Abstract
Background
The triglyceride-glucose (TyG) index is recognized as a robust indicator for evaluating insulin resistance (IR). Despite the well-documented anti-aging biological functions of Klotho protein, its correlation with the TyG index remains unexplored.
Methods
A cross-sectional analysis was conducted involving participants from the National Health and Nutrition Examination Surveys (NHANES) 2007–2016. The TyG index was computed using laboratory data, while serum Klotho concentrations was determined using ELISA kit. After adjusting potential confounding variables, multivariate regression models were employed to evaluate the association between the TyG index and Klotho protein levels among middle-aged and elderly females and males separately. Additionally, smooth curve fitting and segmented regression model were applied to investigate potential threshold effects and identify the inflection point.
Results
A total of 6,573 adults qualified for inclusion, comprising 3,147 (47.88%) males and 3,426 (52.12%) females. Multivariate regression analysis revealed that females with a higher TyG index exhibited significantly lower serum Klotho concentrations (β=-83.41, 95% CI: -124.23 to -42.60, P < 0.0001). This association was not statistically significant in males (β = 15.40, 95% CI: -19.16 to 49.95, P = 0.3827). Subgroup analyses revealed a significant interaction effect by diabetes status in females (P-interaction = 0.0121), where non-diabetic females showed a stronger negative association between TyG index and serum Klotho levels compared to diabetic females. In the female group, when TyG index was divided into quartiles, individuals in the highest quartile of TyG index exhibited reduced levels of Klotho protein (Q4: -88.77 pg/ml) compared to those in the lowest quartile (Q1) after full adjustment (P = 0.0041). Segmented regression analysis indicated a turning point value of 9.4 in females. Notably, a 1-unit increase in TyG index was significantly associated with a decrease in Klotho levels by -111.43 pg/ml (95% CI: -157.34 to -65.52, P < 0.0001) when TyG index was below 9.4, while above this threshold, the association was not significant (Log likelihood ratio test: 0.009).
Conclusions
The findings highlight a non-linear correlation between the TyG index and serum Klotho concentrations among females, indicative of a saturation effect. This relationship was particularly pronounced in non-diabetic women. In contrast, no statistically significant association was observed in male participants.
Keywords: Triglyceride-glucose index, Insulin resistance, Klotho, NHANES, Aging
Background
Insulin resistance (IR) results from a reduced sensitivity and response to the effects of insulin, which is recognized as a critical factor contributing to several disorders, including cardiovascular disease, type 2 diabetes, metabolic syndrome (MetS), and cognitive impairment [1–4]. The hyperinsulinemic-euglycemic clamp (HEC) technique has emerged as the gold standard for IR detection; however, its utility in clinical settings is limited due to its laborious and costly nature [5]. Subsequently, the homeostatic model assessment of insulin resistance (HOMA-IR) was introduced to estimate IR indirectly through fasting glucose and insulin levels [6]. Despite its widespread use, the necessity for insulin measurement, elevated testing expenses, and limited repeatability have restricted its practical application. Recent research has highlighted the triglyceride-glucose (TyG) index, a synthetic marker derived from fasting triglycerides and glucose, as an effective alternative to gold standard IR measurements, due to its considerable connection with HEC [7]. This index has proven to be as effective as, or even superior to, HOMA-IR in assessing IR [8, 9]. Furthermore, the TyG score outperforms traditional indicators in terms of accessibility and cost-effectiveness. Extensive research has demonstrated its association with increased risks of age-related diseases such as cardiovascular disease, arteriosclerosis, dementia, and diabetes [10–12].
The Klotho gene is responsible for producing a transmembrane protein and its soluble variant, which emerges through the proteolytic cleavage of its extracellular domain by membrane-anchored proteases [13]. Serum soluble Klotho (hereinafter referred to as Klotho), acting as a hormone, exhibits diverse health-related functions and remarkable anti-aging properties [14]. The expression of Klotho protein contributes to cardiovascular health by improving endothelial dysfunction and alleviating arteriosclerosis [15, 16]. Conversely, downregulation of this protein has been implicated in various aging-associated disorders, including cancer, MetS, osteoporosis, and cognitive decline [17–20]. Notably, these diseases have also been linked to IR [21]. Moreover, it has been consistently reported that serum Klotho levels were inversely correlated with age in humans [22]. A shorter leukocyte telomere length, a robust marker of biological aging, has been associated with elevated TyG index values [23]. Despite these insights, research into the direct correlation between the TyG index and serum Klotho concentrations remains scarce.
Research into Klotho has unveiled gender-specific disparities, revealing that serum Klotho levels and their associated anti-aging effects vary between females and males, alongside their responses to various diseases [24]. Additionally, gender also affects the degree of IR, with women showing a higher prevalence of impaired fasting glucose [25, 26]. Consequently, this suggests that the association between the TyG index, an indicator for IR, and serum Klotho concentrations could exhibit gender-related variations.
Therefore, we obtained publicly available data from the National Health and Nutrition Examination Survey (NHANES) to investigate the correlation between the TyG index and serum Klotho levels in the middle-aged and elderly population, focusing specifically on identifying any gender-based disparities within this association.
Methods
Study design and participants
NHANES is a cross-sectional and nationally representative survey that annually collects data from approximately 5,000 individuals to assess the health and nutritional status across the United States. Authorized by the National Center for Health Statistics of the Centers for Disease Control and Prevention, the survey ensures all participants give written informed consent, adhering to ethical standards for study conduct.
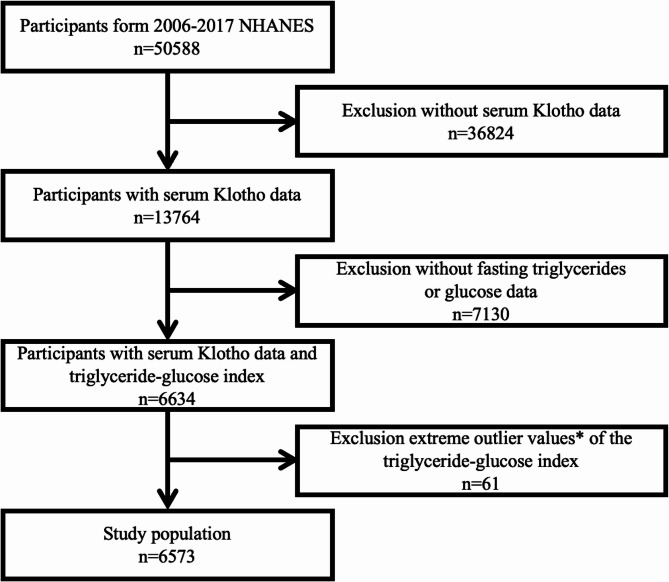
Considering the availability of Klotho, this study extracted NHANES data from 2007 to 2016 with 50,588 participants. Exclusion criteria included missing serum Klotho data, or incomplete information necessary for calculating the TyG index (n = 43,954). Additionally, participants with extreme TyG index values (> mean ± 3 standard deviations, n = 61) were removed [27]. No other inclusion or exclusion criteria were applied. The exclusion process resulted in a final cohort of 6,573 subjects for further detailed analysis. Figure 1 illustrates the selection procedure of the study participants.
Fig. 1.
Flowchart of the study. *Extreme outlier values, defined as those over 3 standard deviations from the mean
Measurement of serum Klotho
Serum samples were collected from participants, then transferred and preserved at -80 °C. The quantification of serum Klotho was performed using an enzyme-linked immunosorbent assay (ImmunoBiological Laboratories, Gunma, Japan) strictly adhering to the manufacturer’s protocol. The assay, with a sensitivity of 6 pg/mL, required that each sample be bisected and analyzed in duplicate. The final result was derived from the average of these two separate measurements. Serum Klotho concentrations were utilized as the outcome variable in our analysis.
Assessment of TyG index
The TyG index was calculated using the formula Ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2] [28]. Morning blood samples, collected after an overnight fast, were analyzed to measure triglyceride and glucose concentrations using enzymatic assays on the Roche Modular P and Roche Cobas 6000 chemistry analyzers, respectively. Additionally, fasting glucose concentrations were determined through a hexokinase-mediated reaction with the Roche/Hitachi Cobas C 501 analyzer. It is essential to emphasize that, within the parameters of our research design, the TyG index functioned as an exposure variable.
Measurement and definitions of covariates
Several covariates were identified as potential confounders affecting the association between the TyG index and serum Klotho levels. Covariates encompassed demographic variables (age, racial/ethnic identification including non-Hispanic White, non-Hispanic Black, other Hispanic, Mexican American, and other races), socioeconomic indicators (Poverty Income Ratio, PIR, and education levels categorized as less than high school, high school, and college or above), physical health metrics (Body Mass Index, BMI, stratified into < 25, 25–30, and ≥ 30 kg/m2 categories, waist circumference), and medication data (use of hypoglycemic and lipid-lowering prescription drugs). Lifestyle factors assessed encompassed tobacco use (categorized by lifetime cigarette consumption into never, former, and current smokers) and alcohol intake (< 12 or ≥ 12 drinks/year). Health status variables included high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), diagnoses of diabetes, hypertension, cancer, stroke, congestive heart failure (CHF), coronary heart disease (CHD), angina, heart attack, chronic obstructive pulmonary disease (COPD), along with estimated glomerular filtration rate (eGFR), computed via the Chronic Kidney Disease Epidemiology Collaboration formula [29]. Diabetes was operationalized as either a clinical diagnosis, current treatment with insulin or diabetes medication, an HbA1c level ≥ 6.5%, or a fasting glucose level ≥ 126 mg/dL. Hypertension was defined by a prior physician diagnosis or current antihypertensive medication use. The inclusion of self-reported medical conditions (cancer, stroke, CHF, CHD, angina, heart attack, and COPD) were classified dichotomously (Yes/No).
Statistical analysis
In our study, continuous variables at baseline were reported as mean ± standard deviation (SD), whereas categorical variables were represented as percentages. The chi-square test assessed differences in categorical variables between genders, while differences in continuous variables were evaluated using the Student’s t-test or the Mann-Whitney U test. The TyG index was examined both as a continuous and a categorical variable (segmented into quartiles), with the first quartile acting as the reference group for trend analyses. Multivariable linear regression was applied to elucidate the independent association between the TyG index and serum Klotho levels, comprising three models of adjustment: Model 1, without any covariate adjustments; Model 2, adjusting for age and race; and Model 3, a fully adjusted model, additionally accounted for PIR, BMI, waist circumference, education level, smoking and alcohol consumption, HDL-C, LDL-C, eGFR, cancer, stroke, CHF, CHD, angina, heart attack, COPD, hypoglycemic drugs or insulin use, lipid-lowering drug use, diabetes, and hypertension, expanding upon Model 2. The investigation of a potential non-linear relationship between the TyG index and serum Klotho involved smoothing curve fittings, with any detected non-linearity further examined through segmented linear regression to identify threshold effects and calculate the inflection point. Interaction and stratification analyses, within the context of Model 3, were systematically conducted to explore subgroup dynamics. All statistical procedures were performed using EmpowerStats (http://www.EmpowerStats.com) and R version 3.6.3, considering a two-tailed P-value of < 0.05 as indicative of statistical significance.
Results
Baseline characteristics
Table 1 presents the clinical and laboratory characteristics of the study participants, stratified by sex, drawing on data from NHANES 2007–2016. The cohort consisted of 6,573 individuals aged 40 to 79 years, including 3,147 (47.88%) males and 3,426 (52.12%) females, with average ages of 56.26 ± 10.38 and 56.59 ± 10.54 years, respectively. Male participants demonstrated a higher propensity for smoking, drinking, and being overweight compared to their female counterparts, who exhibited less favorable income levels. Notably, the TyG index and eGFR were significantly elevated in males (TyG index: 8.77 ± 0.63 vs. 8.62 ± 0.61, P < 0.0001; eGFR: 88.95 ± 20.42 vs. 86.40 ± 18.53 mL/min/1.73m2, P < 0.0001), whereas serum Klotho concentrations were significantly lower (822.88 ± 280.41 vs. 870.02 ± 316.13 pg/mL, P < 0.0001). Additionally, significant differences were observed in lipid profiles, with males having lower HDL-C levels (49.24 ± 14.63 vs. 60.71 ± 17.96 mg/dL, P < 0.0001) and LDL-C levels compared to females (116.18 ± 35.98 vs. 120.81 ± 35.54 mg/dL, P < 0.0001). The use of hypoglycemic drugs or insulin, as well as lipid-lowering medications, was more prevalent among male participants (P < 0.05). Furthermore, the incidence of chronic conditions, including diabetes mellitus, angina, CHF, CHD, and heart attack, was less frequent among female participants (all P < 0.05).
Table 1.
Baseline characteristics of study population
| Variables | Male (N = 3147) |
Female (N = 3426) |
P-value |
|---|---|---|---|
| Age (years) | 56.26 ± 10.38 | 56.59 ± 10.54 | P = 0.1916 |
| Race (%) | P = 0.1572 | ||
| Non-Hispanic White | 74.56 | 73.05 | |
| Non-Hispanic Black | 7.80 | 9.47 | |
| American Mexican | 6.22 | 6.59 | |
| Other Hispanic | 5.05 | 4.68 | |
| Other races | 6.37 | 6.22 | |
| PIR (%) | P < 0.0001 | ||
| ≤ 1.3 | 15.93 | 19.48 | |
| > 1.3 and ≤ 3.5 | 31.83 | 33.94 | |
| > 3.5 | 52.25 | 46.58 | |
| BMI (%) | P < 0.0001 | ||
| Normal (< 25 kg/m2) | 20.57 | 28.30 | |
| Overweight (25–30 kg/m2) | 41.72 | 30.32 | |
| Obesity (≥ 30 kg/m2) | 37.71 | 41.38 | |
| Waist circumference (cm) | 104.79 ± 14.43 | 98.93 ± 15.87 | P < 0.0001 |
| Education (%) | P = 0.5767 | ||
| Less than High school | 17.13 | 16.30 | |
| High school | 20.70 | 21.92 | |
| College or above | 62.16 | 61.74 | |
| Smoking (%) | P < 0.0001 | ||
| Never | 43.87 | 57.89 | |
| Former | 36.78 | 25.55 | |
| Current | 19.35 | 16.56 | |
| Alcohol (%) | P < 0.0001 | ||
| < 12 drinks/year | 13.66 | 32.54 | |
| ≥ 12 drinks/year | 86.34 | 67.46 | |
| Diabetes (%) | P < 0.0001 | ||
| Yes | 21.06 | 17.11 | |
| No | 78.94 | 82.89 | |
| Hypertension (%) | P = 0.7308 | ||
| Yes | 42.89 | 42.47 | |
| No | 57.11 | 57.53 | |
| CHF (%) | P = 0.0045 | ||
| Yes | 3.77 | 2.86 | |
| No | 95.93 | 97.10 | |
| CHD (%) | P < 0.0001 | ||
| Yes | 6.39 | 2.62 | |
| No | 93.27 | 97.03 | |
| Angina (%) | P = 0.0365 | ||
| Yes | 3.27 | 2.24 | |
| No | 96.54 | 97.59 | |
| Heart attack (%) | P < 0.0001 | ||
| Yes | 6.65 | 2.96 | |
| No | 93.28 | 96.87 | |
| Stroke (%) | P = 0.0733 | ||
| Yes | 3.11 | 3.57 | |
| No | 96.66 | 96.39 | |
| Cancer (%) | P = 0.6690 | ||
| Yes | 12.76 | 13.09 | |
| No | 87.15 | 86.75 | |
| COPD (%) | P = 0.8478 | ||
| Yes | 4.88 | 4.44 | |
| No | 95.07 | 95.53 | |
| Hypoglycemic drugs or insulin users (%) | P = 0.0168 | ||
| Yes | 5.80 | 4.49 | |
| No | 94.20 | 95.51 | |
| Lipid-lowering drug users (%) | P < 0.0001 | ||
| Yes | 29.24 | 23.48 | |
| No | 70.76 | 76.52 | |
| HDL-C (mg/dL) | 49.24 ± 14.63 | 60.71 ± 17.96 | P < 0.0001 |
| LDL-C (mg/dL) | 116.18 ± 35.98 | 120.81 ± 35.54 | P < 0.0001 |
| eGFR (mL/min/1.73m2) | 88.95 ± 20.42 | 86.40 ± 18.53 | P < 0.0001 |
| TyG index | 8.77 ± 0.63 | 8.62 ± 0.61 | P < 0.0001 |
| Klotho (pg/ml) | 822.88 ± 280.41 | 870.02 ± 316.13 | P < 0.0001 |
BMI: Body mass index; PIR: Poverty income ratio; CHF: Congestive heart failure; CHD: Coronary heart disease; COPD: Chronic obstructive pulmonary disease; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; eGFR: Estimated glomerular filtration rate
Multivariate regression analysis
Multivariate regression analysis was conducted to evaluate the independent association between the TyG index and serum Klotho concentrations within separate cohorts of middle-aged and elderly females and males. Table 2 delineates the beta coefficients (β) along with their 95% confidence intervals (CIs) across various models. In the female cohort, significant inverse relationships between the TyG index and serum Klotho levels were consistently observed across all three multivariate linear regression models. Specifically, Model 1 reported a β of − 75.48 with a 95% CI ranging from − 92.72 to − 58.24 (P < 0.0001). Model 2 demonstrated a β of − 68.29 (95% CI: −85.84 to − 50.74, P < 0.0001), and Model 3 showed a β of − 83.41 (95% CI: −124.23 to − 42.60, P < 0.0001). These results suggest a consistent decrease in serum Klotho protein levels in females with an elevated TyG index. Furthermore, this significant association remained robust upon categorization of the TyG index into quartiles, with participants in the highest quartile (Q4) experiencing a reduction in Klotho levels by 88.77 pg/ml compared to those in the lowest quartile (Q1), even after comprehensive adjustments. The trend analysis across all models confirmed a significant decline in Klotho levels corresponding with increasing TyG index (all P < 0.01). Conversely, in the male cohort, no statistically significant association was observed in the fully adjusted model (Model 3), with a β of 15.40 (95% CI: −19.16 to 49.95, P = 0.3827), indicating a disparate impact of TyG index on serum Klotho concentrations by sex.
Table 2.
Multivariate linear analysis of the association between TyG index and serum Klotho concentrations
| Exposure | Model 1 β (95% CI) |
P-value | Model 2 β (95% CI) |
P-value | Model 3 β (95% CI) |
P-value |
|---|---|---|---|---|---|---|
| Female group | ||||||
| TyG index | -75.48 (-92.72, -58.24) | < 0.0001 | -68.29 (-85.84, -50.74) | < 0.0001 | -83.41 (-124.23, -42.60) | < 0.0001 |
| TyG index quartiles | ||||||
| Quartile 1 | Reference | Reference | Reference | |||
| Quartile 2 | -53.89 (-82.70, -25.08) | 0.0002 | -48.13 (-76.83, -19.44) | 0.0010 | -29.99 (-81.57, 21.59) | 0.2547 |
| Quartile 3 | -93.00 (-122.40, -63.60) | < 0.0001 | -82.61 (-112.30, -52.93) | < 0.0001 | -85.61 (-145.58, -25.65) | 0.0052 |
| Quartile 4 | -123.32 (-153.26, -93.39) | < 0.0001 | -111.29 (-141.64, -80.93) | < 0.0001 | -88.77 (-155.58, -21.95) | 0.0093 |
| P for trend | < 0.0001 | < 0.0001 | 0.0041 | |||
| Male group | ||||||
| TyG index | 19.78 (4.24, 35.32) | 0.0126 | 18.48 (2.79, 34.16) | 0.0210 | 15.40 (-19.16, 49.95) | 0.3827 |
| TyG index quartiles | ||||||
| Quartile 1 | Reference | Reference | Reference | |||
| Quartile 2 | 28.14 (0.49, 55.79) | 0.0462 | 26.96 (-0.75, 54.68) | 0.0566 | -7.95 (-56.31, 40.40) | 0.7473 |
| Quartile 3 | 22.88 (-4.75, 50.51) | 0.1047 | 22.30 (-5.44, 50.05) | 0.1152 | -27.30 (-77.86, 23.27) | 0.2903 |
| Quartile 4 | 34.73 (6.90, 62.57) | 0.0145 | 32.39 (4.29, 60.48) | 0.0239 | 11.76 (-45.69, 69.20) | 0.6884 |
| P for trend | 0.0275 | 0.0423 | 0.8620 |
Model 1: No covariates were adjusted
Model 2: Age and race were adjusted
Model 3: Age, race, PIR, BMI, waist circumference, education, smoking, alcohol consumption, HDL-C, LDL-C, eGFR, cancer, stroke, CHF, CHD, angina, heart attack, COPD, hypoglycemic drugs or insulin use, lipid-lowering drug use, diabetes, and hypertension were adjusted
BMI: Body mass index; PIR: Poverty income ratio; CHF: Congestive heart failure; CHD: Coronary heart disease; COPD: Chronic obstructive pulmonary disease; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; eGFR: Estimated glomerular filtration rate
Subgroup analysis
A subgroup stratification analysis was performed to examine the robustness of the association between the TyG index and Klotho levels, stratified by age, race, and diabetes status for both males and females, as detailed in Table 3. The analysis revealed no significant interaction effects in the association based on age groups (< 60 vs. ≥60 years) or racial categories (non-Hispanic White, non-Hispanic Black, other Hispanic, Mexican American, and other races) in either gender, with all P-values for interaction exceeding 0.05.
Table 3.
Subgroup analyses for the association between TyG index and serum Klotho concentrations
| Subgroup | β (95% CI) | P Value | P-interaction |
|---|---|---|---|
| Female group | |||
| Age, Years | 0.4883 | ||
| < 60 | -69.03 (-121.81, -16.25) | 0.0105 | |
| ≥ 60 | -96.27 (-156.28, -36.27) | 0.0017 | |
| Race | 0.2850 | ||
| Non-Hispanic White | -88.04 (-136.34, -39.74) | 0.0004 | |
| Non-Hispanic Black | -92.49 (-227.48, 42.50) | 0.1796 | |
| American Mexican | 72.07 (-91.54, 235.67) | 0.3882 | |
| Other Hispanic | -49.52 (-257.92, 158.89) | 0.6415 | |
| Other races | -160.71 (-324.87, 3.45) | 0.0553 | |
| Diabetes | 0.0121 | ||
| Yes | 0.31 (-79.28, 79.89) | 0.9939 | |
| No | -115.46 (-162.63, -68.28) | < 0.0001 | |
| Male group | |||
| Age, Years | 0.2623 | ||
| < 60 | 25.28 (-16.16, 66.72) | 0.2321 | |
| ≥ 60 | -15.04 (-75.13, 45.06) | 0.6239 | |
| Race | 0.8925 | ||
| Non-Hispanic White | 11.80 (-29.03, 52.63) | 0.5712 | |
| Non-Hispanic Black | 1.96 (-123.82, 127.74) | 0.9756 | |
| American Mexican | -49.93 (-204.39, 104.54) | 0.5265 | |
| Other Hispanic | 70.69 (-124.70, 266.07) | 0.4785 | |
| Other races | 17.26 (-140.26, 174.78) | 0.8300 | |
| Diabetes | 0.4046 | ||
| Yes | 34.66 (-25.35, 94.68) | 0.2579 | |
| No | 4.95 (-35.83, 45.73) | 0.8121 |
All analyses were based on model 3. In each subgroup, the model is not adjusted for itself
However, when stratified by diabetes status, a significant interaction was observed in the female group, where the association between the TyG index and Klotho levels was more pronounced in non-diabetic females (P-interaction = 0.0121). In contrast, the male group did not show a significant interaction based on diabetes status (P-interaction = 0.4046).
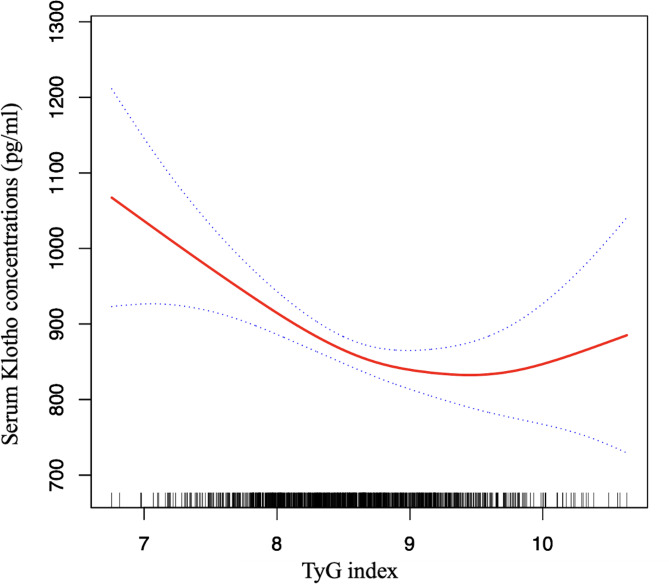
Smoothed curve fitting and threshold effect analysis
In the female cohort, smooth curve fitting was applied to assess the potential non-linear connection between the TyG index and serum Klotho levels. After comprehensive adjustment, the analysis unveiled a non-linear correlation (P for non-linearity = 0.0065; see Fig. 2). Prompted by this finding, a threshold effect analysis was initiated. The analysis via segmented regression identified a critical turning point for the TyG index at 9.4. Below this threshold, a 1-unit elevation in the TyG index corresponded to a substantial reduction in serum Klotho levels by 111.43 pg/ml (95% CI: -157.34 to -65.52, P < 0.0001). Conversely, above this threshold, the association ceased to be significant (β = 119.09, 95% CI: -39.66 to 277.84, P = 0.1418), as evidenced by the log likelihood ratio test (0.009; referenced in Table 4). These findings corroborate the initial smooth curve analysis, indicating a threshold saturation effect in the female population.
Fig. 2.
The relationship between TyG index and serum Klotho concentrations in the female group. Solid line in the middle represents smooth curve fitting between variables. The dotted line on both sides represents a 95% confidence interval (CI) for the fit. The bottom black and white strip shows the population density according to TyG index. All models were adjusted for age, race, PIR, BMI, waist circumference, education, smoking, alcohol consumption, HDL-C, LDL-C, eGFR, cancer, stroke, CHF, CHD, angina, heart attack, COPD, hypoglycemic drugs or insulin use, lipid-lowering drug use, diabetes, and hypertension
Table 4.
Threshold effect analysis for the relationship between TyG index and serum Klotho concentrations in the female group
| Model | Serum Klotho concentrations | |
|---|---|---|
| Adjusted β (95% CI) | P Value | |
| Model I | ||
| The standard linear mode | -83.41 (-124.23, -42.60) | < 0.0001 |
| Model II | ||
| Turning point (K) | ||
| TyG index < 9.4 | -111.43 (-157.34, -65.52) | < 0.0001 |
| TyG index > 9.4 | 119.09 (-39.66, 277.84) | 0.1418 |
| Log likelihood ratio test | 0.009 | |
All models were adjusted for age, race, PIR, BMI, waist circumference, education, smoking, alcohol consumption, HDL-C, LDL-C, eGFR, cancer, stroke, CHF, CHD, angina, heart attack, COPD, hypoglycemic drugs or insulin use, lipid-lowering drug use, diabetes, and hypertension
BMI: Body mass index; PIR: Poverty income ratio; CHF: Congestive heart failure; CHD: Coronary heart disease; COPD: Chronic obstructive pulmonary disease; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; eGFR: Estimated glomerular filtration rate
Discussion
In this cross-section study, we explored the relationship between the TyG index and serum Klotho concentrations using a comprehensive dataset from NHANES. Our analysis disclosed a marked negative correlation between the TyG index and serum Klotho concentrations among female participants. Notably, subgroup analyses revealed that this association was particularly pronounced in non-diabetic females. Conversely, among male participants, this correlation did not reach statistical significance upon full adjustment. Additionally, our findings indicate a non-linear relationship in females, characterized by a saturation effect with a discernible threshold at a TyG index of 9.4. These findings provide new epidemiological evidence contributing to a better understanding of the link between IR and aging.
With the rapid growth of the aging population, aging has emerged as a significant threat to public healthcare safety. While both the TyG index and Klotho protein are implicated in aging and related disorders, their interrelationship has not been previously explored. The Klotho protein is renowned for its anti-aging effects, mediated through several pathways. Notably, it downregulates the Wnt signaling pathway, thereby potentially reversing stem and progenitor cell dysfunction and promoting tissue regeneration. Additionally, Klotho exerts anti-aging effects by inhibiting the IGF-1 signaling pathway, which reduces the increase in reactive oxygen species and diminishes oxidative stress [30]. Further evidence suggests Klotho’s role in enhancing telomerase activity and telomere maintenance, through the upregulation of telomeric repeat-binding factor 1, which counters altered differentiation, cellular senescence, and stem cell apoptosis [31, 32]. These mechanisms underscore Klotho’s contribution to longevity and cellular health. Moreover, Klotho deficiency has been associated with various aging-related conditions such as hypertension, arterial stiffening, endothelial dysfunction, and other chronic diseases, highlighting its significance in aging and age-related pathologies [33].
The established connection between IR and an increased risk of developing various metabolic disorders underscores its significance as a key factor in the aging process [34]. Recent findings have elucidated a relationship between IR and Klotho, with Amaro-Gahete et al. uncovering a negative correlation between Klotho levels and IR in a cohort of healthy middle-aged individuals [35]. Furthermore, a cross-sectional analysis indicated a substantial inverse relationship between MetS and serum Klotho levels in middle-aged and older females [36]. These findings collectively imply that IR could lead to diminished serum Klotho levels. Although the TyG index is recognized as an alternative and reliable marker for IR, investigations into its correlation with serum Klotho levels are limited. Our study aligns with these observations, confirming a negative association between the TyG index and Klotho levels in middle-aged and elderly females, alongside identifying gender-specific differences and a saturation point in this relationship. This introduces a novel perspective to the research on IR and aging, providing comprehensive evidence to support further clinical and basic research. The findings have significant implications for public health and healthcare strategies, highlighting the importance of managing blood glucose and lipid levels to mitigate age-related decline in women. Monitoring the TyG index could be an effective approach for assessing the risk of aging-related diseases. Early detection and intervention for elevated TyG index levels may help delay disease progression, thereby improving the quality of life for middle-aged and elderly women.
The gender disparities observed in our study are consistent with previous research showing a significant inverse correlation between metabolic syndrome and serum Klotho levels, especially among females [36]. The underlying mechanism for these sex differences may be attributed to the role of endogenous estrogens, which are known to enhance insulin sensitivity in women [37]. However, this estrogenic protective effect can be diminished by the presence of conditions such as diabetes and hyperlipidemia [38], which are often exacerbated by a higher TyG index. Furthermore, our study population, aged over 40, predominantly consisted of women undergoing late perimenopause or menopause, a phase marked by reduced estrogen levels. This reduction in estrogen not only diminishes its beneficial effects on insulin sensitivity but also contributes to a redistribution of fat from subcutaneous to visceral depots, thereby increasing metabolic risk [39, 40]. Moreover, estrogen has been shown to upregulate the expression of Klotho [41]. The decrease in estrogen levels may therefore directly impact Klotho expression, contributing to the gender differences observed in our study.
The diabetes-stratified analysis in this study revealed a stronger correlation between the TyG index and Klotho levels in non-diabetic female participants. This finding corresponds with the saturation effect noted within our data, suggesting that at elevated levels of IR, the association between the TyG index and serum Klotho concentrations may reach a plateau or diminish. This pattern implies that in advanced stages of IR, such as diabetic individuals, the regulatory pathways connecting IR to Klotho expression may be overwhelmed by other pathological processes [42]. Further research is essential to elucidate the underlying mechanisms.
The exact molecular mechanism defining the correlation between the TyG index and serum Klotho levels remains elusive. An elevated TyG index is indicative of increased IR, which could lead to an enhanced inflammatory response, thereby affecting Klotho protein expression by altering the secretion of inflammatory mediators. Factors such as tumor necrosis factor-alpha (TNF-α) and TNF-like weak inducer of apoptosis (TWEAK), both associated with IR and metabolic dysfunctions [43, 44], have been proposed to inhibit Klotho expression [45], contributing to the observed inverse relationship between the TyG index and Klotho levels. Moreover, IR is also linked to enhanced oxidative stress [46], known to suppress α-Klotho gene expression and, subsequently, Klotho protein production [47]. Further investigations are warranted to elucidate the detailed mechanism.
Our study possesses several notable strengths. It appears to be the first to document a connection between the TyG index and levels of the anti-aging protein Klotho in humans, offering novel, practical insights into aging delay mechanisms. Additionally, we have identified gender-specific differences in this association. Future health management and treatment strategies should consider these differences. Personalized interventions tailored to the specific needs of men and women could be more effective in mitigating the adverse effects of insulin resistance and aging. Furthermore, our research benefits from analyzing a diverse, multi-ethnic sample from the comprehensive NHANES database, including 6,573 American participants, enhancing the robustness of our findings.
Nevertheless, there are limitations to consider. Firstly, the cross-sectional nature of our study limits our ability to establish causality or the directionality of the observed associations. Future research should delve deeper into the mechanistic links and conduct longitudinal studies to verify our findings and clarify the causal relationship. Additionally, assessing the long-term effects of interventions targeting high TyG index levels on Klotho concentrations and health outcomes would be beneficial. Secondly, while our analysis accounted for a wide range of covariates, the potential for residual confounding factors, such as unavailable metabolic health indicators like muscle mass, visceral fat mass, and maximal oxygen consumption (VO2 max), remains. These factors are critical in evaluating overall metabolic health and their exclusion might limit the interpretation of the associations between the TyG index and serum Klotho levels. Thus, caution is advised when interpreting our findings, and future studies should consider including these metrics to provide a more comprehensive evaluation of health outcomes. Lastly, by focusing exclusively on individuals aged 40 and above, the generalizability of our results to younger populations may be constrained.
Conclusions
In summary, our cross-sectional analysis reveals a saturation effect, indicating a non-linear association between the TyG index and serum Klotho levels in middle-aged and elderly women, particularly among non-diabetic individuals. In contrast, no statistically significant association was found in male subjects. This study highlights the potential future utility of monitoring the TyG index and Klotho levels in clinical settings, particularly for female patients, to improve early detection and intervention strategies for age-related diseases. Integrating these biomarkers into routine healthcare practices could enhance personalized medicine approaches, leading to better management of aging-related health issues. Future research should further investigate these sex-specific dynamics to enhance our understanding of aging mechanisms.
Acknowledgements
The authors have no acknowledgments to report.
Abbreviations
- IR
Insulin resistance
- TyG
Triglyceride glucose
- NHANES
National Health and Nutrition Examination Surveys
- MetS
Metabolic syndrome
- HEC
Hyperinsulinemic-euglycemic clamp
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- CHF
Congestive heart failure
- CHD
Coronary heart disease
- COPD
Chronic obstructive pulmonary disease
- eGFR
Estimated glomerular filtration rate
- SD
Standard deviation
- BMI
Body mass index
Author contributions
C.W.: Funding acquisition, Project administration, Writing – original draft; D.L.: Conceptualization, Writing – review & editing; J.L.: Formal Analysis, Writing – original draft; B.H.: Data curation, Writing – review & editing; B.F.: Data curation, Writing – review & editing; J.Y.: Conceptualization, Writing – review & editing; J.Q.: Supervision, Writing – review & editing; Z.Z.: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Natural Science Foundation of the First Affiliated Hospital of Soochow University (BXQN202229).
Data availability
The dataset underpinning the conclusions drawn in this article is accessible at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the National Health and Nutrition Examination Survey (NHANES) through the National Center for Health Statistics Research Ethics Review Board. All participants provided their informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chen Wang, Dongmei Liu and Jie Lu contributed equally to this work and share first authorship.
References
- 1.Lu S, Li Y, Qian Z, et al. Role of the inflammasome in insulin resistance and type 2 diabetes mellitus. Front Immunol. 2023;14:1052756. 10.3389/fimmu.2023.1052756. Published 2023 Mar 13. 10.3389/fimmu.2023.1052756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambie M, Bonomini M, Davies SJ, Accili D, Arduini A, Zammit V. Insulin resistance in cardiovascular disease, uremia, and peritoneal dialysis. Trends Endocrinol Metab. 2021;32(9):721–30. 10.1016/j.tem.2021.06.001. 10.1016/j.tem.2021.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerr AG, Andersson DP, Rydén M, Arner P. Insulin resistance in adipocytes: novel insights into the pathophysiology of metabolic syndrome. Clin Nutr. 2024;43(2):468–75. 10.1016/j.clnu.2023.12.012. 10.1016/j.clnu.2023.12.012 [DOI] [PubMed] [Google Scholar]
- 4.Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14(10):591–604. 10.1038/s41574-018-0048-7. 10.1038/s41574-018-0048-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–26. 10.1152/ajpendo.00645.2007. 10.1152/ajpendo.00645.2007 [DOI] [PubMed] [Google Scholar]
- 6.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. 10.1007/BF00280883. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 7.Huang D, Ma R, Zhong X, et al. Positive association between different triglyceride glucose index-related indicators and psoriasis: evidence from NHANES. Front Immunol. 2023;14:1325557. 10.3389/fimmu.2023.1325557. Published 2023 Dec 20. 10.3389/fimmu.2023.1325557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. 10.1089/met.2008.0034. 10.1089/met.2008.0034 [DOI] [PubMed] [Google Scholar]
- 9.Vasques AC, Novaes FS, de Oliveira Mda S, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–100. 10.1016/j.diabres.2011.05.030. 10.1016/j.diabres.2011.05.030 [DOI] [PubMed] [Google Scholar]
- 10.Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68. 10.1186/s12933-022-01511-x. Published 2022 May 6. 10.1186/s12933-022-01511-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aimo A, Chiappino S, Clemente A, et al. The triglyceride/HDL cholesterol ratio and TyG index predict coronary atherosclerosis and outcome in the general population. Eur J Prev Cardiol. 2022;29(5):e203–4. 10.1093/eurjpc/zwab164. 10.1093/eurjpc/zwab164 [DOI] [PubMed] [Google Scholar]
- 12.Nayak SS, Kuriyakose D, Polisetty LD, et al. Diagnostic and prognostic value of triglyceride glucose index: a comprehensive evaluation of meta-analysis. Cardiovasc Diabetol. 2024;23(1):310. 10.1186/s12933-024-02392-y. Published 2024 Aug 23. 10.1186/s12933-024-02392-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martín-Vírgala J, Martín-Carro B, Fernández-Villabrille S, et al. Soluble Klotho, a potential biomarker of chronic kidney Disease-Mineral Bone disorders involved in healthy ageing: lights and shadows. Int J Mol Sci. 2024;25(3):1843. 10.3390/ijms25031843. Published 2024 Feb 3. 10.3390/ijms25031843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paquette JS, Rhéaume C, Cordeau P, et al. The longevity protein klotho: a Promising Tool to monitor lifestyle improvements. Metabolites. 2023;13(11):1157. 10.3390/metabo13111157. Published 2023 Nov 16. 10.3390/metabo13111157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu JP, Zeng RX, He MH, Lin SS, Guo LH, Zhang MZ. Associations between serum Soluble α-Klotho and the prevalence of specific Cardiovascular Disease. Front Cardiovasc Med. 2022;9:899307. 10.3389/fcvm.2022.899307. Published 2022 Jun 20. 10.3389/fcvm.2022.899307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen WY. Soluble Alpha-Klotho alleviates Cardiac Fibrosis without altering cardiomyocytes Renewal. Int J Mol Sci. 2020;21(6):2186. 10.3390/ijms21062186. Published 2020 Mar 22. 10.3390/ijms21062186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mencke R, Olauson H, Hillebrands JL. Effects of Klotho on fibrosis and cancer: a renal focus on mechanisms and therapeutic strategies. Adv Drug Deliv Rev. 2017;121:85–100. 10.1016/j.addr.2017.07.009. 10.1016/j.addr.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Lee J, Chae DW et al. Serum klotho is inversely associated with metabolic syndrome in chronic kidney disease: results from the KNOW-CKD study. BMC Nephrol. 2019;20(1):119. Published 2019 Apr 3. 10.1186/s12882-019-1297-y [DOI] [PMC free article] [PubMed]
- 19.Jiang J, Liu Q, Mao Y et al. Klotho reduces the risk of osteoporosis in postmenopausal women: a cross-sectional study of the National Health and Nutrition Examination Survey (NHANES). BMC Endocr Disord. 2023;23(1):151. Published 2023 Jul 14. 10.1186/s12902-023-01380-9 [DOI] [PMC free article] [PubMed]
- 20.Linghui D, Simin Y, Zilong Z, Yuxiao L, Shi Q, Birong D. The relationship between serum klotho and cognitive performance in a nationally representative sample of US adults. Front Aging Neurosci. 2023;15:1053390. 10.3389/fnagi.2023.1053390. Published 2023 Feb 2. 10.3389/fnagi.2023.1053390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian N, Chen S, Han H, Jin J, Li Z. Association between triglyceride glucose index and total bone mineral density: a cross-sectional study from NHANES 2011–2018. Sci Rep. 2024;14(1):4208. Published 2024 Feb 20. 10.1038/s41598-024-54192-9 [DOI] [PMC free article] [PubMed]
- 22.Pedersen L, Pedersen SM, Brasen CL, Rasmussen LM. Soluble serum Klotho levels in healthy subjects. Comparison of two different immunoassays. Clin Biochem. 2013;46(12):1079–83. 10.1016/j.clinbiochem.2013.05.046. 10.1016/j.clinbiochem.2013.05.046 [DOI] [PubMed] [Google Scholar]
- 23.Hu L, Zhang Q, Bai Y, Hu G, Li J. Triglyceride-glucose index correlate with telomere length in healthy adults from the National Health and Nutrition Examination Survey. Front Endocrinol (Lausanne). 2022;13:844073. 10.3389/fendo.2022.844073. Published 2022 Jun 2. 10.3389/fendo.2022.844073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Lu J, Huang S, Chen Y, Fang Q, Cao Y. Sex differences in the association between serum α-Klotho and depression in middle-aged and elderly individuals: a cross-sectional study from NHANES 2007–2016. J Affect Disord. 2023;337:186–94. 10.1016/j.jad.2023.05.073. 10.1016/j.jad.2023.05.073 [DOI] [PubMed] [Google Scholar]
- 25.van Genugten RE, Utzschneider KM, Tong J, et al. Effects of sex and hormone replacement therapy use on the prevalence of isolated impaired fasting glucose and isolated impaired glucose tolerance in subjects with a family history of type 2 diabetes. Diabetes. 2006;55(12):3529–35. 10.2337/db06-0577. 10.2337/db06-0577 [DOI] [PubMed] [Google Scholar]
- 26.Juutilainen A, Kortelainen S, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care. 2004;27(12):2898–904. 10.2337/diacare.27.12.2898. 10.2337/diacare.27.12.2898 [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Zeng L. Non-linear association of triglyceride-glucose index with prevalence of prediabetes and diabetes: a cross-sectional study. Front Endocrinol (Lausanne). 2023;14:1295641. 10.3389/fendo.2023.1295641. Published 2023 Dec 13. 10.3389/fendo.2023.1295641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Tan Z, Huang Y, et al. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1):124. 10.1186/s12933-022-01546-0. Published 2022 Jul 1. 10.1186/s12933-022-01546-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med. 2011;155(6):408]. Ann Intern Med. 2009;150(9):604–612. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed]
- 30.Kuro- OM. The Klotho proteins in health and disease. Nat Rev Nephrol. 2019;15(1):27–44. 10.1038/s41581-018-0078-3. 10.1038/s41581-018-0078-3 [DOI] [PubMed] [Google Scholar]
- 31.Ullah M, Sun Z. Klotho Deficiency accelerates stem cells aging by impairing telomerase activity. J Gerontol Biol Sci Med Sci. 2019;74(9):1396–407. 10.1093/gerona/gly261. 10.1093/gerona/gly261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto M, Clark JD, Pastor JV, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280(45):38029–34. 10.1074/jbc.M509039200. 10.1074/jbc.M509039200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchanan S, Combet E, Stenvinkel P, Shiels PG. Klotho, Aging, and the failing kidney. Front Endocrinol (Lausanne). 2020;11:560. 10.3389/fendo.2020.00560. Published 2020 Aug 27. 10.3389/fendo.2020.00560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Föhr T, Hendrix A, Kankaanpää A, et al. Metabolic syndrome and epigenetic aging: a twin study. Int J Obes (Lond) Published Online January. 2024;25. 10.1038/s41366-024-01466-x. [DOI] [PMC free article] [PubMed]
- 35.Amaro-Gahete FJ, Jurado-Fasoli L, Sanchez-Delgado G, García-Lario JV, Castillo MJ, Ruiz JR. Relationship between plasma S-Klotho and cardiometabolic risk in sedentary adults. Aging. 2020;12(3):2698–710. 10.18632/aging.102771. 10.18632/aging.102771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orces CH. The association between metabolic syndrome and the anti-aging humoral factor klotho in middle-aged and older adults. Diabetes Metab Syndr. 2022;16(6):102522. 10.1016/j.dsx.2022.102522. 10.1016/j.dsx.2022.102522 [DOI] [PubMed] [Google Scholar]
- 37.Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional β-cell mass in diabetes. Nat Rev Endocrinol. 2012;8(6):342–51. 10.1038/nrendo.2011.242. Published 2012 Feb 14. 10.1038/nrendo.2011.242 [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Abudukeremu A, Jiang Y et al. U-shaped association between the triglyceride-glucose index and atrial fibrillation incidence in a general population without known cardiovascular disease. Cardiovasc Diabetol. 2023;22(1):118. Published 2023 May 18. 10.1186/s12933-023-01777-9 [DOI] [PMC free article] [PubMed]
- 39.Hetemäki N, Savolainen-Peltonen H, Tikkanen MJ, Wang F, Paatela H, Hämäläinen E, et al. Estrogen Metabolism in Abdominal Subcutaneous and visceral adipose tissue in Postmenopausal Women. J Clin Endocrinol Metab. 2017;102(12):4588–95. 10.1210/jc.2017-01474 [DOI] [PubMed] [Google Scholar]
- 40.Mattsson C, Olsson T. Estrogens and glucocorticoid hormones in adipose tissue metabolism. Curr Med Chem. 2007;14(27):2918–24. 10.2174/092986707782359972 [DOI] [PubMed] [Google Scholar]
- 41.Tan Z, Li Y, Guan Y, et al. Klotho regulated by Estrogen Plays a key role in sex differences in stress resilience in rats. Int J Mol Sci. 2023;24(2):1206. 10.3390/ijms24021206. Published 2023 Jan 7. 10.3390/ijms24021206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuguang L, Chang Y, Chen N, et al. Serum klotho as a novel biomarker for metabolic syndrome: findings from a large national cohort. Front Endocrinol (Lausanne). 2024;15. 10.3389/fendo.2024.1295927. :1295927. Published 2024 Mar 4. [DOI] [PMC free article] [PubMed]
- 43.Grimble RF. Inflammatory status and insulin resistance. Curr Opin Clin Nutr Metab Care. 2002;5(5):551–9. 10.1097/00075197-200209000-00015. 10.1097/00075197-200209000-00015 [DOI] [PubMed] [Google Scholar]
- 44.Cilekar S, Beysel S, Karatas S, Balci A, Akaslan K, Uncu A. Circulating sTweak is associated with visceral adiposity and severity in patients with obstructive sleep apnea syndrome. Sci Rep. 2021;11(1):22058. 10.1038/s41598-021-01553-3. Published 2021 Nov 11. 10.1038/s41598-021-01553-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreno JA, Izquierdo MC, Sanchez-Niño MD, et al. The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Nephrol. 2011;22(7):1315–25. 10.1681/ASN.2010101073. 10.1681/ASN.2010101073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ziolkowska S, Binienda A, Jabłkowski M, Szemraj J, Czarny P. The interplay between Insulin Resistance, inflammation, oxidative stress, base excision repair and metabolic syndrome in nonalcoholic fatty liver disease. Int J Mol Sci. 2021;22(20):11128. 10.3390/ijms222011128. Published 2021 Oct 15. 10.3390/ijms222011128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Y, Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev. 2015;36(2):174–93. 10.1210/er.2013-1079. 10.1210/er.2013-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset underpinning the conclusions drawn in this article is accessible at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.