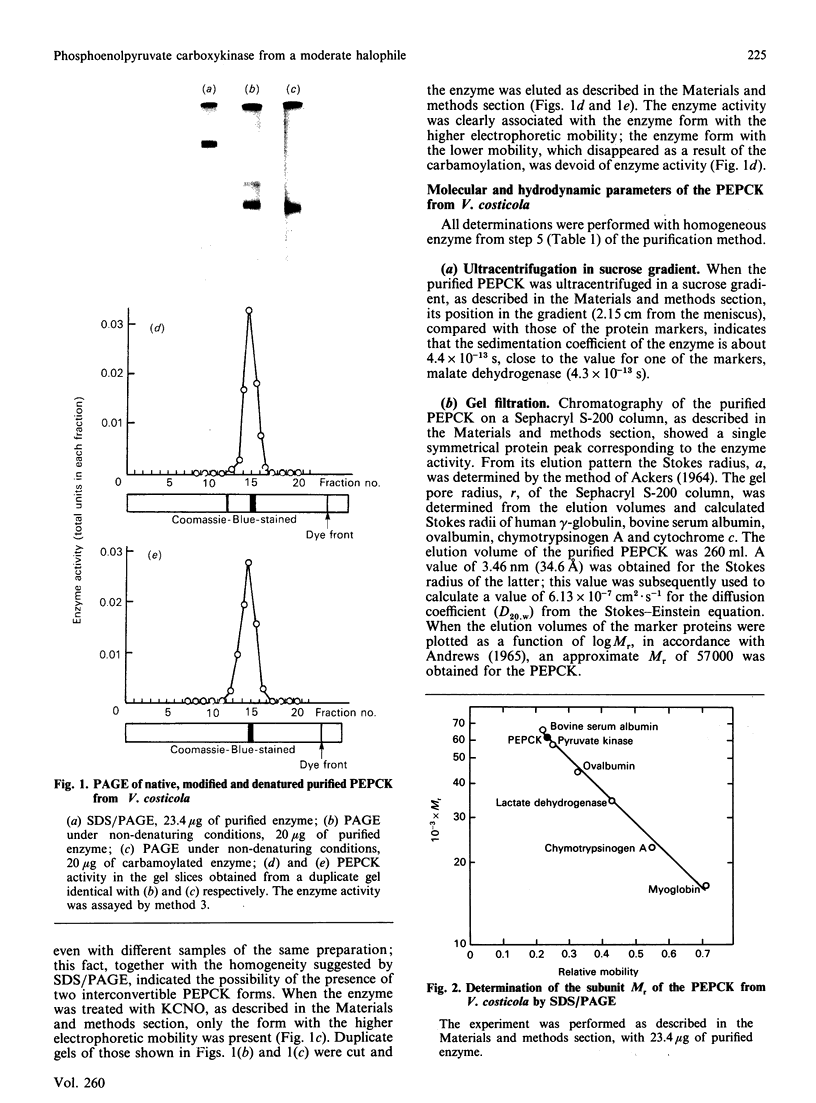
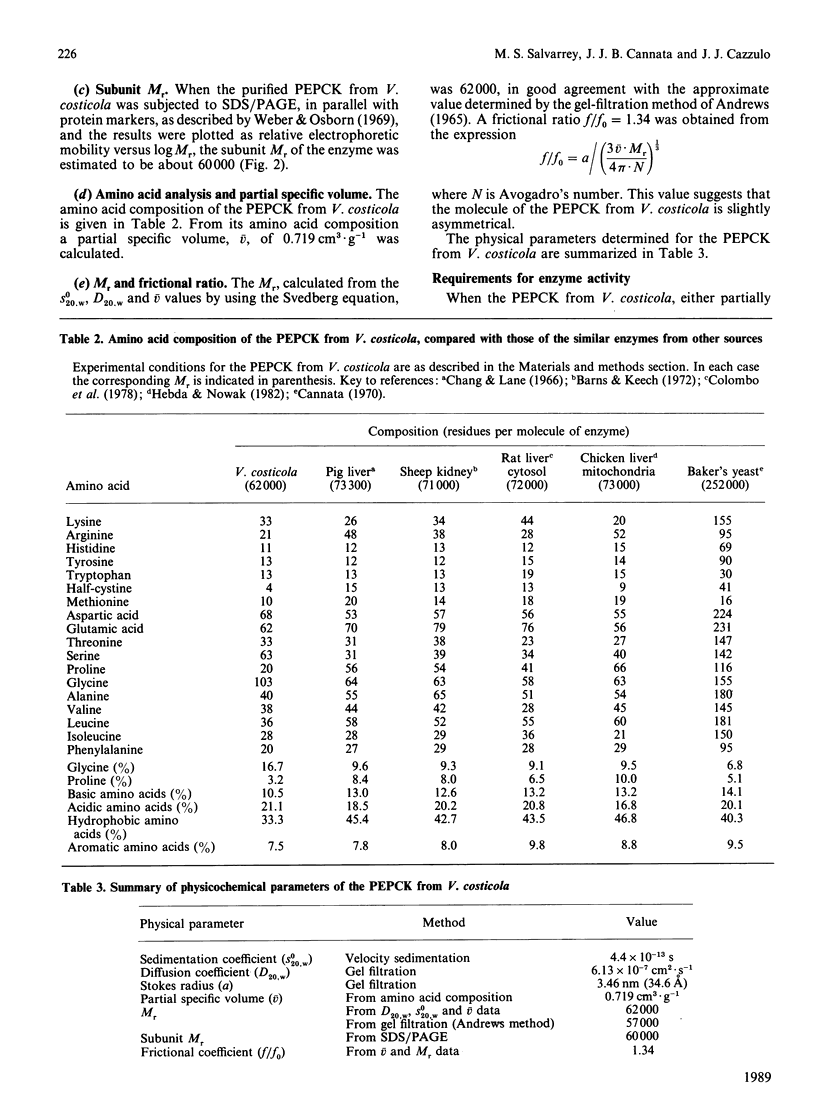
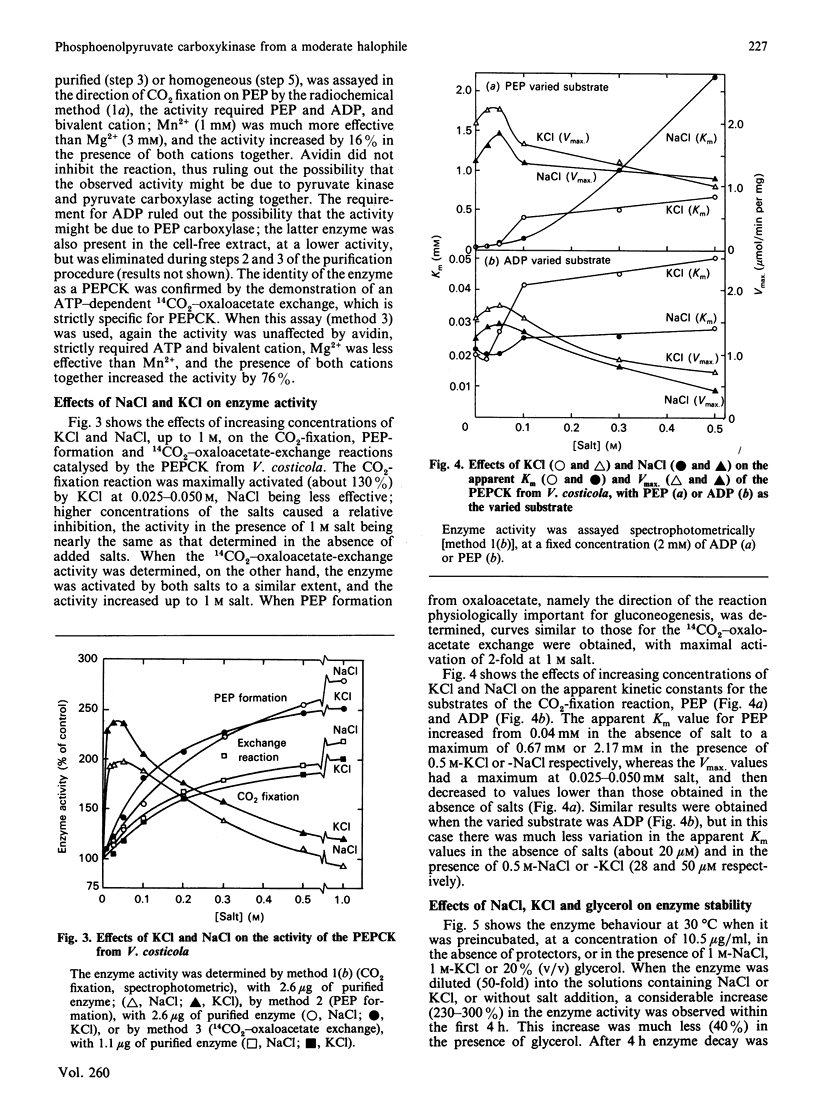
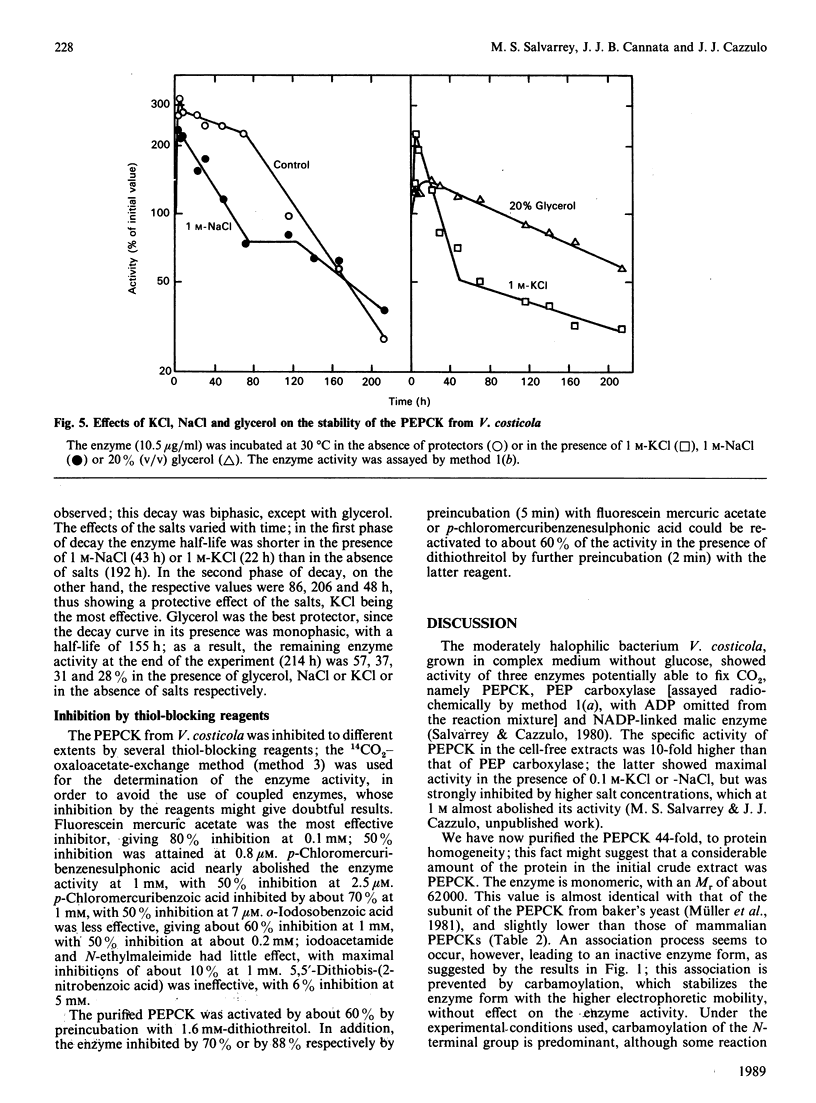
Abstract
Phosphoenolpyruvate carboxykinase (PEPCK) was purified to homogeneity from the moderately halophilic bacterium Vibrio costicola. The enzyme is monomeric, with an Mr of 62,000, as determined by the Svedberg equation, by using values of s0(20,w) 4.4 x 10(-13) s, D20,w 6.13 x 10(-7) cm2.s-1 and v 0.719 cm3.g-1. Compared with other, non-halophilic, PEPCKs, the enzyme from V. costicola had a significantly lower total content of hydrophobic amino acids. The contents of glycine and serine were higher in the V. costicola enzyme (16.7 and 10.22% respectively) than in the non-halophilic PEPCKs (6.8-9.6% and 4.67-6.28% respectively). These results resemble those obtained by De Médicis & Rossignol [(1979) Experientia 35, 1546-1547] with the pyruvate kinase from V. costicola, and agree with the proposal by Lanyi [(1974) Bacteriol. Rev. 38, 272-290] of partial replacement of hydrophobic amino acids by glycine and serine to maintain the balance between hydrophobic and hydrophilic forces in halophilic enzymes. In agreement with this 'halophilic' characteristic, the PEPCK was somewhat stabilized by 1 M-KCl or -NaCl and by 20% (v/v) glycerol, and its oxaloacetate-decarboxylation and 14CO2-oxaloacetate-exchange reactions were activated by KCl and NaCl up to 1 M, whereas the fixation of CO2 on PEP had a maximum at 0.025-0.05 M salt. These facts suggest that the salts, at concentrations probably physiological for the bacterium, increase the formation of the complex of oxaloacetate and ATP with the enzyme, and the liberation of the products, PEP and ADP, thus favouring PEP synthesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K. MOLECULAR EXCLUSION AND RESTRICTED DIFFUSION PROCESSES IN MOLECULAR-SIEVE CHROMATOGRAPHY. Biochemistry. 1964 May;3:723–730. doi: 10.1021/bi00893a021. [DOI] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- Barns R. J., Keech D. B. Sheep kidney phosphoenolpyruvate carboxylase. Purification and properties. Biochim Biophys Acta. 1972 Jul 13;276(1):284–296. doi: 10.1016/0005-2744(72)90031-9. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- CADAVID N. G., PALADINI A. C. AUTOMATIC AMINO ACID ANALYSIS: REAGENT AND INSTRUMENTAL IMPROVEMENTS. Anal Biochem. 1964 Oct;9:170–174. doi: 10.1016/0003-2697(64)90099-5. [DOI] [PubMed] [Google Scholar]
- CHRISTIAN J. H., WALTHO J. A. Solute concentrations within cells of halophilic and non-halophilic bacteria. Biochim Biophys Acta. 1962 Dec 17;65:506–508. doi: 10.1016/0006-3002(62)90453-5. [DOI] [PubMed] [Google Scholar]
- Cannata J. J., De Flombaum M. A. Phosphenolpyruvate carboxykinases from bakers' yeast. Kinetics of phosphoenolpyruvate formation. J Biol Chem. 1974 Jun 10;249(11):3356–3365. [PubMed] [Google Scholar]
- Cannata J. J. Phosphoenolpyruvate carboxykinase from bakers' yeast. Isolation of the enzyme and study of its physical properties. J Biol Chem. 1970 Feb 25;245(4):792–798. [PubMed] [Google Scholar]
- Chang H. C., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. II. Purification and properties of liver mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1966 May 25;241(10):2413–2420. [PubMed] [Google Scholar]
- Colombo G., Carlson G. M., Lardy H. A. Phosphoenolpyruvate carboxykinase (guanosine triphosphate) from rat liver cytosol. Separation of homogeneous forms of the enzyme with high and low activity by chromatography on agarose-hexane-guanosine triphosphate. Biochemistry. 1978 Dec 12;17(25):5321–5329. doi: 10.1021/bi00618a001. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Forsyth M. P., Kushner D. J. Nutrition and distribution of salt response in populations of moderately halophilic bacteria. Can J Microbiol. 1970 Apr;16(4):253–261. doi: 10.1139/m70-047. [DOI] [PubMed] [Google Scholar]
- Foster D. O., Lardy H. A., Ray P. D., Johnston J. B. Alteration of rat liver phosphoenolpyruvate carboxykinase activity by L-tryptophan in vivo and metals in vitro. Biochemistry. 1967 Jul;6(7):2120–2128. doi: 10.1021/bi00859a033. [DOI] [PubMed] [Google Scholar]
- HJERTEN S., LEVIN O., TISELIUS A. Protein chromatography on calcium phosphate columns. Arch Biochem Biophys. 1956 Nov;65(1):132–155. doi: 10.1016/0003-9861(56)90183-7. [DOI] [PubMed] [Google Scholar]
- Haga T., Haga K., Gilman A. G. Hydrodynamic properties of the beta-adrenergic receptor and adenylate cyclase from wild type and varient S49 lymphoma cells. J Biol Chem. 1977 Aug 25;252(16):5776–5782. [PubMed] [Google Scholar]
- Hebda C. A., Nowak T. Phosphoenolpyruvate carboxykinase. Mn2+ and Mn2+ substrate complexes. J Biol Chem. 1982 May 25;257(10):5515–5522. [PubMed] [Google Scholar]
- Lanyi J. K. Salt-dependent properties of proteins from extremely halophilic bacteria. Bacteriol Rev. 1974 Sep;38(3):272–290. doi: 10.1128/br.38.3.272-290.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. H., Hebda C. A., Nowak T. The role of cations in avian liver phosphoenolpyruvate carboxykinase catalysis. Activation and regulation. J Biol Chem. 1981 Dec 25;256(24):12793–12801. [PubMed] [Google Scholar]
- MAEHLY A. C., CHANCE B. The assay of catalases and peroxidases. Methods Biochem Anal. 1954;1:357–424. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Masui M., Wada S. Intracellular concentrations of Na+, K+, and cl minus of a moderately halophilic bacterium. Can J Microbiol. 1973 Oct;19(10):1181–1186. doi: 10.1139/m73-191. [DOI] [PubMed] [Google Scholar]
- Müller M., Müller H., Holzer H. Immunochemical studies on catabolite inactivation of phosphoenolpyruvate carboxykinase in Saccharomyces cerevisiae. J Biol Chem. 1981 Jan 25;256(2):723–727. [PubMed] [Google Scholar]
- Nowicki C., Santomé J. A. Modification of lysine 69 reactivity in bovine growth hormone by carbamylation of its N-terminal group. Int J Pept Protein Res. 1981 Jul;18(1):52–60. doi: 10.1111/j.1399-3011.1981.tb02039.x. [DOI] [PubMed] [Google Scholar]
- Sadler M., McAninch M., Alico R., Hochstein L. I. The intracellular Na+ and K+ composition of the moderately halophilic bacterium, Paracoccus halodenitrificans. Can J Microbiol. 1980 Apr;26(4):496–502. doi: 10.1139/m80-083. [DOI] [PubMed] [Google Scholar]
- Salvarrey M. S., Cazzulo J. J. Some properties of the NADP-specific malic enzyme from the moderate halophile Vibrio costicola. Can J Microbiol. 1980 Jan;26(1):50–57. doi: 10.1139/m80-008. [DOI] [PubMed] [Google Scholar]
- Shindler D. B., Wydro R. M., Kushner D. J. Cell-bound cations of the moderately halophilic bacterium Vibrio costicola. J Bacteriol. 1977 May;130(2):698–703. doi: 10.1128/jb.130.2.698-703.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wydro R. M., Madira W., Hiramatsu T., Kogut M., Kushner D. J. Salt-sensitive in vitro protein synthesis by a moderately halophilic bacterium. Nature. 1977 Oct 27;269(5631):824–825. doi: 10.1038/269824a0. [DOI] [PubMed] [Google Scholar]
- de Médicis E., Rossignol B. Pyruvate kinase from the moderate halophile, Vibrio costicola. Can J Biochem. 1977 Aug;55(8):825–833. doi: 10.1139/o77-122. [DOI] [PubMed] [Google Scholar]
- de Médicis E., Rossignol B. The halophilic properties of pyruvate kinase from Vibrio costicola, a moderate halophile. Experientia. 1979 Dec 15;35(12):1546–1548. doi: 10.1007/BF01953183. [DOI] [PubMed] [Google Scholar]



