Abstract
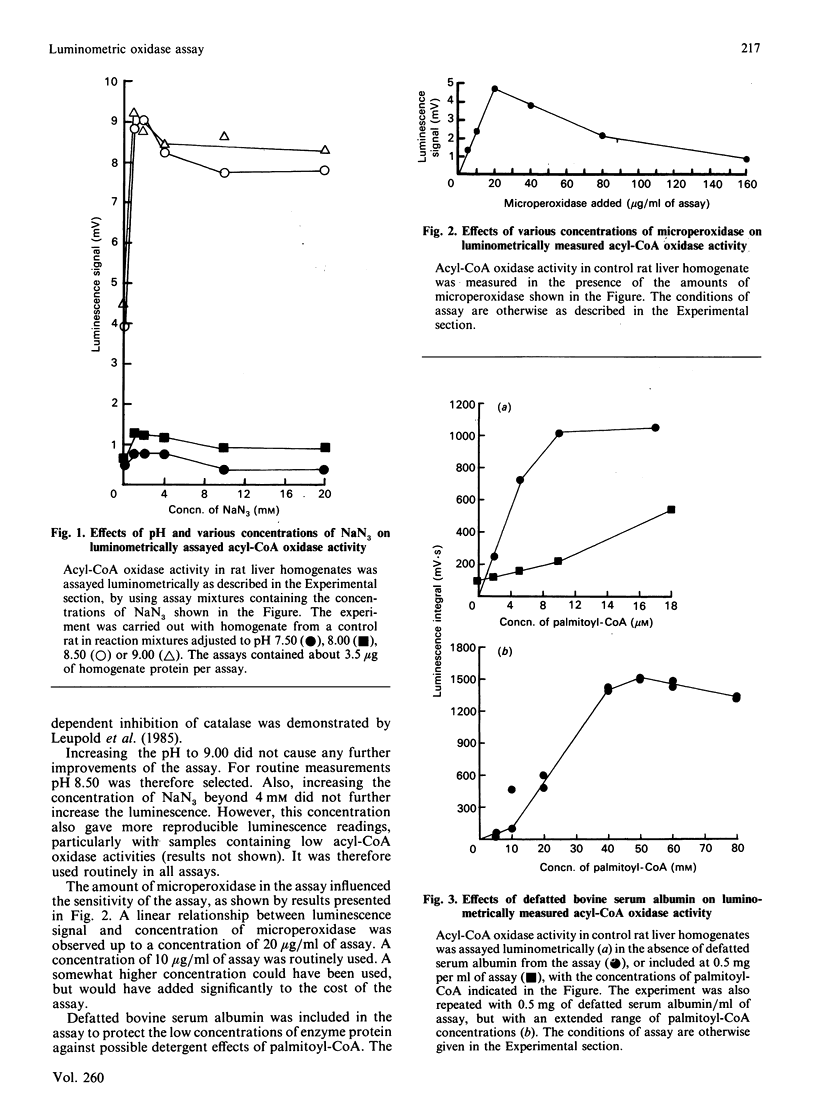
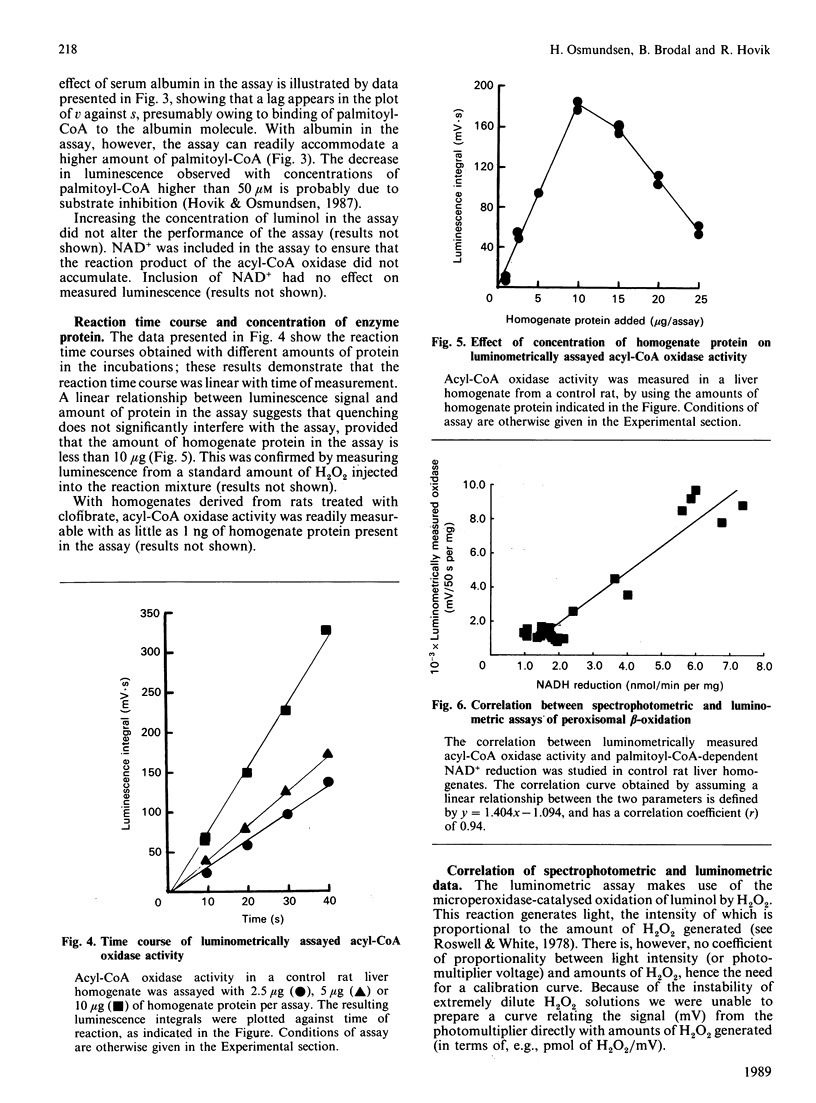
1. A luminometric assay for acyl-CoA oxidase activity is described. The assay uses the luminol/microperoxidase system to monitor continuously acyl-CoA-dependent generation of H2O2. The assay is rapid, convenient, and lends itself to automation with an LKB 1251 luminometer. The assay is extremely sensitive, requiring at the most 10 micrograms of liver-homogenate protein per assay. 2. The assay can also be used to measure other oxidases, e.g. glycollate oxidase (EC 1.1.3.15), D-aspartate oxidase (EC 1.4.3.1) and urate oxidase (EC 1.7.3.3), the only modification being substitution of substrates to appropriate concentration. 3. With rat liver homogenates, spectrophotometrically measured rates of palmitoyl-CoA-dependent NAD+ reduction and acyl-CoA oxidase activity [Hryb & Hogg (1979) Biochem. Biophys. Res. Commun. 87, 1200-1206] was generally found in good agreement with luminometrically measured acyl-CoA oxidase activity. 4. With liver homogenates from streptozotocin-diabetic rats, however, rates of palmitoyl-CoA-dependent NAD+ reduction were consistently lower than the corresponding acyl-CoA oxidase activity. This difference was most marked with respect to luminometrically assayed acyl-CoA oxidase activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berge R. K., Hosøy L. H., Farstad M. N. Influence of dietary status on liver palmitoyl-CoA hydrolase, peroxisomal enzymes, CoASH and long-chain acyl-CoA in rats. Int J Biochem. 1984;16(4):403–410. doi: 10.1016/0020-711x(84)90139-3. [DOI] [PubMed] [Google Scholar]
- Hamilton G. A. Peroxisomal oxidases and suggestions for the mechanism of action of insulin and other hormones. Adv Enzymol Relat Areas Mol Biol. 1985;57:85–178. doi: 10.1002/9780470123034.ch2. [DOI] [PubMed] [Google Scholar]
- Horie S., Ishii H., Suga T. Changes in peroxisomal fatty acid oxidation in the diabetic rat liver. J Biochem. 1981 Dec;90(6):1691–1696. doi: 10.1093/oxfordjournals.jbchem.a133645. [DOI] [PubMed] [Google Scholar]
- Hovik R., Osmundsen H. Peroxisomal beta-oxidation of long-chain fatty acids possessing different extents of unsaturation. Biochem J. 1987 Nov 1;247(3):531–535. doi: 10.1042/bj2470531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryb D. J., Hogg J. F. Chain length specificities of peroxisomal and mitochondrial beta-oxidation in rat liver. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1200–1206. doi: 10.1016/s0006-291x(79)80034-0. [DOI] [PubMed] [Google Scholar]
- Inestrosa N. C., Bronfman M., Leighton F. Detection of peroxisomal fatty acyl-coenzyme A oxidase activity. Biochem J. 1979 Sep 15;182(3):779–788. doi: 10.1042/bj1820779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii H., Horie S., Suga T. Physiological role of peroxisomal beta-oxidation in liver of fasted rats. J Biochem. 1980 Jun;87(6):1855–1858. doi: 10.1093/oxfordjournals.jbchem.a132931. [DOI] [PubMed] [Google Scholar]
- Kawashima Y., Katoh H., Kozuka H. Differential effects of altered hormonal state on the induction of acyl-CoA hydrolases and peroxisomal beta-oxidation by clofibric acid. Biochim Biophys Acta. 1983 Feb 7;750(2):365–372. doi: 10.1016/0005-2760(83)90041-3. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Localization of the glycerol-phosphate dehydrogenase in the outer phase of the mitochondrial inner membrane. Eur J Biochem. 1970 Apr;13(2):247–252. doi: 10.1111/j.1432-1033.1970.tb00924.x. [DOI] [PubMed] [Google Scholar]
- Kramar R., Kremser K., Hohenegger M., Mayer M. Fatty acyl-CoA oxidase in rat kidney and liver after application of thyroxine. Enzyme. 1986;35(1):27–33. doi: 10.1159/000469315. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B. Three hypolipidemic drugs increase hepatic palmitoyl-coenzyme A oxidation in the rat. Science. 1977 Aug 5;197(4303):580–581. doi: 10.1126/science.195342. [DOI] [PubMed] [Google Scholar]
- Leupold C., Völkl A., Fahimi H. D. Luminometric determination of oxidase activity in peroxisomal fractions of rat liver: glycolate oxidase. Anal Biochem. 1985 Nov 15;151(1):63–69. doi: 10.1016/0003-2697(85)90053-3. [DOI] [PubMed] [Google Scholar]
- Mannaerts G. P., Debeer L. J., Thomas J., De Schepper P. J. Mitochondrial and peroxisomal fatty acid oxidation in liver homogenates and isolated hepatocytes from control and clofibrate-treated rats. J Biol Chem. 1979 Jun 10;254(11):4585–4595. [PubMed] [Google Scholar]
- Slauter R. W., Yamazaki R. K. Glucagon and fasting do not activate peroxisomal fatty acid beta-oxidation in rat liver. Arch Biochem Biophys. 1984 Aug 15;233(1):197–202. doi: 10.1016/0003-9861(84)90617-9. [DOI] [PubMed] [Google Scholar]
- Thomassen M. S., Christiansen E. N., Norum K. R. Characterization of the stimulatory effect of high-fat diets on peroxisomal beta-oxidation in rat liver. Biochem J. 1982 Aug 15;206(2):195–202. doi: 10.1042/bj2060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Walusimbi-Kisitu M., Harrison E. H. Fluorometric assay for rat liver peroxisomal fatty acyl-coenzyme A oxidase activity. J Lipid Res. 1983 Aug;24(8):1077–1084. [PubMed] [Google Scholar]
- Yamada R., Nagasaki H., Wakabayashi Y., Iwashima A. Presence of D-aspartate oxidase in rat liver and mouse tissues. Biochim Biophys Acta. 1988 May 12;965(2-3):202–205. doi: 10.1016/0304-4165(88)90057-8. [DOI] [PubMed] [Google Scholar]


