Abstract
Background
In populations with chronic disease, skin autofluorescence (SAF), a measure of long-term fluorescent advanced glycation end-products (AGEs) accumulation in body tissues, has been associated with vascular endothelial function, measured using flow-mediated dilation (FMD). The primary aim of this study was to quantify the relationship between endothelial function and tissue accumulation of AGEs in adults from the general population to determine whether SAF could be used as a marker to predict early impairment of the endothelium.
Methods
A cross-sectional study was conducted with 125 participants (median age: 28.5 y, IQR: 24.4–36.0; 54% women). Endothelial function was measured by fasting FMD. Skin AGEs were measured as SAF using an AGE Reader. Participant anthropometry, blood pressure, and blood biomarkers were also measured. Associations were evaluated using multivariable regression analysis and were adjusted for significant covariates.
Results
FMD was inversely correlated with SAF (ρ = -0.50, P < 0.001) and chronological age (ρ = -0.51, P < 0.001). In the multivariable analysis, SAF, chronological age, and male sex were independently associated with reduced FMD (B [95% CI]; -2.60 [-4.40, -0.80]; -0.10 [-0.16, -0.03]; 1.40 [0.14, 2.67], respectively), with the multivariable model adjusted R2 = 0.31, P < 0.001.
Conclusions
Higher skin AGE levels, as measured by SAF, were associated with lower FMD values, in a predominantly young, healthy population. Additionally, older age and male participants exhibited significantly lower FMD values, corresponding with compromised endothelial function. These results suggest that SAF, a simple and inexpensive marker, could be used to predict endothelial impairment before the emergence of any structural artery pathophysiology or classic cardiovascular disease risk markers.
Trial registration
The study was prospectively registered with the Australian New Zealand Clinical Trials Registry (ACTRN12621000821897) and concurrently entered into the WHO International Clinical Trials Registry Platform under the same ID number.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-024-02428-3.
Keywords: Advanced glycation end products, AGEs, Cardiovascular risk, Endothelial function, Endothelium-dependent vasodilation, Flow-mediated dilation, Skin autofluorescence, Vascular endothelium
Background
Endothelial dysfunction is a predictive risk factor for the development of atherosclerosis and cardiovascular disease (CVD), the leading cause of death worldwide [1]. Atherosclerosis, a chronic inflammatory disease, is the slow progressive accumulation of fatty plaque in the artery wall, causing narrowing and impairment of blood flow [2]. The subclinical asymptomatic changes that occur in the artery can be assessed by specific early-stage tests, including endothelial function by flow-mediated dilation (FMD), arterial stiffness via pulse-wave velocity (PWV), and artery structure through intima-media thickness (IMT) of the carotid artery.
Advanced glycation end-products (AGEs) are a diverse group of compounds formed by the irreversible reaction between amino acids and reducing sugars [3]. AGEs are formed endogenously and accumulate in body tissues throughout life [4–6] and are also derived exogenously from the diet [7]. The accumulation of AGEs in tissues is elevated in individuals with metabolic dysfunction [8], type 1 diabetes [9], type 2 diabetes [10], and renal disease [11]. AGEs can adversely affect the vasculature via pro-atherogenic mechanistic pathways, increasing endothelial dysfunction. AGEs can form cross-links between long-lived extracellular matrix (ECM) proteins such as elastin and collagen [12], which leads to increased artery stiffness [13]. AGEs can also bind with the receptor for AGEs (RAGE) to contribute to downstream proinflammatory signaling, including leading to an increase in oxidative stress by reducing nitric oxide (NO) or endothelial nitric oxide synthase (eNOS) expression, as shown through mechanistic studies [14–19]. RAGE is expressed in many cell types, including vascular endothelial cells [20].
Circulating AGEs concentrations are transient and do not necessarily directly reflect the chronic accumulation of AGEs in tissues [21]. In contrast, long-term deposition of AGEs in tissue can be non-invasively estimated by measuring skin autofluorescence (SAF) using a desktop device known as an AGE Reader [4]. AGEs accumulation in skin tissue measured by the AGE Reader positively correlates with PWV and carotid IMT markers in populations predominantly with chronic disease [22]. Furthermore, there is a positive association between SAF and vascular stiffness, measured by PWV, where the relationship is most marked in men and younger individuals, regardless of glycemic status [23]. While PWV and carotid IMT are structural atherosclerotic biomarkers, FMD is a vascular functional biomarker. Thus, FMD can be used to assess nitric oxide-mediated endothelial dysfunction before any structural changes in the artery occur, providing early prognostic information about endothelial function [24]. Inverse correlations have previously been reported between tissue AGEs accumulation and FMD in four at-risk populations: subjects with uremia [25], older subjects with moderate-to-high CVD risk factors but without chronic kidney disease [25], subjects with diabetes mellitus [26], and community-dwelling older women [27]. As FMD is a functional prognostic marker, tissue AGEs accumulation, measured as SAF, could play an important early role in endothelial dysfunction in apparently healthy people before they develop any classic CVD risk factors such as elevated waist circumference, blood pressure, and LDL cholesterol.
The primary aim of this study was to quantify the relationship between endothelial function, via FMD, and tissue accumulation of AGEs, via SAF, in adults from the general population, to determine whether SAF could be used as a marker to predict early impairment of the endothelium. The secondary aim was to identify clinical risk factors for CVD that could influence the relationship between SAF and FMD.
Methods
Study design and participants
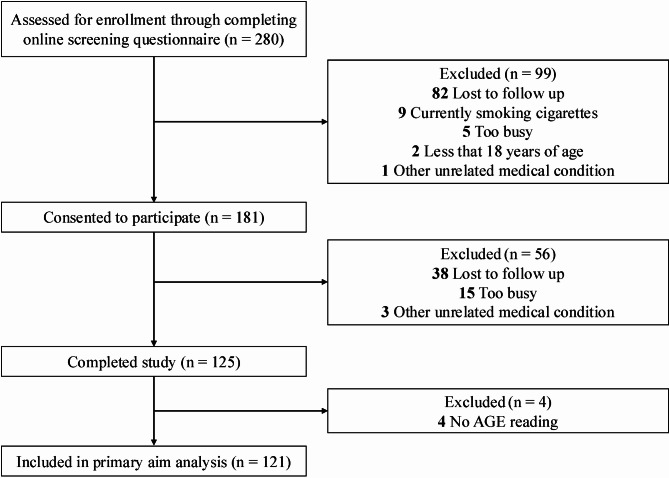
A cross-sectional study on a general population-based cohort was conducted between 2021 and 2022 in Melbourne, Australia. The exclusion criteria were: (1) age < 18 years, (2) current smokers or those who had ceased smoking within six months of testing, (3) women who were pregnant or breastfeeding, or (4) individuals who had an arteriovenous fistula, implantable cardiac defibrillator, cardiac pacemaker, or had made any dramatic dietary changes in the previous three months prior to testing. The study was conducted in accordance with the Declaration of Helsinki for human studies and was approved by the Monash University Human Research Ethics Committee (Project ID: 23731). The study was prospectively registered with the Australian New Zealand Clinical Trials Registry (ACTRN12621000821897). An online screening questionnaire via Qualtrics (Qualtrics, Provo, Utah, USA) was utilized to confirm eligibility before study enrolment. Informed consent was obtained from all participants prior to screening and enrolment. Once enrolled, participants completed a general medical, demographic, physical activity status, and lifestyle online questionnaire, then were invited for a testing visit in the morning following an overnight fast (≥ 12 h). Participants were instructed to avoid exercise for 24 h prior to testing. All medications being taken by participants were noted, along with female participants’ menstrual phase and cycle length. The flow diagram of participant inclusion is displayed in Fig. 1.
Fig. 1.
Flow diagram of participant inclusion. AGE, advanced glycation end-products
Endothelial function
A trained examiner assessed brachial artery endothelial function via FMD, using a 7–12 MHz multifrequency linear array probe attached to a high-resolution ultrasound machine (Venue Go, GE Healthcare, Massachusetts, United States) according to recent expert guidelines [24]. In brief, after supine rest in a semi-darkened, temperature-controlled room, the participant’s left arm was extended from the torso, and a rapid inflation pneumatic cuff (SC12D, Hokanson, Bellevue, WA) was wrapped immediately below the elbow. The brachial artery was scanned in B-mode 2–6 cm longitudinally above the antecubital fossa in the distal third of the upper arm. After an optimal image of the artery diameter was obtained, the probe was held in place until the conclusion of the protocol. Baseline artery diameter was continuously recorded for 1 min, followed by 15 s of recorded pulsed-wave Doppler flow. The pneumatic cuff was then rapidly inflated on the forearm to 220 mmHg for 5 min to produce lower forearm ischemia and induce shear stress. Thirty seconds before cuff deflation, the continuous recording was resumed. Live acquisition of pulsed-wave Doppler flow mode occurred for the first 15 s post-cuff deflation [28] before switching back to B-mode for artery diameter assessment. Recording continued for 3 min post-cuff deflation. The recorded files were analyzed by a trained blinded observer using an offline continuous automated edge-detection software Brachial Analyzer (Medical Imaging Applications, LLC, Iowa, USA) [29]. FMD was calculated as the percentage change in peak reactive hyperemia diameter from baseline artery diameter. The mean shear rate area under the curve was calculated for the 15 s of Doppler flow, and the calculation for shear rate was defined as shear rate = 8 x (mean blood flow velocity [cm/s] / diameter [cm]) [24]. As initial baseline artery diameter can influence the assessment of the percentage change in FMD, all analyses were also conducted on allometrically scaled FMD data according to the method described by Atkinson and Batterham [30].
Skin autofluorescence
Skin autofluorescence (SAF), a marker of long-term AGE accumulation in the skin, was measured using the AGE Reader (Diagnoptics BV, Groningen, The Netherlands). Certain AGEs display fluorescent properties, allowing the AGE Reader to estimate the level of AGE accumulation through excitation via UV light, which penetrates the dermis (methods have been described elsewhere in detail) [4, 31]. Measurements were taken on the ventral surface of the lower forearm, with researchers choosing the lightest section of skin, an approximate 1 cm2 area, free from dark marks or skin abnormalities. Per the manufacturer’s instructions, SAF was measured in triplicate, averaged, and expressed in arbitrary units (AU). The AGE Reader has an intra-individual overall Altman error percentage of 5.03% over repeated SAF measurements on a single day and a 5.87% error percentage for seasonal changes [4].
Anthropometric measures and physical activity
Height was measured twice and averaged using a stadiometer to the nearest 1 mm. Waist circumference was measured twice and averaged to the nearest 1 mm using a constant tension flexible tape measure at the mid-point between the inferior margin of the last rib and the iliac crest [32]. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Weight and body composition were measured using a bioelectrical impedance analysis scale (SECA, 515, Ecomed). Variables, including total body fat-free mass (kg and %), total body fat mass (kg and %), total body water (L and %), total skeletal muscle mass (kg and %), and visceral adipose tissue (L and %) were recorded. The International Physical Activity Questionnaire (IPAQ)- short form was self-administered to assess participants’ habitual physical activity levels [33].
Blood pressure
After 10 min of supine rest in a quiet room, brachial systolic, diastolic, and mean arterial blood pressure were measured with an automated oscillometric blood pressure monitor (Welch Allyn ProBP 3400 Digital). The average of three consecutive congruent measurements defined blood pressure and heart rate.
Biochemical parameters
Fasting plasma samples assessed glucose, insulin, and soluble receptor for advanced glycation end-products (sRAGE). Fasting serum samples assessed total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, and high-sensitivity C-reactive protein (hsCRP). Whole blood was collected for the assessment of glycated hemoglobin (HbA1c). All fasting blood samples were processed and stored at -80 °C.
Plasma glucose, serum lipids, and hsCRP analysis were performed on an Indiko Clinical Chemistry Analyzer (Thermo Fisher Scientific Oy, Vantaa, Finland) using commercially available kits from the manufacturer. Plasma insulin was measured by a human insulin ELISA kit (EZHI-14 K, Merck Millipore, Massachusetts, USA), following the manufacturer’s instructions. Plasma sRAGE was determined through the Quantikine ELISA Human RAGE Immunoassay kit (R&D Systems, Minnesota, USA), following the manufacturer’s protocols. Both sRAGE and insulin were read on an absorbance plate reader (PHERAstar FS, BMG Labtech, Ortenberg, Germany). HbA1c was analyzed using the Capillary 3 HbA1c kit (Sebia, Lisses, France) by Monash Pathology (Monash Medical Centre, Clayton). Homeostatic model assessment for insulin resistance (HOMA-IR) was calculated with the formula [fasting insulin (µU/mL) × fasting glucose (mmol/L)] ÷ 22.5 [34].
Sample size calculation
Based on alpha = 0.05, a power of 80%, and an estimated correlation coefficient of 0.25 or greater between FMD and SAF, a minimum of 120 participants were required.
Statistical analysis
Unless otherwise stated, all data are presented as median (interquartile range) for continuous variables and numbers (percentage) for categorical variables. Participants with missing data were excluded from the analysis. The data were tested for normality using the Kolmogorov-Smirnov test and visual inspection of box plots and histograms using Stata, version 18.0 (StataCorp, College Station, Texas, USA). Correlation analysis was used to quantify the relationship between FMD, SAF, and other clinical variables. Statistically significant variables were then included in a multivariable linear regression model with FMD as the dependent variable. Multivariable regression model 1 was constructed to include sex and age. From the correlation analysis, variables correlated with FMD (p ≤ 0.05) were entered into model 2. Furthermore, variables with a known association with CVD were also entered into model 2 to identify independent predictors of FMD. All variables were tested for collinearity via the vif command in Stata. Two-tailed p values ≤ 0.05 were considered statistically significant.
Results
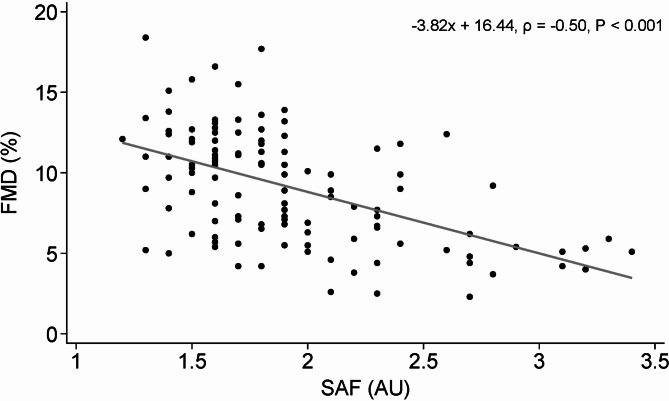
One hundred and twenty-five participants completed a testing visit; baseline characteristics are presented in Table 1, of which data for SAF was missing in 4 individuals. Thus, 121 participants were included in the primary outcome analysis of the relationship between FMD and SAF. While the cohort characteristics were heterogeneous, each variable’s median and interquartile range was generally within the healthy range; thus, the cohort could be considered predominantly young and healthy adults. In unadjusted linear regression and Spearman correlation analysis, SAF was inversely associated with FMD (ρ = -0.50, P < 0.001, N = 121) (Fig. 2). When participants with medical conditions and those who were aged over 50 y were removed from the sample, SAF remained inversely associated with FMD (ρ = -0.31, P < 0.01, N = 92).
Table 1.
Participant characteristics of study subjects
| Median (IQR) or number (%) | Range | |
|---|---|---|
| Age, years | 28.5 (24.4–36.0) | 18.7–72.8 |
| Sex, female | 68 (54.4%) | |
| Skin autofluorescence, AU (N = 121) | 1.8 (1.6–2.1) | 1.2–3.4 |
| FMD, % | 9.7 (6.0-11.9) | 2.3–18.4 |
| Allometrically scaled FMD, % | 8.0 (6.9–9.2) | 5.6–12.4 |
| Baseline diameter, mm | 3.6 (3.1–4.1) | 2.4-5.0 |
| Peak diameter, mm | 4.0 (3.4–4.4) | 2.7–5.4 |
| Absolute FMD change, mm | 0.3 (0.2–0.4) | 0.1–0.7 |
| Shear rate, sec−1 | 1415.1 (1039.4-1743.7) | 712.7-2545.5 |
| BMI, kg/m2 | 22.9 (20.3–25.7) | 16.1–38.2 |
| Waist circumference, cm | 80.0 (72.5–90.0) | 57.8–127.0 |
| Fat-free mass, % | 72.7 (68.9–80.0) | 51.8–94.1 |
| Fat mass, % | 27.3 (20.0-31.1) | 5.9–48.2 |
| Total body water, % | 53.3 (50.0-58.3) | 38.2–67.3 |
| Skeletal muscle mass, % (N = 102) | 34.9 (31.2–38.3) | 20.6–45.4 |
| Visceral adipose tissue, % | 2.2 (1.5–2.9) | 0.1–7.1 |
| Physical activity, MET minutes/week | 2399 (1138–4320) | 0-10479 |
| Systolic blood pressure, mmHg | 108.3 (101.7–116.0) | 90.3-155.3 |
| Diastolic blood pressure, mmHg | 68.7 (64.0–73.0) | 56.3–90.7 |
| Mean arterial pressure, mmHg | 81.6 (76.4–87.7) | 67.7-108.7 |
| Heart rate, beats/min | 60.0 (52.3–67.3) | 37.0-90.7 |
| Glucose, mmol/L (N = 117) | 5.3 (5.0-5.5) | 4.4–6.8 |
| Insulin, pmol/ml (N = 117) | 32.5 (21.3–44.0) | 5.1-170.4 |
| sRAGE, pg/mL (N = 117) | 1832 (1296–2384) | 363–4731 |
| Total Cholesterol, mmol/L (N = 117) | 5.0 (4.4–5.9) | 3.0-22.2 |
| LDL Cholesterol, mmol/L (N = 117) | 3.2 (2.5-4.0) | 1.4–20.2 |
| HDL Cholesterol, mmol/L (N = 117) | 1.7 (1.5-2.0) | 0.9–3.2 |
| Triglycerides, mmol/L (N = 117) | 0.8 (0.7–1.2) | 0.3–3.5 |
| hsCRP, mg/l (N = 117) | 0.6 (0.3–1.4) | 0.0-10.7 |
| HbA1c, % (N = 117) | 5.1 (5.0-5.3) | 4.4–6.6 |
| HOMA-IR, (N = 117) | 1.3 (0.9–1.8) | 0.2–7.4 |
N = 125 unless otherwise stated. Values are shown as median (IQR) or number (percentage). Abbreviations: AU, arbitrary units; BMI, body mass index; FMD, flow-mediated dilation; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment for insulin resistance; hsCRP, high-sensitivity C-reactive protein; IQR, interquartile range; LDL, low-density lipoprotein; MET, metabolic equivalent task; sRAGE, soluble receptor for advanced glycation end-products
Fig. 2.
Unadjusted linear regression and Spearman correlation analysis to evaluate the association between SAF and FMD. N = 121. Scatter plot with linear trend line (solid line). Abbreviations: SAF, skin autofluorescence; AU, arbitrary units; FMD, flow-mediated dilation; N, sample size
Relationships between FMD, SAF, and other predictor variables
Correlation analysis shows the relationships between FMD and other study variables (Table 2). Variables associated with FMD included chronological age (ρ = -0.51, P < 0.001), SAF (ρ = -0.50, P < 0.001), visceral adipose tissue (ρ = -0.24, P < 0.01), total cholesterol (ρ = -0.21, P < 0.05), LDL cholesterol (ρ = -0.23, P < 0.01), and HbA1c (ρ = -0.28, P < 0.01). There were no statistically significant relationships between FMD and the other covariates.
Table 2.
Spearman correlations between FMD and other study variables
| FMD | AlloFMD | Age | SAF^ | BMI | WC | FFM | FM | BW | SMM# | VAT | PA | SBP | DBP | MAP | HR | Glu§ | Insulin§ | sRAGE§ | TC§ | LDL C§ | HDL C§ | TG§ | hsCRP§ | HbA1c§ | HOMA-IR§ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FMD | ||||||||||||||||||||||||||
| AlloFMD | 0.93‡ | |||||||||||||||||||||||||
| Age | -0.51‡ | -0.56‡ | ||||||||||||||||||||||||
| SAF^ | -0.50‡ | -0.45‡ | 0.52‡ | |||||||||||||||||||||||
| BMI | -0.02 | -0.14 | 0.32‡ | 0.16 | ||||||||||||||||||||||
| WC | -0.10 | -0.23† | 0.45‡ | 0.18* | 0.85‡ | |||||||||||||||||||||
| FFM | -0.02 | -0.11 | -0.21* | -0.22* | -0.56‡ | -0.46‡ | ||||||||||||||||||||
| FM | 0.02 | 0.11 | 0.21* | 0.22* | 0.56‡ | 0.46‡ | -1.00‡ | |||||||||||||||||||
| BW | -0.02 | -0.12 | -0.17 | -0.20* | -0.55‡ | -0.45‡ | 0.99‡ | -0.99‡ | ||||||||||||||||||
| SMM# | 0.05 | -0.14 | -0.11 | -0.28† | -0.25† | -0.20* | 0.92‡ | -0.92‡ | 0.92‡ | |||||||||||||||||
| VAT | -0.24† | -0.27† | 0.38‡ | 0.42‡ | 0.48‡ | 0.60‡ | -0.48‡ | 0.48‡ | -0.50‡ | -0.44‡ | ||||||||||||||||
| PA | -0.10 | -0.10 | -0.01 | 0.09 | -0.15 | -0.13 | 0.32‡ | -0.32‡ | 0.34‡ | 0.26† | -0.24† | |||||||||||||||
| SBP | -0.16 | -0.27† | 0.53‡ | 0.27† | 0.41‡ | 0.47‡ | -0.07 | 0.07 | -0.06 | 0.14 | 0.34‡ | 0.10 | ||||||||||||||
| DBP | -0.15 | -0.18* | 0.44‡ | 0.34‡ | 0.41‡ | 0.40‡ | -0.25† | 0.25† | -0.25† | -0.08 | 0.39‡ | 0.03 | 0.88‡ | |||||||||||||
| MAP | -0.15 | -0.22* | 0.49‡ | 0.32‡ | 0.41‡ | 0.43‡ | -0.17 | 0.17 | -0.17 | 0.02 | 0.38‡ | 0.06 | 0.96‡ | 0.98‡ | ||||||||||||
| HR | 0.13 | 0.23† | -0.29† | -0.02 | -0.11 | -0.12 | -0.20* | 0.20* | -0.22* | -0.27† | 0.05 | -0.13 | -0.11 | 0.14 | 0.03 | |||||||||||
| Glu§ | -0.13 | -0.20* | 0.25† | 0.27† | 0.36‡ | 0.38‡ | -0.08 | 0.08 | -0.07 | -0.01 | 0.27† | 0.10 | 0.16 | 0.09 | 0.12 | -0.10 | ||||||||||
| Insulin§ | 0.16 | 0.18 | -0.05 | 0.08 | 0.44‡ | 0.44‡ | -0.50‡ | 0.50‡ | -0.51‡ | -0.36‡ | 0.38‡ | -0.14 | 0.16 | 0.25† | 0.21* | 0.25† | 0.23† | |||||||||
| sRAGE§ | -0.01 | -0.07 | -0.08 | -0.18 | -0.17 | -0.21* | 0.31‡ | -0.31‡ | 0.28† | 0.25† | -0.26† | 0.01 | 0.01 | -0.09 | -0.04 | 0.04 | 0.02 | -0.06 | ||||||||
| TC§ | -0.21* | -0.23* | 0.41‡ | 0.41‡ | 0.32‡ | 0.28† | -0.27† | 0.27† | -0.26† | -0.23* | 0.33‡ | 0.06 | 0.30† | 0.30‡ | 0.30‡ | -0.06 | 0.21* | 0.07 | -0.13 | |||||||
| LDL C§ | -0.23† | -0.29† | 0.41‡ | 0.41‡ | 0.38‡ | 0.36‡ | -0.28† | 0.28† | -0.28† | -0.22* | 0.40‡ | 0.04 | 0.35‡ | 0.33‡ | 0.34‡ | -0.09 | 0.22* | 0.10 | -0.15 | 0.95‡ | ||||||
| HDL C§ | -0.04 | 0.10 | -0.004 | 0.01 | -0.35‡ | -0.39‡ | 0.04 | -0.04 | 0.06 | -0.13 | -0.37‡ | 0.10 | -0.22* | -0.17 | -0.20* | -0.001 | -0.12 | -0.28† | 0.08 | 0.22* | -0.01 | |||||
| TG§ | -0.05 | -0.11 | 0.27† | 0.26† | 0.51‡ | 0.48‡ | -0.33‡ | 0.33‡ | -0.36‡ | -0.18 | 0.60‡ | -0.12 | 0.42‡ | 0.43‡ | 0.44‡ | 0.08 | 0.27† | 0.36‡ | -0.17 | 0.36‡ | 0.41‡ | -0.46‡ | ||||
| hsCRP§ | 0.02 | -0.03 | 0.18* | 0.17 | 0.33‡ | 0.31‡ | -0.30† | 0.30† | -0.31‡ | -0.17 | 0.28† | -0.05 | 0.18* | 0.22* | 0.21* | -0.05 | 0.01 | 0.22* | 0.0003 | 0.19* | 0.18* | -0.09 | 0.33‡ | |||
| HbA1c§ | -0.28† | -0.33‡ | 0.33‡ | 0.40‡ | 0.24† | 0.30‡ | -0.20* | 0.20* | -0.19* | -0.20 | 0.39‡ | 0.10 | 0.12 | 0.16 | 0.14 | -0.08 | 0.38‡ | 0.16 | -0.10 | 0.17 | 0.21* | -0.07 | 0.17 | 0.29† | ||
| HOMA-IR§ | 0.15 | 0.16 | -0.02 | 0.11 | 0.47‡ | 0.47‡ | -0.50‡ | 0.50‡ | -0.50‡ | -0.35‡ | 0.41‡ | -0.14 | 0.17 | 0.25† | 0.21* | 0.25† | 0.32‡ | 0.99‡ | -0.07 | 0.08 | 0.12 | -0.29† | 0.37‡ | 0.21* | 0.19* |
N = 125 unless otherwise stated. * P ≤ 0.05, † P ≤ 0.01, and ‡ P ≤ 0.001 for Spearman’s ρ correlation coefficient. ^ N = 121, # N = 102, § N = 117. Abbreviations: AlloFMD, allometrically scaled FMD; BMI, body mass index; BW, body water; DBP, diastolic blood pressure; FFM, fat-free mass; FM, fat mass; FMD, flow-mediated dilation; Glu, glucose; HbA1c, glycated hemoglobin; HDL C, high-density lipoprotein; HOMA-IR, homeostatic model assessment for insulin resistance; HR, heart rate; hsCRP, high-sensitivity C-reactive protein; LDL C, low-density lipoprotein; MAP, mean arterial pressure; N, sample size; PA, physical activity; SAF, skin autofluorescence; SBP, systolic blood pressure; SMM, skeletal muscle mass; sRAGE, soluble receptor for advanced glycation end-products; TC, total cholesterol; TG, Triglycerides; VAT, visceral adipose tissue; WC, waist circumference.
In multivariable model 1, FMD was negatively associated with chronological age (B = -0.12; 95% CI, -0.16, -0.08; P < 0.001) (Table 3). Only significant variables from the correlation analysis, with the removal of collinear variables (total cholesterol) plus the addition of any variables with known biological associations with CVD or FMD (sex, waist circumference, and systolic BP), were included in the multivariable model 2 (Table 3). After the exclusion of 12 participants who had incomplete data, FMD was associated with age (B = -0.10; 95% CI, -0.16, -0.03; P = 0.005), sex (B = 1.40; 95% CI, 0.14, 2.67; P = 0.030), SAF (B = -2.60; 95% CI, -4.40, -0.80; P = 0.005), and systolic blood pressure (B = 0.07; 95% CI, 0.01, 0.14; P = 0.033) (N = 113). Waist circumference, visceral adipose tissue, LDL cholesterol, and HbA1c were not independently associated with FMD after adjustment in the regression model. The combined covariates in model 2 explained 31% of the variance in FMD.
Table 3.
Multivariable regression analysis exploring the associations between FMD and covariates
| Covariate | Unstandardized coefficient |
Standardized coefficient |
t-value | P-value | 95% CI Lower, Upper |
Obs (N) | R2 | Adjusted R2 | |
|---|---|---|---|---|---|---|---|---|---|
| B | Std. error | Beta | |||||||
| Model 1 | |||||||||
| Intercept | 12.73 | 0.82 | 0.00 | 15.43 | < 0.001* | 11.10, 14.36 | 125 | 0.244 | 0.23 |
| Age | -0.12 | 0.02 | -0.47 | -5.90 | < 0.001* | -0.16, -0.08 | |||
| Sex | 0.79 | 0.57 | 0.11 | 1.39 | 0.167 | -0.34, 1.91 | |||
| Model 2 | |||||||||
| Intercept | 2.09 | 7.02 | 0.000 | 0.30 | 0.766 | -11.82, 16.00 | 113 | 0.359 | 0.31 |
| Age | -0.10 | 0.03 | -0.37 | -2.88 | 0.005* | -0.16, -0.03 | |||
| Sex | 1.40 | 0.64 | 0.20 | 2.21 | 0.030* | 0.14, 2.67 | |||
| SAF | -2.60 | 0.91 | -0.35 | -2.87 | 0.005* | -4.40, -0.80 | |||
| WC | 0.05 | 0.03 | 0.18 | 1.49 | 0.138 | -0.02, 0.12 | |||
| VAT | -0.17 | 0.32 | -0.06 | -0.52 | 0.607 | -0.81, 0.47 | |||
| Systolic BP | 0.07 | 0.03 | 0.25 | 2.16 | 0.033* | 0.01, 0.14 | |||
| LDL Cholesterol | -0.26 | 0.17 | -0.13 | -1.50 | 0.137 | -0.59, 0.08 | |||
| HbA1c (%) | 0.65 | 1.12 | 0.06 | 0.58 | 0.564 | -1.57, 2.87 | |||
Linear multivariable regression analysis was performed to determine the predictors of FMD. Abbreviations: BP, blood pressure; FMD, flow-mediated dilation; HbA1c, hemoglobin A1c; LDL, low-density lipoprotein; n, sample size; Obs, observations; SAF, skin autofluorescence; std error, standard error; VAT, visceral adipose tissue; WC, waist circumference.
Relationships between allometrically scaled FMD, SAF, and other predictor variables
Analyses were also conducted on allometrically scaled FMD data as baseline artery diameter can potentially influence the calculation of percent change FMD. Correlation analysis showed that allometric FMD was correlated with age (ρ = -0.56, P < 0.001), SAF (ρ = -0.45, P < 0.001), waist circumference (ρ = -0.23, P < 0.01), visceral adipose tissue (ρ = -0.27, P < 0.01), systolic blood pressure (ρ = -0.27, P < 0.01), diastolic blood pressure (ρ = -0.18, P < 0.05), mean arterial pressure (ρ = -0.22, P < 0.05), heart rate (ρ = 0.23, P < 0.01), glucose (ρ = -0.20, P < 0.05), total cholesterol (ρ = -0.23, P < 0.05), LDL cholesterol (ρ = -0.29, P < 0.01), and HbA1c (ρ = -0.33, P < 0.00) (Table 2). No statistically significant relationship existed between allometrically scaled FMD and the other covariates. Waist circumference, systolic blood pressure, diastolic blood pressure, mean arterial pressure, heart rate, and glucose were only correlated in the allometric scaled dataset and not in the raw FMD dataset.
Chronological age (B = -0.05; 95% CI, -0.06, -0.03; P < 0.001) and sex (B = -0.04; 95% CI, -0.07, -0.02; P < 0.01) were negatively associated with allometrically scaled FMD in multivariable model 1 (Additional file 1: Table S1). In model 2 for 113 participants, allometrically scaled FMD was associated with chronological age (B = -0.04; 95% CI, -0.07, -0.02; P = 0.001), sex (B = 1.21; 95% CI, 0.70, 1.72; P < 0.001), SAF (B = -0.84; 95% CI, -1.54, -0.14; P < 0.05), and systolic blood pressure (B = 0.03; 95% CI: 0.01, 0.06; P < 0.05) (Additional file 1: Table S1). After adjustment in the regression model, waist circumference, visceral adipose tissue, systolic BP, diastolic BP, heart rate, glucose, total cholesterol, LDL cholesterol, and HbA1c were not independently associated with allometrically scaled FMD. The combined covariates in model 2 explained 40% of the variance in allometrically scaled FMD.
Discussion
In this study, SAF, age, and sex were associated with brachial artery FMD in a predominantly young and apparently healthy population. Higher SAF levels were associated with lower FMD values, indicating impaired endothelial function. Furthermore, older individuals and males in the sample were more likely to have lower FMD values.
Correlation analysis in the present study showed an inverse correlation of -0.50 between FMD and SAF, which differs from previously reported correlations in some populations with existing metabolic dysfunction. For example, lower inverse correlations were reported in older adults with moderate-to-high CVD risk factors but without chronic kidney disease (r = -0.46, P < 0.01) [25], adults with diabetes mellitus (r = -0.26, P = 0.002) [26], and community-dwelling older women (r = -0.37, P < 0.05) [27]. However, participants with uremia have demonstrated a higher inverse correlation compared with the present study (r = -0.80, P < 0.01) [25]. Increased tissue AGEs are observed in people with impaired renal function [35]. Kidney dysfunction impairs the urinary excretion of AGEs [36], but the presence of other underlying comorbidities could also contribute to increased AGE generation in people with chronic kidney disease, affecting FMD.
In the multivariable model, our results showed stronger relationships between SAF and FMD in a predominantly young and healthy adult sample than in populations with chronic disease. We found that for every 1 arbitrary unit (AU) increase in SAF, there was a corresponding 2.60% reduction in FMD. In comparison, Wang et al. [25] reported that in patients with uremia on hemodialysis, every 1 AU increase in SAF was associated with a 1.60% reduction in FMD (mean age ± SD: 59.60 ± 11.63 y, N = 119). AGEs are not filtered through a hemodialysis membrane, and patients on dialysis longer would possibly have higher AGE accumulation [25], affecting the association with FMD. In people with CVD risk factors but no kidney disease, every 1 AU increase in SAF has been independently associated with a 1.85% reduction in FMD (mean age 58.72 ± 10.50 y, N = 57) [25]. However, over half the CVD risk factor group used either angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), beta-blockers, or statins, which can impact FMD. Statins can upregulate eNOS [37] while ACEIs/ARBs reduce angiotensin II levels and increase bradykinin levels [38], both pathways leading to increased NO production and hence vasodilation and improved FMD. Furthermore, SAF is an independent predictor of FMD in participants with diabetes mellitus, though chronological age and BMI were stronger predictors [26]. In diabetes mellitus, other factors can affect NO and oxidative stress, potentially contributing to the FMD and SAF values. For example, increased insulin resistance, postprandial acute hyperglycemia, or chronic hyperglycemia can stimulate O2− (superoxide) production, increasing oxidative stress [39, 40], meaning increased endothelial dysfunction misaligned with SAF values.
While most previous studies have recruited participants considered at high risk for CVD, FMD as a prognostic technique is potentially only related to cardiovascular risk factors in low-risk populations [41]. Indeed, participants with older age or CVD risk factors show larger intra-individual variations in FMD, which impair FMD reproducibility [42]. FMD is approximately 70% nitric oxide-mediated [43], and as the artery stiffens with age or more CVD risk factors are present, such as hypertension, hypercholesterolemia, physical inactivity, and diabetes, there may be alternative limitations on the capacity of the artery to dilate. Thus, measuring the FMD in high-risk groups is not entirely an indication of NO-dependent dilation.
Poor nitric oxide production or bioavailability is the precursor to impaired endothelial function and the first step toward atherosclerosis. Mechanistic studies have shown that a greater accumulation of AGEs may directly influence endothelial function through NO. The interaction of AGEs with their receptor RAGE, particularly on endothelial and smooth muscle cells [20], contributes to increased activation of the vascular nicotinamide adenine dinucleotide phosphate (NADPH) oxidase pathway [44, 45], plus increased production of cytokines, chemokines, adhesion molecules and inflammatory factors including transcription factor nuclear factor kappa B (NF-κB) [46, 47]. These factors lead to the generation of reactive oxygen species, including increased superoxide production [40], upregulation of endothelin-1 [48], impairment of eNOS [16, 49], and ultimately quenching or inhibition of NO [14]. Moreover, vasodilation of the artery can also be impaired via AGEs-induced protein cross-linking, altering of extracellular matrix properties [20], and thickening and remodeling of the arterial wall, which can lead to vascular stiffness [50]. Thus, increased skin AGEs accumulation in the dermis and epidermis of the skin is associated with decreased FMD, indicating decreased NO bioavailability, potentially promoting pro-atherogenic effects. SAF measured via the AGE reader is a potential biomarker for early detection of suboptimal endothelial function before any traditional clinical CVD risk factors are present.
In the present study, for every 1-year increase in chronological age, FMD decreased by 0.1%. Vascular aging mechanisms can impact the arterial wall to promote endothelial dysfunction, affecting eNOS through decreased expression, known as eNOS uncoupling [51, 52]. L-arginine and tetrahydrobiopterin (BH4) are cofactors needed to produce NO, which are reduced with age [53, 54]. Baseline artery diameter can overtly influence the FMD percent change calculation. For example, a cross-sectional study with 5695 male subjects aged 30 to 74 showed that larger baseline artery diameter was positively correlated with age [55]. Similarly, in 376 women with chest pain who ranged in age from 20 to 82 years, larger resting brachial artery diameter, measured by B-mode ultrasonography, was positively associated with age [56]. This relationship was consistent in our sample, where baseline artery diameter was inversely correlated with FMD (ρ = − 0.28, P = 0.001) and positively correlated with age (ρ = 0.40, P < 0.0001) (data not shown). Thus, to account for these variations in baseline diameter, FMD data were allometrically scaled [57], and results showed similar age and sex values compared to raw FMD percentage change, indicating that differences in age-related changes in baseline vessel diameter do not entirely account for the associations in this sample.
Biological sex was another factor that was predictive of endothelial function; in this study, FMD values were, on average, 1.5% higher in females than males. A study on 457 healthy adults aged 20–91 demonstrated reduced endothelial function with aging and showed that cardiovascular risk occurs 20 years earlier in men [58]. The sex hormone estrogen can exert cardio-protective effects. Estrogen, but not testosterone binding to its receptor, upregulates the release of endothelial NO via increased activity of eNOS in human umbilical vein endothelial cells (HUVEC) [59]. Furthermore, endothelial function declines across the stages of menopause with prolonged estrogen deficiency [60]. Thus, female participants could have had greater cardio-protection, leading to higher FMD in females than males.
Systolic blood pressure was independently associated with FMD. However, in this study, the gradient was nearly zero (B = 0.07), leading to statistical significance but not biological significance. In a group of 5314 Japanese adults (mean age ± SD: 46 ± 13 y, 77.7% male), systolic and diastolic blood pressure were independent predictors of FMD [61], with increases in blood pressure impairing endothelial function. Other classic cardiovascular risk factors, including waist circumference, visceral adipose tissue, and LDL cholesterol, were significant in the correlation analysis but not in the multivariable regression analysis. Our cohort could have potentially been too young to have been exhibiting any of these CVD risk factors in levels high enough to associate with FMD.
The study’s strengths include measuring well-defined outcomes concerning CVD risk factors, including anthropometry, blood pressure, vascular function, diabetes risk, inflammation, and metabolism, using well-accepted methods in a large, predominantly young and healthy population. The present study has some limitations. The 30–45-year-old age bracket was difficult to recruit, which could have affected our analysis; a wider population span is needed to validate our associations even after statistical adjustment. FMD can be affected by the female menstrual cycle [62], and while it was not controlled for in this study, the first day of female participants’ last menstrual cycles were recorded and showed no associations with FMD. The AGE Reader uses ultraviolet light to measure only the AGEs with fluorescent properties in the skin, so it cannot estimate all AGE accumulation, as not all AGEs fluoresce. However, there is a significant correlation between the assessment of AGE accumulation from skin biopsies and the SAF levels calculated by the AGE Reader [4, 5, 10]. Lastly, approximately 9 subjects had dark pigmented skin (Fitzpatrick Type V and VI), which cannot be measured as reliably by the AGE Reader due to higher melanin being present, which absorbs more emission light and more excitation light [63] which can overall affect the SAF reading.
Conclusions
For the first time, across a predominantly young and healthy adult cohort, increased AGE accumulation quantified by SAF was associated with lower FMD values independent of traditional CVD risk factors. Older age and male sex were also associated with lower FMD values, corresponding with compromised endothelial function. Measurement of SAF is a non-invasive, easy-to-replicate method that could be used as a complementary marker for endothelial function in healthy individuals prior to the appearance of traditional CVD risk markers.
Electronic supplementary material
Acknowledgements
The authors wish to thank all study participants. JJF thanks Mr Anthony Wald for his support and training in ultrasound measurements.
Abbreviations
- ACEIs
Angiotensin-converting enzyme inhibitors
- AGEs
Advanced glycation end-products
- ARBs
Angiotensin II receptor blockers
- AU
Arbitrary unit
- BH4
Tetrahydrobiopterin
- BMI
Body mass index
- BP
Blood pressure
- CVD
Cardiovascular disease
- ECM
Extracellular matrix
- ELISA
Enzyme-linked immunosorbent assay
- eNOS
Endothelial nitric oxide synthase
- FMD
Flow-mediated dilation
- HbA1c
Glycated hemoglobin
- HDL
High-density lipoprotein cholesterol
- HOMA-IR
Homeostatic model assessment for insulin resistance
- hsCRP
High-sensitivity C-reactive protein
- HUVEC
Human umbilical vein endothelial cells
- IMT
Intima-media thickness
- IPAQ
The International Physical Activity Questionnaire
- IQR
Interquartile range
- LDL
Low-density lipoprotein cholesterol
- MET
Metabolic equivalent task
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NF-κB
Nuclear factor kappa B
- NO
Nitric oxide
- O2−
Superoxide
- PWV
Pulse-wave velocity
- RAGE
Receptor for AGEs
- SAF
Skin autofluorescence
- sRAGE
Soluble receptor for advanced glycation end-products
- VAT
Visceral adipose tissue
- WC
Waist circumference
Author contributions
JJF, NJK, GW, and ALD: developed the concept and designed the research; JJF: wrote the ethics application, which was edited and revised by NJK, GW, ALD, and MM; GW: resources, supervision; NJK and ALD: supervision; JJF: carried out the FMD, SAF, blood pressure, anthropometric and biomarker measurements; JJF and NJK: carried out statistical analysis and interpretation of data; JJF, NJK, GW, and ALD: discussion of data, findings, implications, novelty and relevance to literature; JJF: drafted the manuscript and prepared all tables and figures; JJF, NJK, GW, ALD, and MM: read, revised, and edited the manuscript; JJF, NJK, GW, MM, and ALD: approved the final manuscript.
Funding
This work was supported by a Monash University PhD Scholarship to JJF, a National Health & Medical Research Council (NHMRC) Early Career Research Fellowship (APP1164771) awarded to NJK, and Monash University funding to GW.
Availability of data and materials
The data sets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the declaration of Helsinki for human studies and was approved by the Monash University Human Research Ethics Committee (Project ID: 23731). Informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, et al. European society of cardiology: cardiovascular disease statistics 2019. Eur Heart J. 2020;41:12–85. 10.1093/eurheartj/ehz859 [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc Res. 2021;117:2525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fishman SL, Sonmez H, Basman C, Singh V, Poretsky L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: a review. Mol Med. 2018;24:59. 10.1186/s10020-018-0060-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meerwaldt R, Graaff R, Oomen PHN, Links TP, Jager JJ, Alderson NL, et al. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47:1324–30. 10.1007/s00125-004-1451-2 [DOI] [PubMed] [Google Scholar]
- 5.Meerwaldt R, Links T, Graaff R, Thorpe SR, Baynes JW, Hartog J, et al. Simple noninvasive measurement of skin autofluorescence. Ann N Y Acad Sci. 2005;1043:290–8. 10.1196/annals.1333.036 [DOI] [PubMed] [Google Scholar]
- 6.Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol Biol Sci Med Sci. 2010;65A:963–75. 10.1093/gerona/glq074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad C, Davis KE, Imrhan V, Juma S, Vijayagopal P. Advanced glycation end products and risks for chronic diseases: intervening through lifestyle modification. Am J Lifestyle Med. 2017;13:384–404. 10.1177/1559827617708991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Waateringe RP, Slagter SN, van Beek AP, van der Klauw MM, van Vliet-Ostaptchouk JV, Graaff R, et al. Skin autofluorescence, a non-invasive biomarker for advanced glycation end products, is associated with the metabolic syndrome and its individual components. Diabetol Metab Syndr. 2017;9:42. 10.1186/s13098-017-0241-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanc-Bisson C, Velayoudom-Cephise FL, Cougnard-Gregoire A, Helmer C, Rajaobelina K, Delcourt C, et al. Skin autofluorescence predicts major adverse cardiovascular events in patients with type 1 diabetes: a 7-year follow-up study. Cardiovasc Diabetol. 2018;17:82. 10.1186/s12933-018-0718-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutgers HL, Graaff R, Links TP, Ubink-Veltmaat LJ, Bilo HJ, Gans RO, et al. Skin autofluorescence as a noninvasive marker of vascular damage in patients with type 2 diabetes. Diabetes Care. 2006;29:2654–9. 10.2337/dc05-2173 [DOI] [PubMed] [Google Scholar]
- 11.McIntyre NJ, Fluck RJ, McIntyre CW, Taal MW. Skin autofluorescence and the association with renal and cardiovascular risk factors in chronic kidney disease stage 3. Clin J Am Soc Nephrol. 2011;6:2356–63. 10.2215/CJN.02420311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–31. 10.1074/jbc.M006700200 [DOI] [PubMed] [Google Scholar]
- 13.Hofmann B, Jacobs K, Navarrete Santos A, Wienke A, Silber RE, Simm A. Relationship between cardiac tissue glycation and skin autofluorescence in patients with coronary artery disease. Diabetes Metab. 2015;41:410–5. 10.1016/j.diabet.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 14.Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991;87:432–8. 10.1172/JCI115014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu F, Feng J-Z, Qiu Y-H, Yu F-B, Zhang J-Z, Zhou W, et al. Activation of receptor for advanced glycation end products contributes to aortic remodeling and endothelial dysfunction in sinoaortic denervated rats. Atherosclerosis. 2013;229:287–94. 10.1016/j.atherosclerosis.2013.04.033 [DOI] [PubMed] [Google Scholar]
- 16.Ren X, Ren L, Wei Q, Shao H, Chen L, Liu N. Advanced glycation end-products decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Cardiovasc Diabetol. 2017;16:52. 10.1186/s12933-017-0531-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267:14987–97. 10.1016/S0021-9258(18)42137-0 [DOI] [PubMed] [Google Scholar]
- 18.Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–5004. 10.1016/S0021-9258(18)42138-2 [DOI] [PubMed] [Google Scholar]
- 19.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. 10.1016/S0092-8674(00)80801-6 [DOI] [PubMed] [Google Scholar]
- 20.Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–29. 10.1016/j.redox.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odrovicsné-Tóth A, Thauerer B, Stritzinger B, Kullich W, Salzer A, Skoumal M, et al. The patient’s physiological status at the start determines the success of the inpatient cardiovascular rehabilitation program. J Clin Med. 2023;12:1735. 10.3390/jcm12051735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saz-Lara A, Álvarez-Bueno C, Martínez-Vizcaíno V, Notario-Pacheco B, Sequí-Dominguez I, Cavero-Redondo I. Are advanced glycation end products in skin associated with vascular dysfunction markers? A meta-analysis. Int J Environ Res Public Health. 2020;17:6936. 10.3390/ijerph17186936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birukov A, Cuadrat R, Polemiti E, Eichelmann F, Schulze MB. Advanced glycation end-products, measured as skin autofluorescence, associate with vascular stiffness in diabetic, pre-diabetic and normoglycemic individuals: a cross-sectional study. Cardiovasc Diabetol. 2021;20:110. 10.1186/s12933-021-01296-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thijssen DHJ, Bruno RM, van Mil ACCM, Holder SM, Faita F, Greyling A, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J. 2019;40:2534–47. 10.1093/eurheartj/ehz350 [DOI] [PubMed] [Google Scholar]
- 25.Wang C-C, Wang Y-C, Wang G-J, Shen M-Y, Chang Y-L, Liou S-Y, et al. Skin autofluorescence is associated with endothelial dysfunction in uremic subjects on hemodialysis. PLoS ONE. 2016;11:e0147771. 10.1371/journal.pone.0147771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ninomiya H, Katakami N, Sato I, Osawa S, Yamamoto Y, Takahara M, et al. Association between subclinical atherosclerosis markers and the level of accumulated advanced glycation end-products in the skin of patients with diabetes. J Atheroscler Thromb. 2018;25:1274–84. 10.5551/jat.44859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato K, Matsuda N, Takahata M, Koseki C, Yamaki M, Sato T. Relationship between occlusal force and endothelial function in community-dwelling elderly women: a pilot study. Clin Exp Dent Res. 2022;8:1207–12. 10.1002/cre2.630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial artery reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. 10.1016/S0735-1097(01)01746-6 [DOI] [PubMed] [Google Scholar]
- 29.Sonka M, Liang W, Lauer RM. Automated analysis of brachial ultrasound image sequences: early detection of cardiovascular disease via surrogates of endothelial function. IEEE Trans Med Imaging. 2002;21:1271–9. 10.1109/TMI.2002.806288 [DOI] [PubMed] [Google Scholar]
- 30.Atkinson G, Batterham AM. Allometric scaling of diameter change in the original flow-mediated dilation protocol. Atherosclerosis. 2013;226:425–7. 10.1016/j.atherosclerosis.2012.11.027 [DOI] [PubMed] [Google Scholar]
- 31.Meerwaldt R, Hartog JWL, Graaff R, Huisman RJ, Links TP, den Hollander NC, et al. Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J Am Soc Nephrol. 2005;16:3687. 10.1681/ASN.2005020144 [DOI] [PubMed] [Google Scholar]
- 32.Marfell-Jones MJ, Stewart AD, de Ridder JH. International standards for anthropometric assessment. Wellington, New Zealand: International Society for the Advancement of Kinanthropometry; 2012.
- 33.Lee PH, Macfarlane DJ, Lam T, Stewart SM. Validity of the international physical activity questionnaire short form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8:115. 10.1186/1479-5868-8-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 35.Tanaka K, Nakayama M, Kanno M, Kimura H, Watanabe K, Tani Y, et al. Skin autofluorescence is associated with the progression of chronic kidney disease: a prospective observational study. PLoS ONE. 2013;8:e83799. 10.1371/journal.pone.0083799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fotheringham AK, Gallo LA, Borg DJ, Forbes JM. Advanced glycation end products (AGEs) and chronic kidney disease: does the modern diet AGE the kidney? Nutrients. 2022;14:2675. 10.3390/nu14132675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Gong D, Li S, Zhou X. Meta-analysis of the effects of statin therapy on endothelial function in patients with diabetes mellitus. Atherosclerosis. 2012;223:78–85. 10.1016/j.atherosclerosis.2012.01.031 [DOI] [PubMed] [Google Scholar]
- 38.Shahin Y, Khan JA, Samuel N, Chetter I. Angiotensin converting enzyme inhibitors effect on endothelial dysfunction: a meta-analysis of randomised controlled trials. Atherosclerosis. 2011;216:7–16. 10.1016/j.atherosclerosis.2011.02.044 [DOI] [PubMed] [Google Scholar]
- 39.Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795–804. 10.2337/diabetes.52.11.2795 [DOI] [PubMed] [Google Scholar]
- 40.Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;20:742–52. 10.1681/ASN.2008050514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witte DR, Westerink J, de Koning EJ, van der Graaf Y, Grobbee DE, Bots ML. Is the association between flow-mediated dilation and cardiovascular risk limited to low-risk populations? J Am Coll Cardiol. 2005;45:1987–93. 10.1016/j.jacc.2005.02.073 [DOI] [PubMed] [Google Scholar]
- 42.van Mil ACCM, Greyling A, Zock PL, Geleijnse JM, Hopman MT, Mensink RP, et al. Impact of volunteer-related and methodology-related factors on the reproducibility of brachial artery flow-mediated vasodilation: analysis of 672 individual repeated measurements. J Hypertens. 2016;34:1738. 10.1097/HJH.0000000000001012 [DOI] [PubMed] [Google Scholar]
- 43.Green DJ, Dawson EA, Groenewoud HMM, Jones H, Thijssen DHJ. Is flow-mediated dilation nitric oxide mediated? Hypertension. 2014;63:376–82. 10.1161/HYPERTENSIONAHA.113.02044 [DOI] [PubMed] [Google Scholar]
- 44.Wautier J-L, Wautier M-P. Cellular and molecular aspects of blood cell–endothelium interactions in vascular disorders. Int J Mol Sci. 2020;21:5315. 10.3390/ijms21155315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basta G, Lazzerini G, Del Turco S, Ratto GM, Schmidt AM, De Caterina R. At least 2 distinct pathways generating reactive oxygen species mediate vascular cell adhesion molecule-1 induction by advanced glycation end products. Arterioscler Thromb Vasc Biol. 2005;25:1401–7. 10.1161/01.ATV.0000167522.48370.5e [DOI] [PubMed] [Google Scholar]
- 46.Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889–97. 10.1016/S0021-9258(17)36966-1 [DOI] [PubMed] [Google Scholar]
- 47.Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM. Activation of the receptor for advanced glycation end products triggers a p21 ras -dependent mitogen-activated protein kinase pathway regulated by oxidant stress. J Biol Chem. 1997;272:17810–4. 10.1074/jbc.272.28.17810 [DOI] [PubMed] [Google Scholar]
- 48.Adamopoulos C, Piperi C, Gargalionis AN, Dalagiorgou G, Spilioti E, Korkolopoulou P, et al. Advanced glycation end products upregulate lysyl oxidase and endothelin-1 in human aortic endothelial cells via parallel activation of ERK1/2–NF-κB and JNK–AP-1 signaling pathways. Cell Mol Life Sci. 2016;73:1685–98. 10.1007/s00018-015-2091-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biao X, Chibber R, Ruggiero D, Kohner E, Ritter J, Ferro A. Impairment of vascular endothelial nitric oxide synthase activity by advanced glycation end products. FASEB J. 2003;17:1289–91. 10.1096/fj.02-0490fje [DOI] [PubMed] [Google Scholar]
- 50.Ma J, Li Y, Yang X, Liu K, Zhang X, Zuo X, et al. Signaling pathways in vascular function and hypertension: molecular mechanisms and therapeutic interventions. Sig Transduct Target Ther. 2023;8:1–30. 10.1038/s41392-023-01430-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoon HJ, Cho SW, Ahn BW, Yang SY. Alterations in the activity and expression of endothelial NO synthase in aged human endothelial cells. Mech Ageing Dev. 2010;131:119–23. 10.1016/j.mad.2009.12.010 [DOI] [PubMed] [Google Scholar]
- 52.Kielstein JT, Bode-Böger SM, Frölich JC, Ritz E, Haller H, Fliser D. Asymmetric dimethylarginine, blood pressure, and renal perfusion in elderly subjects. Circulation. 2003;107:1891–5. 10.1161/01.CIR.0000060496.23144.A7 [DOI] [PubMed] [Google Scholar]
- 53.Paneni F, Diaz Cañestro C, Libby P, Lüscher TF, Camici GG. The aging cardiovascular system: understanding it at the cellular and clinical levels. J Am Coll Cardiol. 2017;69:1952–67. 10.1016/j.jacc.2017.01.064 [DOI] [PubMed] [Google Scholar]
- 54.Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–67. 10.1161/CIRCRESAHA.118.311378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, et al. Brachial artery diameter as a marker for cardiovascular risk assessment: FMD-J study. Atherosclerosis. 2018;268:92–8. 10.1016/j.atherosclerosis.2017.11.022 [DOI] [PubMed] [Google Scholar]
- 56.Holubkov R, Karas RH, Pepine CJ, Rickens CR, Reichek N, Rogers WJ, et al. Large brachial artery diameter is associated with angiographic coronary artery disease in women. Am Heart J. 2002;143:802–7. 10.1067/mhj.2002.121735 [DOI] [PubMed] [Google Scholar]
- 57.Atkinson G, Batterham AM, Thijssen DHJ, Green DJ. A new approach to improve the specificity of flow-mediated dilation for indicating endothelial function in cardiovascular research. J Hypertens. 2013;31:287–91. 10.1097/HJH.0b013e32835b8164 [DOI] [PubMed] [Google Scholar]
- 58.Königstein K, Wagner J, Frei M, Knaier R, Klenk C, Carrard J, et al. Endothelial function of healthy adults from 20 to 91 years of age: prediction of cardiovascular risk by vasoactive range. J Hypertens. 2021;39:1361–9. 10.1097/HJH.0000000000002798 [DOI] [PubMed] [Google Scholar]
- 59.Hayashi T, Yamada K, Esaki T, Kuzuya M, Satake S, Ishikawa T, et al. Estrogen increases endothelial nitric oxide by a receptor mediated system. Biochem Biophys Res Commun. 1995;214:847–55. 10.1006/bbrc.1995.2364 [DOI] [PubMed] [Google Scholar]
- 60.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab. 2012;97:4692–700. 10.1210/jc.2012-2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, et al. Relationship between flow-mediated vasodilation and cardiovascular risk factors in a large community-based study. Heart. 2013;99:1837–42. 10.1136/heartjnl-2013-304739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K, et al. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation. 1995;92:3431–5. 10.1161/01.CIR.92.12.3431 [DOI] [PubMed] [Google Scholar]
- 63.Atzeni IM, Boersema J, Pas HH, Diercks GFH, Scheijen JLJM, Schalkwijk CG, et al. Is skin autofluorescence (SAF) representative of dermal advanced glycation endproducts (AGEs) in dark skin? A pilot study. Heliyon. 2020;6:e05364. 10.1016/j.heliyon.2020.e05364 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated and/or analysed during the current study are available from the corresponding author on reasonable request.