57. doi: 10.1016/j.canlet.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bertho A., Iturri L., Brisebard E., et al. Evaluation of the role of the immune system response after minibeam radiation therapy. Int J Radiat Oncol Biol Phys. 2023;115:426–439. doi: 10.1016/j.ijrobp.2022.08.011. [DOI] [PubMed] [Google Scholar]
- 47.Prezado Y., Fois G.R. Proton-minibeam radiation therapy: a proof of concept. Med Phys. 2013;40 doi: 10.1118/1.4791648. [DOI] [PubMed] [Google Scholar]
- 48.Prezado Y., Jouvion G., Guardiola C., et al. Tumor control in RG2 glioma-bearing rats: a comparison between proton minibeam therapy and standard proton therapy. Int J Radiat Oncol Biol Phys. 2019;104:266–271. doi: 10.1016/j.ijrobp.2019.01.080. [DOI] [PubMed] [Google Scholar]
- 49.Lamirault C., Brisebard E., Patriarca A., et al. Spatially modulated proton minibeams results in the same increase of lifespan as a uniform target dose coverage in F98-glioma-bearing rats. Radiat Res. 2020;194:715–723. doi: 10.1667/RADE-19-00013.1. [DOI] [PubMed] [Google Scholar]
- 50.Bertho A., Ortiz R., Juchaux M., et al. First evaluation of temporal and spatial fractionation in proton minibeam radiation therapy of glioma-bearing rats. Cancers. 2021;13 doi: 10.3390/cancers13194865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castro J.R. Results of heavy ion radiotherapy. Radiat Environ Biophys. 1995;34:45–48. doi: 10.1007/BF01210545. [DOI] [PubMed] [Google Scholar]
- 52.Dilmanian F.A., Eley J.G., Krishnan S. Minibeam therapy with protons and light ions: physical feasibility and potential to reduce radiation side effects and to facilitate hypofractionation. Int J Radiat Oncol Biol Phys. 2015;92:469–474. doi: 10.1016/j.ijrobp.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prezado Y., Hirayama R., Matsufuji N., et al. A potential renewed use of very heavy ions for therapy: neon minibeam radiation therapy. Cancers. 2021;13 doi: 10.3390/cancers13061356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eley J.G., Haga C.W., Keller A., et al. Heavy ion minibeam therapy: side effects in normal brain. Cancers. 2021;13:6207. doi: 10.3390/cancers13246207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barth R.F. A critical assessment of boron neutron capture therapy: an overview. J Neurooncol. 2003;62:1–5. doi: 10.1007/BF02699929. [DOI] [PubMed] [Google Scholar]
- 56.Barth R.F., H Vicente M.G., Harling O.K., et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat Oncol. 2012;7:146. doi: 10.1186/1748-717X-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fukuda H. Boron neutron capture therapy (BNCT) for cutaneous malignant melanoma using 10B-p-Boronophenylalanine (BPA) with special reference to the radiobiological basis and clinical results. Cells. 2021;10:2881. doi: 10.3390/cells10112881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zonta A., Prati U., Roveda L., et al. Clinical lessons from the first applications of BNCT on unresectable liver metastases. J Phys Conf Ser. 2006;41:484–495. doi: 10.1088/1742-6596/41/1/054. [DOI] [Google Scholar]
- 59.Suzuki M., Kato I., Aihara T., et al. Boron neutron capture therapy outcomes for advanced or recurrent head and neck cancer. J Radiat Res. 2014;55:146–153. doi: 10.1093/jrr/rrt098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nedunchezhian K. Boron neutron capture therapy - a literature review. J Clin Diagn Res. 2016;10:ZE01–ZE04. doi: 10.7860/JCDR/2016/19890.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diaz A.Z. Assessment of the results from the phase I/II boron neutron capture therapy trials at the Brookhaven National Laboratory from a clinician’s point of view. J Neurooncol. 2003;62:101–109. doi: 10.1007/BF02699937. [DOI] [PubMed] [Google Scholar]
- 62.Messner K., Vuong B., Tranmer G.K. The boron advantage: the evolution and diversification of boron’s applications in medicinal chemistry. Pharmaceuticals. 2022;15:264. doi: 10.3390/ph15030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Busse P.M., Harling O.K., Palmer M.R., et al. A critical examination of the results from the Harvard-MIT NCT program phase I clinical trial of neutron capture therapy for intracranial disease. J Neurooncol. 2003;62:111–121. doi: 10.1007/BF02699938. [DOI] [PubMed] [Google Scholar]
- 64.Field S.B. An historical survey of radiobiology and radiotherapy with fast neutrons. Curr Top Radiat Res Q. 1976;11:1–86. [PubMed] [Google Scholar]
- 65.Sgouros G. Dosimetry, radiobiology and synthetic lethality: radiopharmaceutical therapy (RPT) with alpha-particle-emitters. Semin Nucl Med. 2020;50:124–132. doi: 10.1053/j.semnuclmed.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bolch W.E., Eckerman K.F., Sgouros G., Thomas S.R. MIRD Pamphlet No. 21: a generalized schema for radiopharmaceutical dosimetry—Standardization of nomenclature. J Nucl Med. 2009;50:477–484. doi: 10.2967/jnumed.108.056036. [DOI] [PubMed] [Google Scholar]
- 67.Sgouros G., Roeske J.C., McDevitt M.R., et al. MIRD Pamphlet No. 22 (Abridged): radiobiology and dosimetry of α-particle emitters for targeted radionuclide therapy. J Nucl Med. 2010;51:311–328. doi: 10.2967/jnumed.108.058651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sgouros G., Bolch W.E., Chiti A., et al. ICRU REPORT 96, dosimetry-guided radiopharmaceutical therapy. J ICRU. 2021;21:1–212. doi: 10.1177/14736691211060117. [DOI] [Google Scholar]
- 69.O’Donoghue J., Zanzonico P., Humm J., Kesner A. Dosimetry in radiopharmaceutical therapy. J Nucl Med. 2022;63:1467–1474. doi: 10.2967/jnumed.121.262305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baeyens A., Abrantes A.M., Ahire V., et al. In: Radiobiology Textbook. Baatout S., editor. Springer International Publishing; 2023. Basic concepts of radiation biology; pp. 25–81. [Google Scholar]
- 71.Murray I., Flux G. Applying radiobiology to clinical molecular radiotherapy. Nucl Med Biol. 2021;100–101:1–3. doi: 10.1016/j.nucmedbio.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 72.Khazaei Monfared Y., Heidari P., Klempner S.J., et al. DNA damage by radiopharmaceuticals and mechanisms of cellular repair. Pharmaceutics. 2023;15:2761. doi: 10.3390/pharmaceutics15122761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dietrich A., Koi L., Zöphel K., et al. Improving external beam radiotherapy by combination with internal irradiation. Br J Radiol. 2015;88:20150042. doi: 10.1259/bjr.20150042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rassamegevanon T., Feindt L., Koi L., et al. Molecular response to combined molecular- and external radiotherapy in head and neck squamous cell carcinoma (HNSCC) Cancers. 2021;13:5595. doi: 10.3390/cancers13225595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suman S.K., Subramanian S., Mukherjee A. Combination radionuclide therapy: a new paradigm. Nucl Med Biol. 2021;98–99:40–58. doi: 10.1016/j.nucmedbio.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 76.Melzig C., Golestaneh A.F., Mier W., et al. Combined external beam radiotherapy with carbon ions and tumor targeting endoradiotherapy. Oncotarget. 2018;9:29985–30004. doi: 10.18632/oncotarget.25695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park C., Papiez L., Zhang S., Story M., Timmerman R.D. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:847–852. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 78.Sheu T., Molkentine J., Transtrum M.K., et al. Use of the LQ model with large fraction sizes results in underestimation of isoeffect doses. Radiother Oncol. 2013;109:21–25. doi: 10.1016/j.radonc.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 79.Song C.W., Glatstein E., Marks L.B., et al. Biological principles of stereotactic body radiation therapy (SBRT) and stereotactic radiation surgery (SRS): indirect cell death. Int J Radiat Oncol Biol Phys. 2021;110:21–34. doi: 10.1016/j.ijrobp.2019.02.047. [DOI] [PubMed] [Google Scholar]
- 80.Fuks Z., Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 81.Park H.J., Griffin R.J., Hui S., Levitt S.H., Song C.W. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS) Radiat Res. 2012;177:311–327. doi: 10.1667/rr2773.1. [DOI] [PubMed] [Google Scholar]
- 82.Lee Y., Auh S.L., Wang Y., et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim D.W.N., Medin P.M., Timmerman R.D. Emphasis on repair, not just avoidance of injury, facilitates prudent stereotactic ablative radiotherapy. Semin Radiat Oncol. 2017;27:378–392. doi: 10.1016/j.semradonc.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 84.Tsujii H, Kamada T, Shirai T, Noda K, Tsuji H, Karasawa K, eds. Carbon-Ion Radiotherapy. Springer; 2014. ISBN 978-4-431–54456-2.
- 85.Ando K., Koike S., Uzawa A., et al. Biological gain of carbon-ion radiotherapy for the early response of tumor growth delay and against early response of skin reaction in mice. J Radiat Res. 2005;46:51–57. doi: 10.1269/jrr.46.51. [DOI] [PubMed] [Google Scholar]
- 86.Kamada T., Tsujii H., Blakely E.A., et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol. 2015;16:e93–e100. doi: 10.1016/S1470-2045(14)70412-7. [DOI] [PubMed] [Google Scholar]
- 87.Bert C., Durante M. Particle radiosurgery: a new frontier of physics in medicine. Phys Med. 2014;30:535–538. doi: 10.1016/j.ejmp.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 88.Sokol O., Scifoni E., Tinganelli W., et al. Oxygen beams for therapy: advanced biological treatment planning and experimental verification. Phys Med Biol. 2017;62:7798. doi: 10.1088/1361-6560/aa88a0. [DOI] [PubMed] [Google Scholar]
- 89.Inaniwa T., Krämer M., Mairani A., Scifoni E., Sokol O. In: Monte Carlo in Heavy Charged Particle Therapy. Cirrone P., Petringa G., editors. CRC Press; 2024. Chapter 16. Towards multiple ion applications in paricle therapy. ISBN 9781003023920. [Google Scholar]
- 90.Mairani A., Mein S., Blakely E., et al. Roadmap: helium ion therapy. Phys Med Biol. 2022;67:15TR02. doi: 10.1088/1361-6560/ac65d3. [DOI] [PubMed] [Google Scholar]
- 91.Tessonnier T., Ecker S., Besuglow J., et al. Commissioning of helium ion therapy and the first patient treatment with active beam delivery. Int J Radiat Oncol Biol Phys. 2023;116:935–948. doi: 10.1016/j.ijrobp.2023.01.015. [DOI] [PubMed] [Google Scholar]
- 92.Schnelzauer L., Valentin S., Traykov E., Arbor N., Finck C., Vanstalle M. Short-lived radioactive 8Li and 8He ions for hadrontherapy: a simulation study. Phys Med Biol. 2023;68 doi: 10.1088/1361-6560/acb88b. [DOI] [PubMed] [Google Scholar]
- 93.Formenti S.C., Rudqvist N.P., Golden E., et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24:1845–1851. doi: 10.1038/s41591-018-0232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spigel D.R., Faivre-Finn C., Gray J.E., et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40:1301–1311. doi: 10.1200/JCO.21.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Galluzzi L., Buqué A., Kepp O., Zitvogel L., Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 96.Lee N.Y., Ferris R.L., Psyrri A., et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22:450–462. doi: 10.1016/S1470-2045(20)30737-3. [DOI] [PubMed] [Google Scholar]
- 97.Marciscano A.E., Ghasemzadeh A., Nirschl T.R., et al. Elective nodal irradiation attenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy. Clin Cancer Res. 2018;24:5058–5071. doi: 10.1158/1078-0432.CCR-17-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Durante M., Debus J., Loeffler J.S. Physics and biomedical challenges of cancer therapy with accelerated heavy ions. Nat Rev Phys. 2021;3:777–790. doi: 10.1038/s42254-021-00368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Durante M. Kaplan lecture 2023: lymphopenia in particle therapy. Int J Radiat Biol. 2024;100:669–677. doi: 10.1080/09553002.2024.2324472. [DOI] [PubMed] [Google Scholar]
- 100.Galluzzi L., Vitale I., Warren S., et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer. 2020;8:1–22. doi: 10.1136/jitc-2019-000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Galon J., Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 102.Helm A., Totis C., Durante M., Fournier C. Are charged particles a good match for combination with immunotherapy? Current knowledge and perspectives. Int Rev Cell Mol Biol. 2023;376:1–36. doi: 10.1016/bs.ircmb.2023.01.001. [DOI] [PubMed] [Google Scholar]
- 103.Wei J., Montalvo-Ortiz W., Yu L., et al. Sequence of ΑPD-1 relative to local tumor irradiation determines the induction of abscopal antitumor immune responses. Sci Immunol. 2021;6:1–13. doi: 10.1126/sciimmunol.abg0117. [DOI] [PubMed] [Google Scholar]
- 104.Moore C., Hsu C.C., Chen W.M., et al. Personalized ultrafractionated stereotactic adaptive radiotherapy (PULSAR) in preclinical models enhances single-agent immune checkpoint blockade. Int J Radiat Oncol Biol Phys. 2021;110:1306–1316. doi: 10.1016/j.ijrobp.2021.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.He K., Barsoumian H.B., Sezen D., et al. Pulsed radiation therapy to improve systemic control of metastatic cancer. Front Oncol. 2021;11:1–9. doi: 10.3389/fonc.2021.737425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tubin S., Popper H.H., Brcic L. Novel stereotactic body radiation therapy (SBRT)-based partial tumor irradiation targeting hypoxic segment of bulky tumors (SBRT-PATHY): improvement of the radiotherapy outcome by exploiting the bystander and abscopal effects. Radiat Oncol. 2019;14:1–11. doi: 10.1186/s13014-019-1227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tubin S., Yan W., Mourad W.F., Fossati P., Khan M.K. The future of radiation-induced abscopal response: beyond conventional radiotherapy approaches. Future Oncol. 2020;16:1137–1151. doi: 10.2217/fon-2020-0063. [DOI] [PubMed] [Google Scholar]
- 108.Tubin S., Gupta S., Grusch M., et al. Shifting the immune-suppressive to predominant immune-stimulatory radiation effects by SBRT-PArtial tumor irradiation targeting HYpoxic segment (SBRT-PATHY) Cancers. 2020;13:50. doi: 10.3390/cancers13010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tubin S., Fossati P., Carlino A., et al. Novel carbon ion and proton partial irradiation of recurrent unresectable bulky tumors (particle-PATHY): early indication of effectiveness and safety. Cancers. 2022;14:1–18. doi: 10.3390/cancers14092232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Meric I., Alagoz E., Hysing L.B., et al. A hybrid multi-particle approach to range assessment-based treatment verification in particle therapy. Sci Rep. 2023;13:6709. doi: 10.1038/s41598-023-33777-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schauer J., Wieser H.P., Huang Y., et al. Proton beam range verification by means of ionoacoustic measurements at clinically relevant doses using a correlation-based evaluation. Front Oncol. 2022;12 doi: 10.3389/FONC.2022.925542/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parodi K., Polf J.C. In vivo range verification in particle therapy. Med Phys. 2018;45:e1036–e1050. doi: 10.1002/MP.12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lacombe S., Porcel E., Scifoni E. Particle therapy and nanomedicine: state of art and research perspectives. Cancer Nanotechnol. 2017;8:9. doi: 10.1186/s12645-017-0029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gong L., Zhang Y., Liu C., Zhang M., Han S. Application of radiosensitizers in cancer radiotherapy. Int J Nanomed. 2021;16:1083–1102. doi: 10.2147/IJN.S290438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Montoro A., Obrador E., Mistry D., et al. Radioprotectors, radiomitigators, and radiosensitizers. Radiobiol Textbook. 2023;11:571–628. doi: 10.1007/978-3-031-18810-7_11. [DOI] [Google Scholar]
- 116.Song X., Sun Z., Li L., Zhou L., Yuan S. Application of nanomedicine in radiotherapy sensitization. Front Oncol. 2023;13:1088878. doi: 10.3389/FONC.2023.1088878/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Baatout S. In: Radiobiology Textbook. Baatout S., editor. Springer International Publishing; 2023. Chapter 11: Radioprotectors, Radiomitigators, and Radiosensitizers. ISBN 978-3-031-18809-1. [Google Scholar]
- 118.Low J.M., Rodriguez-Berriguete G., Higgins G.S. Repurposing radiosensitising medicines for radiotherapy: an overview. BMJ Oncol. 2024;3:192. doi: 10.1136/bmjonc-2023-000192. [DOI] [Google Scholar]
- 119.Komorowska D., Radzik T., Kalenik S., Rodacka A. Natural radiosensitizers in radiotherapy: cancer treatment by combining ionizing radiation with resveratrol. Int J Mol Sci. 2022;23:10627. doi: 10.3390/IJMS231810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chuang Y.-C., Wu P.-H., Shen Y.-A., et al. Recent advances in metal-based nanoenhancers for particle therapy. Nanomaterials. 2023;13:1011. doi: 10.3390/nano13061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Conde J., Dias J.T., Grazú V., Moros M., Baptista P.V., de la Fuente J.M. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front Chem. 2014;2:48. doi: 10.3389/fchem.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Heuskin A.-C., Gallez B., Feron O., Martinive P., Michiels C., Lucas S. Metallic nanoparticles irradiated by low-energy protons for radiation therapy: are there significant physical effects to enhance the dose delivery? Med Phys. 2017;44:4299–4312. doi: 10.1002/mp.12362. [DOI] [PubMed] [Google Scholar]
- 123.Subiel A., Ashmore R., Schettino G. Standards and methodologies for characterizing radiobiological impact of High-Z nanoparticles. Theranostics. 2016;6:1651–1671. doi: 10.7150/THNO.15019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ahmad R., Schettino G., Royle G., et al. Radiobiological implications of nanoparticles following radiation treatment. Part Part Syst Charact. 2020;37 doi: 10.1002/PPSC.201900411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Horie M., Shimizu K., Tabei Y. Validation of metallothionein, interleukin-8, and heme oxygenase-1 as markers for the evaluation of cytotoxicity caused by metal oxide nanoparticles. Toxicol Mech Methods. 2018;28:630–638. doi: 10.1080/15376516.2018.1486931. [DOI] [PubMed] [Google Scholar]
- 126.Shen H., Huang H., Jiang Z. Nanoparticle-based radiosensitization strategies for improving radiation therapy. Front Pharmacol. 2023;14:1145551. doi: 10.3389/FPHAR.2023.1145551/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bilynsky C., Millot N., Papa A.L. Radiation nanosensitizers in cancer therapy—From preclinical discoveries to the outcomes of early clinical trials. Bioeng Transl Med. 2021;7 doi: 10.1002/BTM2.10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arif M., Nawaz A.F., Ullah khan S., et al. Nanotechnology-based radiation therapy to cure cancer and the challenges in its clinical applications. Heliyon. 2023;9 doi: 10.1016/J.HELIYON.2023.E17252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bonvalot S., Le Pechoux C., De Baere T., et al. First-in-human study testing a new radioenhancer using nanoparticles (NBTXR3) activated by radiation therapy in patients with locally advanced soft tissue sarcomas. Clin Cancer Res. 2017;23:908–917. doi: 10.1158/1078-0432.CCR-16-1297/274421/AM/FIRST-HUMAN-STUDY-TESTING-A-NEW-RADIO-ENHANCER. [DOI] [PubMed] [Google Scholar]
- 130.Bonvalot S., Rutkowski P.L., Thariat J., et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): a multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. 2019;20:1148–1159. doi: 10.1016/S1470-2045(19)30326-2. [DOI] [PubMed] [Google Scholar]
- 131.Bagley A.F., Ludmir E.B., Maitra A., et al. NBTXR3, a first-in-class radioenhancer for pancreatic ductal adenocarcinoma: report of first patient experience. Clin Transl Radiat Oncol. 2022;33:66–69. doi: 10.1016/J.CTRO.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thivat E., Casile M., Moreau J., et al. Phase I/II study testing the combination of AGuIX nanoparticles with radiochemotherapy and concomitant temozolomide in patients with newly diagnosed glioblastoma (NANO-GBM trial protocol) BMC Cancer. 2023;23:344. doi: 10.1186/S12885-023-10829-Y/TABLES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang C.W., Hu H.M., Hsu W.H., et al. A phase Ib/II trial of PEP503 (NBTXR3, radioenhancer) with radiotherapy and chemotherapy in patients with rectal cancer. Nanomedicine. 2023;18:511–524. doi: 10.2217/NNM-2022-0186/ASSET/IMAGES/LARGE/FIGURE1.JPEG. [DOI] [PubMed] [Google Scholar]
- 134.Rehman M.U., Jawaid P., Kondo T. Dual effects of nanoparticles on radiation therapy: as radiosensitizers and radioprotectors. Radiat Environ Med. 2016;5:40–45. doi: 10.51083/RADIATENVIRONMED.5.1_40. [DOI] [Google Scholar]
- 135.Krokosz A., Lichota A., Nowak K.E., Grebowski J. Carbon nanoparticles as possible radioprotectors in biological systems. Radiat Phys Chem. 2016;128:143–150. doi: 10.1016/J.RADPHYSCHEM.2016.07.006. [DOI] [Google Scholar]
- 136.Xie J., Wang C., Zhao F., Gu Z., Zhao Y. Application of multifunctional nanomaterials in radioprotection of healthy tissues. Adv Healthc Mater. 2018;7 doi: 10.1002/ADHM.201800421. [DOI] [PubMed] [Google Scholar]
- 137.Guo J., Zhao Z., Shang Z.F., Tang Z., Zhu H., Zhang K. Nanodrugs with intrinsic radioprotective exertion: turning the double-edged sword into a single-edged knife. Exploration. 2023;3 doi: 10.1002/EXP.20220119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pereira S., Orlandi E., Deneuve S., et al. The normal, the radiosensitive, and the ataxic in the era of precision radiotherapy: a narrative review. Cancers. 2022;14 doi: 10.3390/cancers14246252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chua M.L.K., Rothkamm K. Biomarkers of radiation exposure: can they predict normal tissue radiosensitivity? Clin Oncol. 2013;25:610–616. doi: 10.1016/j.clon.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 140.Larionova I., Rakina M., Ivanyuk E., Trushchuk Y., Chernyshova A., Denisov E. Radiotherapy resistance: identifying universal biomarkers for various human cancers. J Cancer Res Clin Oncol. 2022;148:1015–1031. doi: 10.1007/s00432-022-03923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chang L., Graham P., Hao J., et al. Proteomics discovery of radioresistant cancer biomarkers for radiotherapy. Cancer Lett. 2015;369:289–297. doi: 10.1016/j.canlet.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 142.He K., Zhang S., Pang J., et al. Genomic profiling reveals novel predictive biomarkers for chemo-radiotherapy toxicity and efficacy in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2021;111 doi: 10.1016/j.ijrobp.2021.07.1240. [DOI] [Google Scholar]
- 143.Penninckx S., Pariset E., Cekanaviciute E., Costes S.V. Quantification of radiation-induced DNA double strand break repair foci to evaluate and predict biological responses to ionizing radiation. NAR Cancer. 2021;3 doi: 10.1093/narcan/zcab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Willers H., Dahm-Daphi J., Powell S.N. Repair of radiation damage to DNA. Br J Cancer. 2004;90:1297–1301. doi: 10.1038/sj.bjc.6601729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Willers H., Gheorghiu L., Liu Q., et al. DNA damage response assessments in human tumor samples provide functional biomarkers of radiosensitivity. Semin Radiat Oncol. 2015;25:237–250. doi: 10.1016/j.semradonc.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Willers H., Pfäffle H.N., Zou L. In: DNA Repair in Cancer Therapy. Kelley M.R., editor. Elsevier; 2012. Targeting homologous recombination repair in cancer; pp. 119–160. [Google Scholar]
- 147.Garcia-Higuera I., Taniguchi T., Ganesan S., et al. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol Cell. 2001;7:249–262. doi: 10.1016/s1097-2765(01)00173-3. [DOI] [PubMed] [Google Scholar]
- 148.Digweed M., Rothe S., Demuth I., et al. Attenuation of the formation of DNA-repair foci containing RAD51 in Fanconi anaemia. Carcinogenesis. 2002;23:1121–1126. doi: 10.1093/carcin/23.7.1121. [DOI] [PubMed] [Google Scholar]
- 149.Godthelp B.C., Wiegant W.W., Waisfisz Q., et al. Inducibility of nuclear Rad51 foci after DNA damage distinguishes All Fanconi anemia complementation groups from D1/BRCA2. Mutat Res. 2006;594:39–48. doi: 10.1016/j.mrfmmm.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 150.Klokov D., MacPhail S.M., Banáth J.P., Byrne J.P., Olive P.L. Phosphorylated histone H2AX in relation to cell survival in tumor cells and xenografts exposed to single and fractionated doses of X-rays. Radiother Oncol. 2006;80:223–229. doi: 10.1016/j.radonc.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 151.Menegakis A., Yaromina A., Eicheler W., et al. Prediction of clonogenic cell survival curves based on the number of residual DNA double strand breaks measured by GammaH2AX staining. Int J Radiat Biol. 2009;85:1032–1041. doi: 10.3109/09553000903242149. [DOI] [PubMed] [Google Scholar]
- 152.Kunogi H., Sakanishi T., Sueyoshi N., Sasai K. Prediction of radiosensitivity using phosphorylation of histone H2AX and apoptosis in human tumor cell lines. Int J Radiat Biol. 2014;90:587–593. doi: 10.3109/09553002.2014.907518. [DOI] [PubMed] [Google Scholar]
- 153.Fokas E., Prevo R., Pollard J.R., et al. Targeting ATR in vivo using the novel inhibitor VE-822 results in selective sensitization of pancreatic tumors to radiation. Cell Death Dis. 2012;3 doi: 10.1038/cddis.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fasih A., Elbaz H.A., Hüttemann M., Konski A.A., Zielske S.P. Radiosensitization of pancreatic cancer cells by metformin through the AMPK pathway. Radiat Res. 2014;182:50–59. doi: 10.1667/RR13568.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Marková E., Schultz N., Belyaev I.Y. Kinetics and dose-response of residual 53BP1/Gamma-H2AX foci: co-localization, relationship with DSB repair and clonogenic survival. Int J Radiat Biol. 2007;83:319–329. doi: 10.1080/09553000601170469. [DOI] [PubMed] [Google Scholar]
- 156.Djuzenova C.S., Elsner I., Katzer A., et al. Radiosensitivity in breast cancer assessed by the histone γ-H2AX and 53BP1 foci. Radiat Oncol. 2013;8:98. doi: 10.1186/1748-717X-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Barcellini A., Charalampopoulou A., De Cecco L., et al. Ovarian cancer radiosensitivity: what have we understood so far? Life. 2022;13:6. doi: 10.3390/life13010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sprong D., Janssen H.L., Vens C., Begg A.C. Resistance of hypoxic cells to ionizing radiation is influenced by homologous recombination status. Int J Radiat Oncol Biol Phys. 2006;64:562–572. doi: 10.1016/j.ijrobp.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 159.Chi J.-T., Wang Z., Nuyten D.S.A., et al. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Toustrup K., Sørensen B.S., Nordsmark M., et al. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res. 2011;71:5923–5931. doi: 10.1158/0008-5472.CAN-11-1182. [DOI] [PubMed] [Google Scholar]
- 161.Halle C., Andersen E., Lando M., et al. Hypoxia-induced gene expression in chemoradioresistant cervical cancer revealed by dynamic contrast-enhanced MRI. Cancer Res. 2012;72:5285–5295. doi: 10.1158/0008-5472.CAN-12-1085. [DOI] [PubMed] [Google Scholar]
- 162.Müller J., Leger S., Zwanenburg A., et al. Radiomics-based tumor phenotype determination based on medical imaging and tumor microenvironment in a preclinical setting. Radiother Oncol. 2022;169:96–104. doi: 10.1016/j.radonc.2022.02.020. [DOI] [PubMed] [Google Scholar]
- 163.Andreassen C.N., Rosenstein B.S., Kerns S.L., et al. Individual patient data meta-analysis shows a significant association between the ATM Rs1801516 SNP and toxicity after radiotherapy in 5456 breast and prostate cancer patients. Radiother Oncol. 2016;121:431–439. doi: 10.1016/j.radonc.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ho A.Y., Fan G., Atencio D.P., et al. Possession of ATM sequence variants as predictor for late normal tissue responses in breast cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69:677–684. doi: 10.1016/j.ijrobp.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 165.Angèle S., Romestaing P., Moullan N., et al. ATM haplotypes and cellular response to DNA damage: association with breast cancer risk and clinical radiosensitivity. Cancer Res. 2003;63:8717–8725. [PubMed] [Google Scholar]
- 166.Andreassen C.N., Alsner J., Overgaard M., Overgaard J. Prediction of normal tissue radiosensitivity from polymorphisms in candidate genes. Radiother Oncol. 2003;69:127–135. doi: 10.1016/j.radonc.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 167.Zschenker O., Raabe A., Boeckelmann I.K., et al. Association of single nucleotide polymorphisms in ATM, GSTP1, SOD2, TGFB1, XPD and XRCC1 with clinical and cellular radiosensitivity. Radiother Oncol. 2010;97:26–32. doi: 10.1016/j.radonc.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 168.Seibold P., Webb A., Aguado-Barrera M.E., et al. REQUITE: a prospective multicentre cohort study of patients undergoing radiotherapy for breast, lung or prostate cancer. Radiother Oncol. 2019;138:59–67. doi: 10.1016/j.radonc.2019.04.034. [DOI] [PubMed] [Google Scholar]
- 169.Avanzini S., Kurtz D.M., Chabon J.J., et al. A mathematical model of CtDNA shedding predicts tumor detection size. Sci Adv. 2020;6 doi: 10.1126/sciadv.abc4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chabon J.J., Hamilton E.G., Kurtz D.M., et al. Integrating genomic features for non-invasive early lung cancer detection. Nature. 2020;580:245–251. doi: 10.1038/s41586-020-2140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chaudhuri A.A., Chabon J.J., Lovejoy A.F., et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov. 2017;7:1394–1403. doi: 10.1158/2159-8290.CD-17-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chin R.-I., Chen K., Usmani A., et al. Detection of solid tumor molecular residual disease (MRD) using circulating tumor DNA (CtDNA) Mol Diagn Ther. 2019;23:311–331. doi: 10.1007/s40291-019-00390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dudley J.C., Schroers-Martin J., Lazzareschi D.V., et al. Detection and surveillance of bladder cancer using urine tumor DNA. Cancer Discov. 2019;9:500–509. doi: 10.1158/2159-8290.CD-18-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mutschelknaus L., Peters C., Winkler K., et al. Exosomes derived from squamous head and neck cancer promote cell survival after ionizing radiation. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang J., Shin T.-S., Kim J.S., Jee Y.-K., Kim Y.-K. A new horizon of precision medicine: combination of the microbiome and extracellular vesicles. Exp Mol Med. 2022;54:466–482. doi: 10.1038/s12276-022-00748-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yang Z., Zhong W., Yang L., Wen P., Luo Y., Wu C. The emerging role of exosomes in radiotherapy. Cell Commun Signal. 2022;20:171. doi: 10.1186/s12964-022-00986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Summerer I., Niyazi M., Unger K., et al. Changes in circulating MicroRNAs after radiochemotherapy in head and neck cancer patients. Radiat Oncol. 2013;8:296. doi: 10.1186/1748-717X-8-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yu Q., Li B., Li P., et al. Plasma MicroRNAs to predict the response of radiotherapy in esophageal squamous cell carcinoma patients. Am J Transl Res. 2015;7:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 179.Mäurer M., Schott D., Pizon M., et al. Increased circulating epithelial tumor cells (CETC/CTC) over the course of adjuvant radiotherapy is a predictor of less favorable outcome in patients with early-stage breast cancer. Curr Oncol. 2022;30:261–273. doi: 10.3390/curroncol30010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Martin O.A., Anderson R.L., Russell P.A., et al. Mobilization of viable tumor cells into the circulation during radiation therapy. Int J Radiat Oncol Biol Phys. 2014;88:395–403. doi: 10.1016/j.ijrobp.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 181.Goodman C.R., Seagle B.-L.L., Friedl T.W.P., et al. Association of circulating tumor cell status with benefit of radiotherapy and survival in early-stage breast cancer. JAMA Oncol. 2018;4 doi: 10.1001/jamaoncol.2018.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Buglione M., Grisanti S., Almici C., et al. Circulating tumour cells in locally advanced head and neck cancer: preliminary report about their possible role in predicting response to non-surgical treatment and survival. Eur J Cancer. 2012;48:3019–3026. doi: 10.1016/j.ejca.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 183.Lowes L.E., Lock M., Rodrigues G., et al. Circulating tumour cells in prostate cancer patients receiving salvage radiotherapy. Clin Transl Oncol. 2012;14:150–156. doi: 10.1007/s12094-012-0775-5. [DOI] [PubMed] [Google Scholar]
- 184.Morosin T., Ashford B., Ranson M., et al. Circulating tumour cells in regionally metastatic cutaneous squamous cell carcinoma: a pilot study. Oncotarget. 2016;7:47111–47115. doi: 10.18632/oncotarget.9946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xu Y., Ren X., Jiang T., et al. Circulating tumor cells (CTCs) and HTERT gene expression in CTCs for radiotherapy effect with lung cancer. BMC Cancer. 2023;23:475. doi: 10.1186/s12885-023-10979-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Frick M.A., Feigenberg S.J., Jean-Baptiste S.R., et al. Circulating tumor cells are associated with recurrent disease in patients with early-stage non–small cell lung cancer treated with stereotactic body radiotherapy. Clin Cancer Res. 2020;26:2372–2380. doi: 10.1158/1078-0432.CCR-19-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chaudhuri A.A., Binkley M.S., Osmundson E.C., Alizadeh A.A., Diehn M. Predicting radiotherapy responses and treatment outcomes through analysis of circulating tumor DNA. Semin Radiat Oncol. 2015;25:305–312. doi: 10.1016/j.semradonc.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Proctor M.J., Morrison D.S., Talwar D., et al. A comparison of inflammation-based prognostic scores in patients with cancer. A glasgow inflammation outcome study. Eur J Cancer. 2011;47:2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 189.Choi N., Kim J.H., Chie E.K., Gim J., Kang H.-C. A meta-analysis of the impact of neutrophil-to-lymphocyte ratio on treatment outcomes after radiotherapy for solid tumors. Medicine. 2019;98 doi: 10.1097/MD.0000000000015369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vaupel P., Mayer A., Höckel M. Impact of hemoglobin levels on tumor oxygenation: the higher, the better? Strahlenther Onkol. 2006;182:63–71. doi: 10.1007/s00066-006-1543-7. [DOI] [PubMed] [Google Scholar]
- 191.Barcellini A., Fontana G., Filippini D.M., et al. Exploring the role of neutrophil-to-lymphocyte ratio and blood chemistry in head and neck adenoid cystic carcinomas treated with carbon ion radiotherapy. Radiother Oncol. 2022;177:143–151. doi: 10.1016/j.radonc.2022.10.027. [DOI] [PubMed] [Google Scholar]
- 192.Ramteke P., Deb A., Shepal V., Bhat M.K. Hyperglycemia associated metabolic and molecular alterations in cancer risk, progression, treatment, and mortality. Cancers. 2019;11 doi: 10.3390/cancers11091402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Duan W., Shen X., Lei J., et al. Hyperglycemia, a neglected factor during cancer progression. Biomed Res Int. 2014;2014 doi: 10.1155/2014/461917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Scott J.G., Berglund A., Schell M.J., et al. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol. 2017;18:202–211. doi: 10.1016/S1470-2045(16)30648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Eschrich S.A., Pramana J., Zhang H., et al. A gene expression model of intrinsic tumor radiosensitivity: prediction of response and prognosis after chemoradiation. Int J Radiat Oncol Biol Phys. 2009;75:489–496. doi: 10.1016/j.ijrobp.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Eschrich S., Zhang H., Zhao H., et al. Systems biology modeling of the radiation sensitivity network: a biomarker discovery platform. Int J Radiat Oncol Biol Phys. 2009;75:497–505. doi: 10.1016/j.ijrobp.2009.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Eschrich S.A., Fulp W.J., Pawitan Y., et al. Validation of a radiosensitivity molecular signature in breast cancer. Clin Cancer Res. 2012;18:5134–5143. doi: 10.1158/1078-0432.CCR-12-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mohammadi H., Prince A., Figura N.B., et al. Using the radiosensitivity index (RSI) to predict pelvic failure in endometrial cancer treated with adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2020;106:496–502. doi: 10.1016/j.ijrobp.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Strom T., Hoffe S.E., Fulp W., et al. Radiosensitivity index predicts for survival with adjuvant radiation in resectable pancreatic cancer. Radiother Oncol. 2015;117:159–164. doi: 10.1016/j.radonc.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Scott J.G., Sedor G., Ellsworth P., et al. Pan-cancer prediction of radiotherapy benefit using genomic-adjusted radiation dose (GARD): a cohort-based pooled analysis. Lancet Oncol. 2021;22:1221–1229. doi: 10.1016/S1470-2045(21)00347-8. [DOI] [PubMed] [Google Scholar]
- 201.Mistry H.B. Radiosensitivity index is not fit to be used for dose adjustments: a pan-cancer analysis. Clin Oncol. 2023;35:565–570. doi: 10.1016/j.clon.2023.02.018. [DOI] [PubMed] [Google Scholar]
- 202.Speers C., Zhao S., Liu M., Bartelink H., Pierce L.J., Feng F.Y. Development and validation of a novel radiosensitivity signature in human breast cancer. Clin Cancer Res. 2015;21:3667–3677. doi: 10.1158/1078-0432.CCR-14-2898. [DOI] [PubMed] [Google Scholar]
- 203.Foy J.-P., Bazire L., Ortiz-Cuaran S., et al. A 13-gene expression-based radioresistance score highlights the heterogeneity in the response to radiation therapy across HPV-negative HNSCC molecular subtypes. BMC Med. 2017;15:165. doi: 10.1186/s12916-017-0929-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Langen B., Vorontsov E., Spetz J., et al. Age and sex effects across the blood proteome after ionizing radiation exposure can bias biomarker screening and risk assessment. Sci Rep. 2022;12:7000. doi: 10.1038/s41598-022-10271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gillies R.J., Kinahan P.E., Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278:563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Aerts H.J.W.L., Velazquez E.R., Leijenaar R.T.H., et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Murakami Y., Soyano T., Kozuka T., et al. Dose-based radiomic analysis (dosiomics) for intensity modulated radiation therapy in patients with prostate cancer: correlation between planned dose distribution and biochemical failure. Int J Radiat Oncol Biol Phys. 2022;112:247–259. doi: 10.1016/j.ijrobp.2021.07.1714. [DOI] [PubMed] [Google Scholar]
- 208.Wu A., Li Y., Qi M., et al. Dosiomics improves prediction of locoregional recurrence for intensity modulated radiotherapy treated head and neck cancer cases. Oral Oncol. 2020;104 doi: 10.1016/j.oraloncology.2020.104625. [DOI] [PubMed] [Google Scholar]
- 209.Liang B., Yan H., Tian Y., et al. Dosiomics: extracting 3D spatial features from dose distribution to predict incidence of radiation pneumonitis. Front Oncol. 2019;9:269. doi: 10.3389/fonc.2019.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Buizza G., Paganelli C., D’Ippolito E., et al. Radiomics and dosiomics for predicting local control after carbon-ion radiotherapy in skull-base chordoma. Cancers. 2021;13:339. doi: 10.3390/cancers13020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Matsumoto S., Lee S.H., Imai R., et al. Unresectable chondrosarcomas treated with carbon ion radiotherapy: relationship between dose-averaged linear energy transfer and local recurrence. Anticancer Res. 2020;40:6429–6435. doi: 10.21873/anticanres.14664. [DOI] [PubMed] [Google Scholar]
- 212.Molinelli S., Magro G., Mairani A., et al. How LEM-based RBE and dose-averaged LET affected clinical outcomes of sacral chordoma patients treated with carbon ion radiotherapy. Radiother Oncol. 2021;163:209–214. doi: 10.1016/j.radonc.2021.08.024. [DOI] [PubMed] [Google Scholar]
- 213.Morelli L., Parrella G., Molinelli S., et al. A dosiomics analysis based on linear energy transfer and biological dose maps to predict local recurrence in sacral chordomas after carbon-ion radiotherapy. Cancers. 2022;15:33. doi: 10.3390/cancers15010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Collins G.S., Reitsma J.B., Altman D.G., Moons K.G.M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Br J Cancer. 2015;112:251–259. doi: 10.1038/bjc.2014.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lambin P., Leijenaar R.T.H., Deist T.M., et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749–762. doi: 10.1038/nrclinonc.2017.141. [DOI] [PubMed] [Google Scholar]
- 216.Dünker N., Jendrossek V. Implementation of the chick chorioallantoic membrane (CAM) model in radiation biology and experimental radiation oncology research. Cancers. 2019;11 doi: 10.3390/cancers11101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Stillger M.N., Li M.J., Hönscheid P., von Neubeck C., Föll M.C. Advancing rare cancer research by MALDI mass spectrometry imaging: applications, challenges, and future perspectives in sarcoma. Proteomics. 2024;24 doi: 10.1002/pmic.202300001. [DOI] [PubMed] [Google Scholar]
- 218.Jakob B., Dubiak-Szepietowska M., Janiel E., Schmidt A., Durante M., Taucher-Scholz G. Differential repair protein recruitment at sites of clustered and isolated DNA double-strand breaks produced by high-energy heavy ions. Sci Rep. 2020;10:1443. doi: 10.1038/s41598-020-58084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yokota Y., Wada Y., Funayama T. Distinct modes of death in human neural stem and glioblastoma cells irradiated with carbon-ion radiation and gamma-rays. Int J Radiat Biol. 2020;96:172–178. doi: 10.1080/09553002.2020.1683639. [DOI] [PubMed] [Google Scholar]
- 220.Merz F., Gaunitz F., Dehghani F., et al. Organotypic slice cultures of human glioblastoma reveal different susceptibilities to treatments. Neuro Oncol. 2013;15:670–681. doi: 10.1093/neuonc/not003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Verwer R.W.H., Hermens W.T.J.M.C., Dijkhuizen P.A., et al. Cells in human postmortem brain tissue slices remain alive for several weeks in culture. FASEB J. 2002;16:54–60. doi: 10.1096/fj.01-0504com. [DOI] [PubMed] [Google Scholar]
- 222.Verhaegen F., Granton P., Tryggestad E. Small animal radiotherapy research platforms. Phys Med Biol. 2011;56:R55. doi: 10.1088/0031-9155/56/12/R01. [DOI] [PubMed] [Google Scholar]
- 223.Verhaegen F., Dubois L., Gianolini S., et al. ESTRO ACROP: technology for precision small animal radiotherapy research: optimal use and challenges. Radiother Oncol. 2018;126:471–478. doi: 10.1016/j.radonc.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 224.Puspitasari A., Koganezawa N., Ishizuka Y., et al. X irradiation induces acute cognitive decline via transient synaptic dysfunction. Radiat Res. 2016;185:423–430. doi: 10.1667/rr14236.1. [DOI] [PubMed] [Google Scholar]
- 225.Parihar V.K., Pasha J., Tran K.K., Craver B.M., Acharya M.M., Limoli C.L. Persistent changes in neuronal structure and synaptic plasticity caused by proton irradiation. Brain Struct Funct. 2015;220:1161–1171. doi: 10.1007/s00429-014-0709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Debus J., Scholz M., Haberer T., et al. Radiation tolerance of the rat spinal cord after single and split doses of photons and carbon ions. Radiat Res. 2003;160(7):536–542. doi: 10.1667/3063. [DOI] [PubMed] [Google Scholar]
- 227.Saager M., Peschke P., Welzel T., et al. Late normal tissue response in the rat spinal cord after carbon ion irradiation. Radiat Oncol. 2018;13:5. doi: 10.1186/s13014-017-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hodge R.D., Bakken T.E., Miller J.A., et al. Conserved cell types with divergent features in human versus mouse cortex. Nature. 2019;573:61–68. doi: 10.1038/s41586-019-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lancaster M.A., Corsini N.S., Wolfinger S., et al. Guided self-organization and cortical plate formation in human brain organoids. Nat Biotechnol. 2017;35:659–666. doi: 10.1038/nbt.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Quadrato G., Nguyen T., Macosko E.Z., et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature. 2017;545:48–53. doi: 10.1038/nature22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dutta D., Heo I., Clevers H. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol Med. 2017;23:393–410. doi: 10.1016/j.molmed.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 232.Pasch C.A., Favreau P.F., Yueh A.E., et al. Patient-derived cancer organoid cultures to predict sensitivity to chemotherapy and radiation. Clin Cancer Res. 2019;25:5376–5387. doi: 10.1158/1078-0432.Ccr-18-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Durante M., Bender T., Schickel E., et al. Aberrant choroid plexus formation in human cerebral organoids exposed to radiation. Res Sq. 2023 doi: 10.21203/rs.3.rs-3445801/v1. [DOI] [Google Scholar]
- 234.Giandomenico S.L., Mierau S.B., Gibbons G.M., et al. Cerebral organoids at the air–liquid interface generate diverse nerve tracts with functional output. Nat Neurosci. 2019;22:669–679. doi: 10.1038/s41593-019-0350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wu Q., Liu J., Wang X., et al. Organ-on-a-chip: recent breakthroughs and future prospects. Biomed Eng Online. 2020;19:9. doi: 10.1186/s12938-020-0752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ingber D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat Rev Genet. 2022;23:467–491. doi: 10.1038/s41576-022-00466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Liu X., Su Q., Zhang X., et al. Recent advances of organ-on-a-chip in cancer modeling research. Biosensors. 2022;12:1045. doi: 10.3390/bios12111045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jalili-Firoozinezhad S., Prantil-Baun R., Jiang A., et al. Modeling radiation injury-induced cell death and countermeasure drug responses in a human gut-on-a-chip. Cell Death Dis. 2018;9:223. doi: 10.1038/s41419-018-0304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yi H.-G., Jeong Y.H., Kim Y., et al. A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat Biomed Eng. 2019;3:509–519. doi: 10.1038/s41551-019-0363-x. [DOI] [PubMed] [Google Scholar]
- 240.Dasgupta Q., Jiang A., Wen A.M., et al. A human lung alveolus-on-a-chip model of acute radiation-induced lung injury. Nat Commun. 2023;14:6506. doi: 10.1038/s41467-023-42171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Riedel N.C., de Faria F.W., Alfert A., Bruder J.M., Kerl K. Three-dimensional cell culture systems in pediatric and adult brain tumor precision medicine. Cancers. 2022;14:5972. doi: 10.3390/cancers14235972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.International Atomic Energy AgencyRelative Biological Effectiveness in Ion Beam Therapy, Technical Reports Series No. 461; 2008.
- 243.Scholz M., Kellerer A.M., Kraft-Weyrather W., Kraft G. Computation of cell survival in heavy ion beams for therapy: the model and its approximation. Radiat Environ Biophys. 1997;36:59–66. doi: 10.1007/s004110050055. [DOI] [PubMed] [Google Scholar]
- 244.Hawkins R.B. A microdosimetric-kinetic model of cell death from exposure to ionizing radiation of any LET, with experimental and clinical applications. Int J Radiat Biol. 1996;69:739–755. doi: 10.1080/095530096145481. [DOI] [PubMed] [Google Scholar]
- 245.Inaniwa T., Furukawa T., Kase Y., et al. Treatment planning for a scanned carbon beam with a modified microdosimetric kinetic model. Phys Med Biol. 2010;55:6721–6737. doi: 10.1088/0031-9155/55/22/008. [DOI] [PubMed] [Google Scholar]
- 246.Elsässer T., Weyrather W.K., Friedrich T., et al. Quantification of the relative biological effectiveness for ion beam radiotherapy: direct experimental comparison of proton and carbon ion beams and a novel approach for treatment planning. Int J Radiat Oncol Biol Phys. 2010;78:1177–1183. doi: 10.1016/j.ijrobp.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 247.Parisi A., Beltran C.J., Furutani K.M. The Mayo Clinic Florida microdosimetric kinetic model of clonogenic survival: formalism and first benchmark against in vitro and in silico data. Phys Med Biol. 2022;67 doi: 10.1088/1361-6560/ac7375. [DOI] [PubMed] [Google Scholar]
- 248.Bellinzona V.E., Cordoni F., Missiaggia M., et al. Linking microdosimetric measurements to biological effectiveness in ion beam therapy: a review of theoretical aspects of MKM and other models. Front Phys. 2021;8 doi: 10.3389/fphy.2020.578492. [DOI] [Google Scholar]
- 249.Cordoni F., Missiaggia M., Attili A., Welford S.M., Scifoni E., La Tessa C. Generalized stochastic microdosimetric model: the main formulation. Phys Rev E. 2021;103 doi: 10.1103/PhysRevE.103.012412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Faddegon B., Blakely E.A., Burigo L., et al. Ionization detail parameters and cluster dose: a mathematical model for selection of nanodosimetric quantities for use in treatment planning in charged particle radiotherapy. Phys Med Biol. 2023;68 doi: 10.1088/1361-6560/acea16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Mairani A., Magro G., Dokic I., et al. Data-driven RBE parameterization for helium ion beams. Phys Med Biol. 2016;61:888–905. doi: 10.1088/0031-9155/61/2/888. [DOI] [PubMed] [Google Scholar]
- 252.Papakonstantinou D., Zanni V., Nikitaki Z., Vasileiou C., Kousouris K., Georgakilas A.G. Using machine learning techniques for asserting cellular damage induced by high-LET particle radiation. Radiation. 2021;1:45–64. doi: 10.3390/radiation1010005. [DOI] [Google Scholar]
- 253.Tian L., Lühr A. Data-driven ion-independent relative biological effectiveness modeling using the beam quality Q. Phys Med Biol. 2023;68 doi: 10.1088/1361-6560/acc9f9. [DOI] [PubMed] [Google Scholar]
- 254.Cordoni F.G., Missiaggia M., Scifoni E., La Tessa C. An artificial intelligence-based model for cell killing prediction: development, validation and explainability analysis of the ANAKIN model. Phys Med Biol. 2023;68 doi: 10.1088/1361-6560/acc71e. [DOI] [PubMed] [Google Scholar]
- 255.Steinsträter O., Grün R., Scholz U., Friedrich T., Durante M., Scholz M. Mapping of RBE-weighted doses between HIMAC- and LEM-based treatment planning systems for carbon ion therapy. Int J Radiat Oncol Biol Phys. 2012;84:854–860. doi: 10.1016/j.ijrobp.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 256.Fossati P., Molinelli S., Matsufuji N., et al. Dose prescription in carbon ion radiotherapy: a planning study to compare NIRS and LEM approaches with a clinically-oriented strategy. Phys Med Biol. 2012;57:7543–7554. doi: 10.1088/0031-9155/57/22/7543. [DOI] [PubMed] [Google Scholar]
- 257.ICRU Report 93 Prescribing, recording, and reporting light ion beam therapy. J ICRU. 2016;16:1–2. [Google Scholar]
- 258.Paganetti H., Blakely E., Carabe-Fernandez A., et al. Report of the AAPM TG-256 on the relative biological effectiveness of proton beams in radiation therapy. Med Phys. 2019;46:e53–e78. doi: 10.1002/mp.13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rørvik E., Fjæra L.F., Dahle T.J., et al. Exploration and application of phenomenological RBE models for proton therapy. Phys Med Biol. 2018;63 doi: 10.1088/1361-6560/aad9db. [DOI] [PubMed] [Google Scholar]
- 260.Gardner L.L., O’Connor J.D., McMahon S.J. Benchmarking proton RBE models. Phys Med Biol. 2024;69 doi: 10.1088/1361-6560/ad3329. [DOI] [PubMed] [Google Scholar]
- 261.Grün R., Friedrich T., Krämer M., Scholz M. Systematics of relative biological effectiveness measurements for proton radiation along the spread out Bragg peak: experimental validation of the local effect model. Phys Med Biol. 2017;62:890–908. doi: 10.1088/1361-6560/62/3/890. [DOI] [PubMed] [Google Scholar]
- 262.Debrot E., Tran L., Chartier L., et al. SOI microdosimetry and modified MKM for evaluation of relative biological effectiveness for a passive proton therapy radiation field. Phys Med Biol. 2018;63 doi: 10.1088/1361-6560/aaec2f. [DOI] [PubMed] [Google Scholar]
- 263.Bertolet A., Cortés-Giraldo M.A., Carabe-Fernandez A. Implementation of the microdosimetric kinetic model using analytical microdosimetry in a treatment planning system for proton therapy. Phys Med. 2021;81:69–76. doi: 10.1016/j.ejmp.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 264.Friedrich T., Pfuhl T., Scholz M. Update of the particle irradiation data ensemble (PIDE) for cell survival. J Radiat Res. 2021;62:645–655. doi: 10.1093/jrr/rrab034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Pfuhl T., Friedrich T., Scholz M. Comprehensive comparison of local effect model IV predictions with the particle irradiation data ensemble. Med Phys. 2022;49:714–726. doi: 10.1002/mp.15343. [DOI] [PubMed] [Google Scholar]
- 266.Suárez-García D., Cortés-Giraldo M.A., Bertolet A. A systematic analysis of the particle irradiation data ensemble in the key of the microdosimetric kinetic model: should clonogenic data be used for clinical relative biological effectiveness? Radiother Oncol. 2023;185 doi: 10.1016/j.radonc.2023.109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Furusawa Y., Fukutsu K., Aoki M., et al. Inactivation of aerobic and hypoxic cells from three different cell lines by accelerated (3)He-, (12)C- and (20)Ne-ion beams. Radiat Res. 2000;154:485–496. doi: 10.1667/0033-7587(2000)154. [DOI] [PubMed] [Google Scholar]
- 268.Bronk L., Guan F., Patel D., et al. Mapping the relative biological effectiveness of proton, helium and carbon ions with high-throughput techniques. Cancers. 2020;12 doi: 10.3390/cancers12123658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Langendijk J.A., Lambin P., De Ruysscher D., Widder J., Bos M., Verheij M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiother Oncol. 2013;107:267–273. doi: 10.1016/j.radonc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 270.Brodin N.P., Kabarriti R., Garg M.K., Guha C., Tomé W.A. Systematic review of normal tissue complication models relevant to standard fractionation radiation therapy of the head and neck region published after the QUANTEC reports. Int J Radiat Oncol Biol Phys. 2018;100:391–407. doi: 10.1016/j.ijrobp.2017.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Brüningk S.C., Kamp F., Wilkens J.J. EUD-based biological optimization for carbon ion therapy. Med Phys. 2015;42:6248–6257. doi: 10.1118/1.4932219. [DOI] [PubMed] [Google Scholar]
- 272.Kaderka R., Liu K.-C., Liu L., et al. Toward automatic beam angle selection for pencil-beam scanning proton liver treatments: a deep learning-based approach. Med Phys. 2022;49:4293–4304. doi: 10.1002/mp.15676. [DOI] [PubMed] [Google Scholar]
- 273.Battestini M., Schwarz M., Krämer M., Scifoni E. Including volume effects in biological treatment plan optimization for carbon ion therapy: generalized equivalent uniform dose-based objective in TRiP98. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.826414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Friedrich T., Henthorn N., Durante M. Modeling radioimmune response—Current status and perspectives. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.647272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sung W., Hong T.S., Poznansky M.C., Paganetti H., Grassberger C. Mathematical modeling to simulate the effect of adding radiation therapy to immunotherapy and application to hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2022;112:1055–1062. doi: 10.1016/j.ijrobp.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Friedrich T., Scholz M., Durante M. A predictive biophysical model of the combined action of radiation therapy and immunotherapy of cancer. Int J Radiat Oncol Biol Phys. 2022;113:872–884. doi: 10.1016/j.ijrobp.2022.03.030. [DOI] [PubMed] [Google Scholar]
- 277.Cella L., Monti S., Pacelli R., Palma G. Modeling frameworks for radiation induced lymphopenia: a critical review. Radiother Oncol. 2024;190 doi: 10.1016/j.radonc.2023.110041. [DOI] [PubMed] [Google Scholar]
- 278.Dal Bello R., Becher T., Fuss M.C., Krämer M., Seco J. Proposal of a chemical mechanism for mini-beam and micro-beam efficacy. Front Phys. 2020;8 doi: 10.3389/fphy.2020.564836. [DOI] [Google Scholar]
- 279.Masilela T.A.M., Prezado Y. Monte Carlo study of the free radical yields in minibeam radiation therapy. Med Phys. 2023;50:5115–5134. doi: 10.1002/mp.16475. [DOI] [PubMed] [Google Scholar]
- 280.Zhang T., García‐Calderón D., Molina‐Hernández M., Leitão J., Hesser J., Seco J. A theoretical study of H2O2 as the surrogate of dose in minibeam radiotherapy, with a diffusion model considering radical removal process. Med Phys. 2023;50:5262–5272. doi: 10.1002/mp.16570. [DOI] [PubMed] [Google Scholar]
- 281.Pouget J.-P., Georgakilas A.G., Ravanat J.-L. Targeted and off-target (bystander and abscopal) effects of radiation therapy: redox mechanisms and risk/benefit analysis. Antioxid Redox Signal. 2018;29:1447–1487. doi: 10.1089/ars.2017.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Mukherjee S., Chakraborty A. Radiation-induced bystander phenomenon: insight and implications in radiotherapy. Int J Radiat Biol. 2019;95:243–263. doi: 10.1080/09553002.2019.1547440. [DOI] [PubMed] [Google Scholar]
- 283.Kundrát P., Friedland W. Mechanistic modelling of radiation-induced bystander effects. Radiat Prot Dosimetry. 2015;166:148–151. doi: 10.1093/rpd/ncv170. [DOI] [PubMed] [Google Scholar]
- 284.van Luijk P., Bijl H.P., Konings A.W.T., van der Kogel A.J., Schippers J.M. Data on dose–volume effects in the rat spinal cord do not support existing NTCP models. Int J Radiat Oncol Biol Phys. 2005;61:892–900. doi: 10.1016/j.ijrobp.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 285.Asperud J., Arous D., Edin N.F.J., Malinen E. Spatially fractionated radiotherapy: tumor response modelling including immunomodulation. Phys Med Biol. 2021;66 doi: 10.1088/1361-6560/ac176b. [DOI] [PubMed] [Google Scholar]
- 286.Brenner D.J., Hlatky L.R., Hahnfeldt P.J., Huang Y., Sachs R.K. The linear-quadratic model and most other common radiobiological models result in similar predictions of time-dose relationships. Radiat Res. 1998;150:83–91. [PubMed] [Google Scholar]
- 287.Wardman P. Radiotherapy using high-intensity pulsed radiation beams (FLASH): a radiation-chemical perspective. Radiat Res. 2020;194:607–617. doi: 10.1667/RADE-19-00016. [DOI] [PubMed] [Google Scholar]
- 288.Thompson S.J., Prise K.M., McMahon S.J. Investigating the potential contribution of inter-track interactions within ultra-high dose-rate proton therapy. Phys Med Biol. 2023;68 doi: 10.1088/1361-6560/acb88a. [DOI] [PubMed] [Google Scholar]
- 289.Boscolo D., Krämer M., Fuss M.C., Durante M., Scifoni E. Impact of target oxygenation on the chemical track evolution of ion and electron radiation. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21020424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ramos-Méndez J., Domínguez-Kondo N., Schuemann J., McNamara A., Moreno-Barbosa E., Faddegon B. LET-dependent intertrack yields in proton irradiation at ultra-high dose rates relevant for FLASH therapy. Radiat Res. 2020;194:351–362. doi: 10.1667/RADE-20-00084.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Battestini M., Missiaggia M., Attili A., et al. Across the stages: a multiscale extension of the generalized stochastic microdosimetric model (MS-GSM2) to include the ultra-high dose rate. Front Phys. 2023;11 doi: 10.3389/fphy.2023.1274064. [DOI] [Google Scholar]
- 292.Zakaria A.M., Colangelo N.W., Meesungnoen J., Azzam E.I., Plourde M.-É., Jay-Gerin J.-P. Ultra-high dose-rate, pulsed (FLASH) radiotherapy with carbon ions: generation of early, transient, highly oxygenated conditions in the tumor environment. Radiat Res. 2020;194:587–593. doi: 10.1667/RADE-19-00015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Böhlen T.T., Germond J.-F., Bourhis J., et al. Normal tissue sparing by FLASH as a function of single-fraction dose: a quantitative analysis. Int J Radiat Oncol Biol Phys. 2022;114:1032–1044. doi: 10.1016/j.ijrobp.2022.05.038. [DOI] [PubMed] [Google Scholar]