Abstract
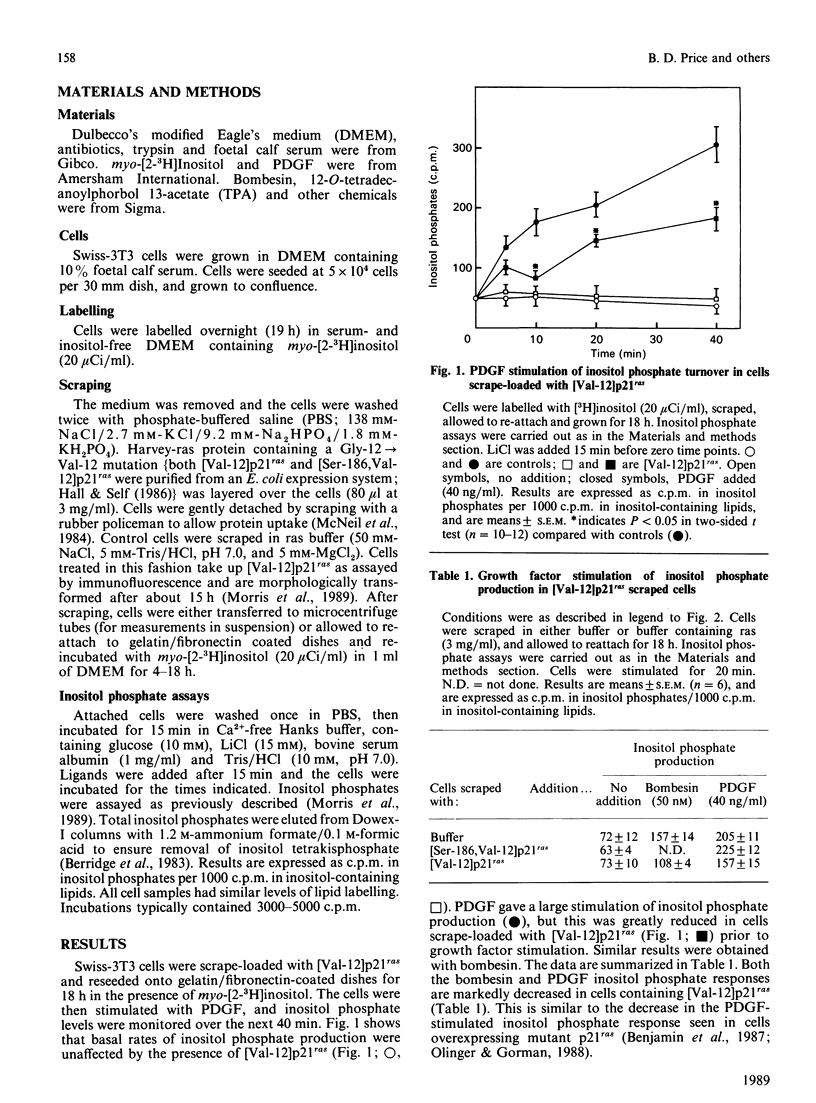
The effect of scrape-loaded [Val-12]p21ras on agonist-stimulated phosphatidylinositol 4,5-bisphosphate (PIP2) turnover in Swiss-3T3 cells was studied. Previously [Morris, Price, Lloyd, Marshall & Hall (1989) Oncogene 4, 27-31] we demonstrated that [Val-12]p21ras activates protein kinase C within 10 min of scrape loading. Here, we show that [Val-12]p21ras inhibits bombesin and platelet-derived growth factor-stimulated PIP2 breakdown 1.5-4 h after scrape loading. This effect persisted for at least 18 h and could be mimicked in control cells by activation of protein kinase C with 12-O-tetradecanoyl 13-acetate (TPA) 15 min prior to ligand stimulation. When protein kinase C was down-regulated by chronic TPA treatment, [Val-12]p21ras was no longer able to inhibit agonist-stimulated inositol phosphate production. These results indicate that changes in inositol phosphate levels caused by ras protein are probably due to activation of protein kinase C and not to an interaction of ras with phospholipase C.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso T., Morgan R. O., Marvizon J. C., Zarbl H., Santos E. Malignant transformation by ras and other oncogenes produces common alterations in inositol phospholipid signaling pathways. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4271–4275. doi: 10.1073/pnas.85.12.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986 Sep 5;233(4768):1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Benjamin C. W., Connor J. A., Tarpley W. G., Gorman R. R. NIH-3T3 cells transformed by the EJ-ras oncogene exhibit reduced platelet-derived growth factor-mediated Ca2+ mobilization. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4345–4349. doi: 10.1073/pnas.85.12.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin C. W., Tarpley W. G., Gorman R. R. Loss of platelet-derived growth factor-stimulated phospholipase activity in NIH-3T3 cells expressing the EJ-ras oncogene. Proc Natl Acad Sci U S A. 1987 Jan;84(2):546–550. doi: 10.1073/pnas.84.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M., Hamon M. H., Laurie M. S., Corps A. N. Protein kinase C-mediated negative-feedback inhibition of unstimulated and bombesin-stimulated polyphosphoinositide hydrolysis in Swiss-mouse 3T3 cells. Biochem J. 1987 Aug 1;245(3):631–639. doi: 10.1042/bj2450631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman L. F., Chahwala S. B., Cantley L. ras-transformed cells: altered levels of phosphatidylinositol-4,5-bisphosphate and catabolites. Science. 1986 Jan 24;231(4736):407–410. doi: 10.1126/science.3001936. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Marshall C. J., McKay I. A., Gardner S., Houslay M. D., Hall A., Wakelam M. J. Mutant but not normal p21 ras elevates inositol phospholipid breakdown in two different cell systems. Oncogene. 1988 Aug;3(2):187–193. [PubMed] [Google Scholar]
- Kamata T., Sullivan N. F., Wooten M. W. Reduced protein kinase C activity in a ras-resistant cell line derived from Ki-MSV transformed cells. Oncogene. 1987 Mar;1(1):37–46. [PubMed] [Google Scholar]
- Lacal J. C., Fleming T. P., Warren B. S., Blumberg P. M., Aaronson S. A. Involvement of functional protein kinase C in the mitogenic response to the H-ras oncogene product. Mol Cell Biol. 1987 Nov;7(11):4146–4149. doi: 10.1128/mcb.7.11.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal J. C., Moscat J., Aaronson S. A. Novel source of 1,2-diacylglycerol elevated in cells transformed by Ha-ras oncogene. Nature. 1987 Nov 19;330(6145):269–272. doi: 10.1038/330269a0. [DOI] [PubMed] [Google Scholar]
- Maly K., Doppler W., Oberhuber H., Meusburger H., Hofmann J., Jaggi R., Grunicke H. H. Desensitization of the Ca2+-mobilizing system to serum growth factors by Ha-ras and v-mos. Mol Cell Biol. 1988 Oct;8(10):4212–4216. doi: 10.1128/mcb.8.10.4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- McNeil P. L., Murphy R. F., Lanni F., Taylor D. L. A method for incorporating macromolecules into adherent cells. J Cell Biol. 1984 Apr;98(4):1556–1564. doi: 10.1083/jcb.98.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. D., Price B., Lloyd A. C., Self A. J., Marshall C. J., Hall A. Scrape-loading of Swiss 3T3 cells with ras protein rapidly activates protein kinase C in the absence of phosphoinositide hydrolysis. Oncogene. 1989 Jan;4(1):27–31. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Olinger P. L., Gorman R. R. NIH-3T3 cells expressing high levels of the c-ras proto-oncogene display reduced platelet derived growth factor-stimulated phospholipase activity. Biochem Biophys Res Commun. 1988 Feb 15;150(3):937–941. doi: 10.1016/0006-291x(88)90719-x. [DOI] [PubMed] [Google Scholar]
- Parries G., Hoebel R., Racker E. Opposing effects of a ras oncogene on growth factor-stimulated phosphoinositide hydrolysis: desensitization to platelet-derived growth factor and enhanced sensitivity to bradykinin. Proc Natl Acad Sci U S A. 1987 May;84(9):2648–2652. doi: 10.1073/pnas.84.9.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
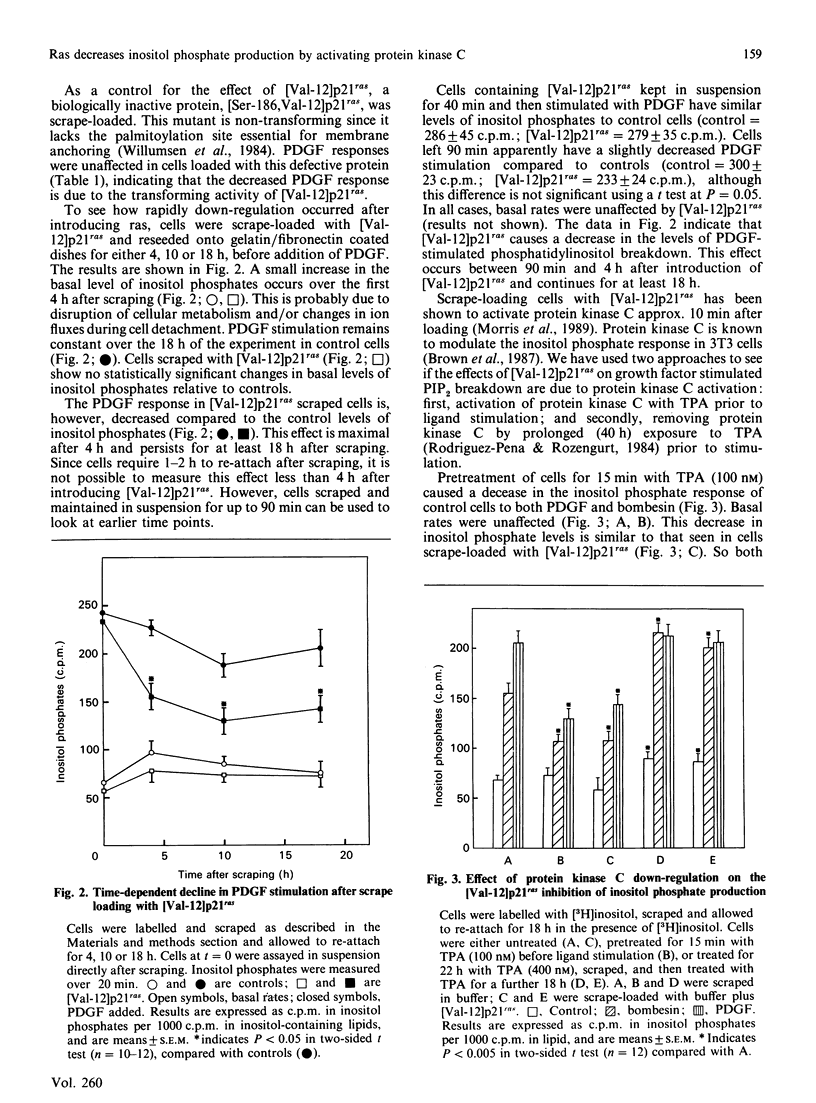
- Trahey M., McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987 Oct 23;238(4826):542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Banks-Schlegel S. P., Pastan I. H. Immunocytochemical localization in normal and transformed human cells in tissue culture using a monoclonal antibody to the src protein of the Harvey strain of murine sarcoma virus. Exp Cell Res. 1983 Nov;149(1):141–149. doi: 10.1016/0014-4827(83)90387-7. [DOI] [PubMed] [Google Scholar]
- Willumsen B. M., Christensen A., Hubbert N. L., Papageorge A. G., Lowy D. R. The p21 ras C-terminus is required for transformation and membrane association. Nature. 1984 Aug 16;310(5978):583–586. doi: 10.1038/310583a0. [DOI] [PubMed] [Google Scholar]
- Wolfman A., Macara I. G. Elevated levels of diacylglycerol and decreased phorbol ester sensitivity in ras-transformed fibroblasts. Nature. 1987 Jan 22;325(6102):359–361. doi: 10.1038/325359a0. [DOI] [PubMed] [Google Scholar]
- Wolfman A., Wingrove T. G., Blackshear P. J., Macara I. G. Down-regulation of protein kinase C and of an endogenous 80-kDa substrate in transformed fibroblasts. J Biol Chem. 1987 Dec 5;262(34):16546–16552. [PubMed] [Google Scholar]


