Abstract
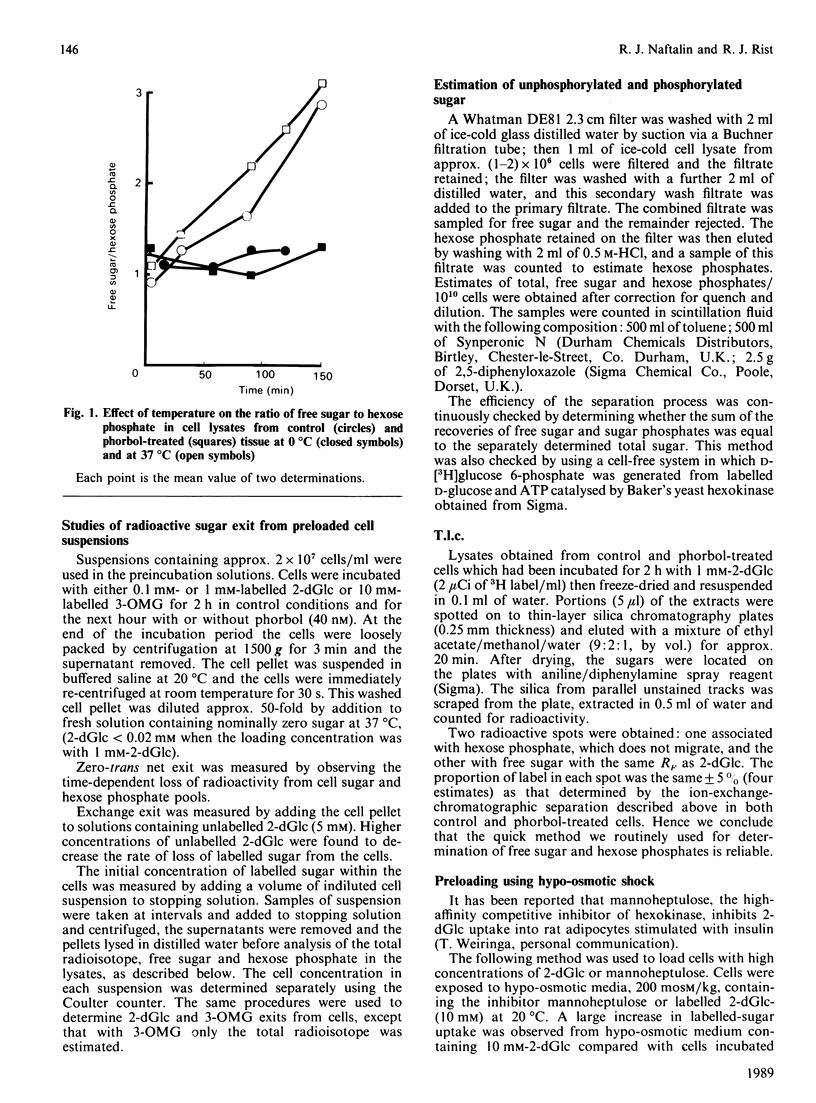
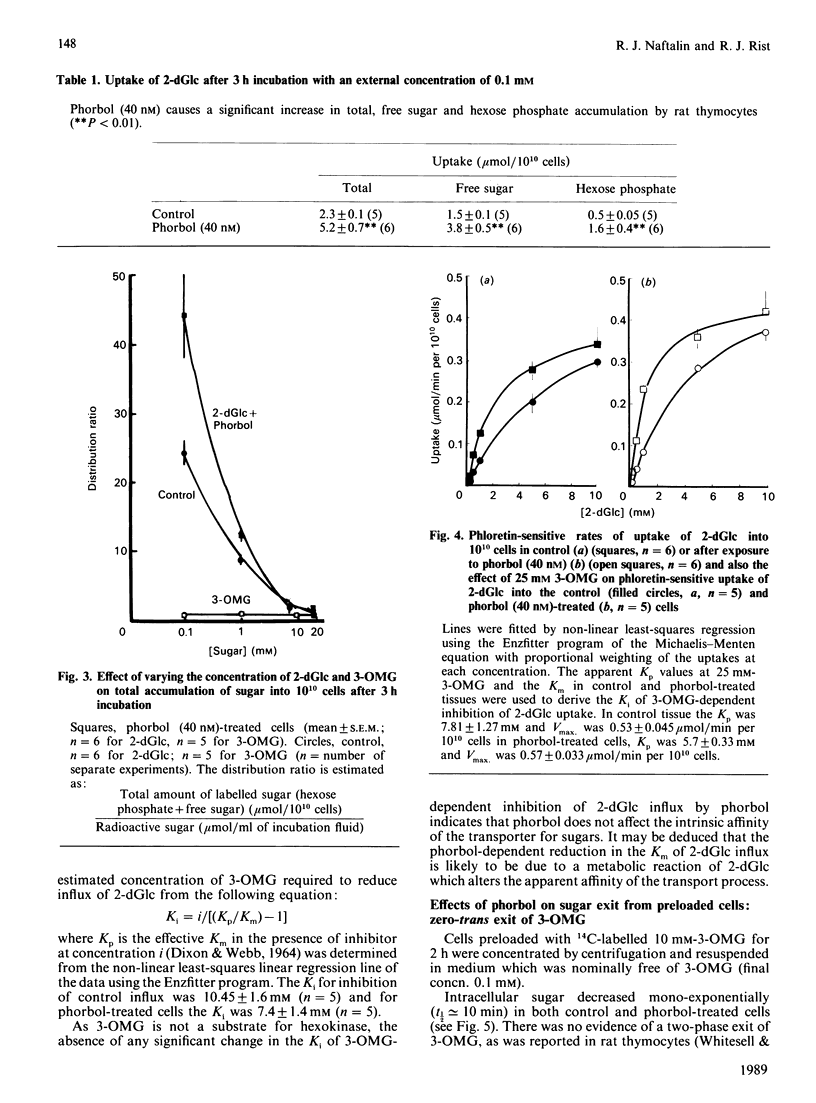
1. Suspensions of rat thymocytes accumulate free 2-deoxy-D-glucose (2-dGlc) within the cytosol to a concentration approx. 25-fold above the external concentration. This active accumulation was enhanced by 40 nM-phorbol 12-myristate 13-acetate (phorbol). 2. The Km for zero-trans uptake in control cells was 2.3 +/- 0.14 mM and Vmax. was 0.41 +/- 0.08 mumol/min per 10(10) cells (n = 6). In cells treated with phorbol (40 nM) the Km for zero-trans uptake was 1.2 +/- 0.13 mM and Vmax. 0.46 +/- 0.03 mumol/min per 10(10) cells (n = 6). The Km was decreased significantly by phorbol (P less than 0.01). 3. Phorbol-dependent activation of thymocytes delayed exit of free 2-dGlc into sugar-free solution and prevented exchange exit. Activation had no effect on 3-O-methyl D-glucoside (3-OMG) exit. 4. Coupling of 2-dGlc transport to hexokinase activity was determined by observing the effects of various concentrations of unlabelled cytosolic 2-dGlc on influx of labelled 2-dGlc into the hexose phosphate pool. In control cells this coupling was 0.81 +/- 0.02 and in phorbol-activated cells it was 0.92 +/- 0.01 (P less than 0.01). 5. The high-affinity inhibitor of hexokinase, mannoheptulose, inhibited uptake of 2-dGlc in both control and phorbol-treated cells. These data are consistent with a model for activation of sugar transport in which hexokinase activity is integrated with the sugar transporter at the endofacial surface. The results suggest that phorbol increases the degree of coupling transport with hexokinase activity, thereby leading to an increase in the rate of uptake of 2-dGlc, a decrease in exit of free 2-dGlc from the cytosol and an increase in free 2-dGlc accumulation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Randle P. J. Enzymes of glucose metabolism in normal mouse pancreatic islets. Biochem J. 1970 Aug;119(1):5–15. doi: 10.1042/bj1190005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIHLER I., HAWKINS K. A., CRANE R. K. Studies on the mechanism of intestinal absorption of sugars. VI. The specificity and other properties of Na ion-dependent entrance of sugars into intestinal tissue under anaerobic conditions, in vitro. Biochim Biophys Acta. 1962 May 7;59:94–102. doi: 10.1016/0006-3002(62)90700-x. [DOI] [PubMed] [Google Scholar]
- Carter-Su C., Okamoto K. Effect of glucocorticoids on hexose transport in rat adipocytes. Evidence for decreased transporters in the plasma membrane. J Biol Chem. 1985 Sep 15;260(20):11091–11098. [PubMed] [Google Scholar]
- Christopher C. W., Kohlbacher M. S., Amos H. Transport of sugars in chick-embryo fibroblasts. Evidence for a low-affinity system and a high-affinity system for glucose transport. Biochem J. 1976 Aug 15;158(2):439–450. doi: 10.1042/bj1580439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coore H. G., Randle P. J. Inhibition of glucose phosphorylation by mannoheptulose. Biochem J. 1964 Apr;91(1):56–59. doi: 10.1042/bj0910056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amore T., Lo T. C. Hexose transport in L6 rat myoblasts. I. Rate-limiting step, kinetic properties, and evidence for two systems. J Cell Physiol. 1986 Apr;127(1):95–105. doi: 10.1002/jcp.1041270113. [DOI] [PubMed] [Google Scholar]
- Elsas L. J., 2nd, Macdonell R. C., Jr Hexose transport and phosphorylation by hamster kidney cortex slices and averted jejunal rings. Biochim Biophys Acta. 1972 Mar 17;255(3):948–959. doi: 10.1016/0005-2736(72)90405-1. [DOI] [PubMed] [Google Scholar]
- Foley J. E., Gliemann J. Accumulation of 2-deoxyglucose against its concentration gradient in rat adipocytes. Biochim Biophys Acta. 1981 Oct 20;648(1):100–106. doi: 10.1016/0005-2736(81)90129-2. [DOI] [PubMed] [Google Scholar]
- Graff J. C., Wohlhueter R. M., Plagemann P. G. Deoxyglucose and 3-O-methylglucose transport in untreated and ATP-depleted Novikoff rat hepatoma cells. Analysis by a rapid kinetic technique, relationship to phosphorylation and effects of inhibitors. J Cell Physiol. 1978 Aug;96(2):171–188. doi: 10.1002/jcp.1040960206. [DOI] [PubMed] [Google Scholar]
- Haspel H. C., Wilk E. W., Birnbaum M. J., Cushman S. W., Rosen O. M. Glucose deprivation and hexose transporter polypeptides of murine fibroblasts. J Biol Chem. 1986 May 25;261(15):6778–6789. [PubMed] [Google Scholar]
- Jaspers H. T., van Steveninck J. Transport-associated phosphorylation of 2-deoxy-D-glucose in Saccharomyces fragilis. Biochim Biophys Acta. 1975 Oct 17;406(3):370–385. doi: 10.1016/0005-2736(75)90017-6. [DOI] [PubMed] [Google Scholar]
- Kleinzeller A., McAvoy E. M. Transport and phosphorylation of 2-deoxy-D-galactase in renal cortical cells. Biochim Biophys Acta. 1976 Nov 11;455(1):126–143. doi: 10.1016/0005-2736(76)90158-9. [DOI] [PubMed] [Google Scholar]
- Klip A., Mack E., Cragoe E. J., Jr, Grinstein S. Regulation of amino acid uptake by phorbol esters and hypertonic solutions in rat thymocytes. J Cell Physiol. 1986 May;127(2):244–252. doi: 10.1002/jcp.1041270209. [DOI] [PubMed] [Google Scholar]
- Klip A., Rothstein A., Mack E. Stimulation of hexose uptake in rat thymic lymphocytes by phorbol ester. A role for Ca2+ and Na+/H+ exchange? Biochem Biophys Res Commun. 1984 Oct 15;124(1):14–22. doi: 10.1016/0006-291x(84)90909-4. [DOI] [PubMed] [Google Scholar]
- Matthaei S., Olefsky J. M., Karnieli E. Cycloheximide decreases glucose transporters in rat adipocyte plasma membranes without affecting insulin-stimulated glucose transport. Biochem J. 1988 Apr 15;251(2):491–497. doi: 10.1042/bj2510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftalin R. J., Smith P. M. A model for accelerated uptake and accumulation of sugars arising from phosphorylation at the inner surface of the cell membrane. Biochim Biophys Acta. 1987 Feb 12;897(1):93–111. doi: 10.1016/0005-2736(87)90318-x. [DOI] [PubMed] [Google Scholar]
- Nordenberg J., Stenzel K. H., Novogrodsky A. 12-0-tetradecanoylphorbol-13-acetate and concanavalin A enhance glucose uptake in thymocytes by different mechanisms. J Cell Physiol. 1983 Nov;117(2):183–188. doi: 10.1002/jcp.1041170208. [DOI] [PubMed] [Google Scholar]
- Segal J., Ingbar S. H. Studies of the mechanism by which 3,5,3'- triiodothyronine stimulates 2-deoxyglucose uptake in rat thymocytes in vitro. Role of calcium and adenosine 3':5'-monophosphate. J Clin Invest. 1981 Jul;68(1):103–110. doi: 10.1172/JCI110224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal J. The effect of trypsin on sugar uptake in rat thymocytes. Modulation of cellular cyclic AMP concentration and the sugar-transport system. Biochem J. 1987 Sep 15;246(3):561–566. doi: 10.1042/bj2460561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weringa T., van Dam K., Bos M. P., van Putten J. P., Krans H. M. The effects of 1-methyl-3-isobutylxanthine on insulin-sensitive 2-deoxyglucose transport. Biochim Biophys Acta. 1984 Mar 23;803(3):123–128. doi: 10.1016/0167-4889(84)90001-6. [DOI] [PubMed] [Google Scholar]
- Whitesell R. R., Abumrad N. A. Increased affinity predominates in insulin stimulation of glucose transport in the adipocyte. J Biol Chem. 1985 Mar 10;260(5):2894–2899. [PubMed] [Google Scholar]
- Whitesell R. R., Regen D. M. Glucose transport characteristics of quiescent thymocytes. J Biol Chem. 1978 Oct 25;253(20):7289–7294. [PubMed] [Google Scholar]


